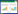The article information
- Sergio Manzetti,Francesco Enrichi
- State-of-the-art developments in metal and carbon-based semiconducting nanomaterials: applications and functions in spintronics, nanophotonics, and nanomagnetics
- Advances in Manufacturing, 2017, 5(2): 105-119.
- http://dx.doi.org/10.1007/s40436-017-0172-y
-
Article history
- Received: 10 August, 2016
- Accepted: 10 April, 2017
- Published online: 14 June, 2017
2 Computational Biology and Bioinformatics, Uppsala University, 751 24 Uppsala, Sweden
3 Historical Museum of Physics and Research and Study Center Enrico Fermi, 00184 Rome, Italy
4 Division of Materials Science, Department of Engineering Sciences and Mathematics, Luleå University of Technology, 97187 Luleå, Sweden
5 Department of Molecular and Nanosystems Sciences, University of Ca'Foscari Venice, 30172 Mestre, Venetia, Italy
6 Institute of Photonics and Nanotechnologies, CSMFO Lab. & FBK-CMM, IFN-CNR, 38123 Povo, Trento, Italy
Applications of metal and semiconductor nanomaterials represent one of the most intriguing areas in nanotechnology for the development of future technologies for energy harvesting, green and renewable energy, portable microelectronic devices, advanced electronics, and beyond-stateof-the-art computational units and devices [1-5]. Metal and semiconductor nanomaterials are associated with three disciplinary areas, namely spintronics, nanophotonics, and nanomagnetics [6-14], which have derived a series of applications for nanomaterials in recent years [11, 15-17]. Many of these technologies and their inherent methods of manufacturing are dependent on the electronic properties of metal alloys and scarce transition metals, including rareearth metals. These properties are critical for beyond-stateof-the-art engineering and are outlined in theoretical and practical engineering for development of advanced materials.
2 General theoretical foundations for advanced metallonanomaterials: classification and physicsAdvanced technologies that devise transition metals and high-electronic-energy elements often depend on the spintronic properties of metallonanomaterials. Spintronic devices depend on quantum effects such as spin hall effects [18-21] and quantum hall effects (QHEs) [22], which respectively give the possibility of tuning the spin of electrons [21] in a metallic/semiconductor material, and localizing free electrons in a defined two-dimensional (2D) space in a metallic or semiconductor lattice or quantum fluid [22, 23]. The implications of spintronics in nanometals and semiconductor nanomaterials yield primary applications of the materials in computational processing units, such as RAM and memory units [24-26], and form a basis for future quantum computing units. Furthermore, advanced metal nanomaterials, semiconductor and carbon-based nanomaterials, including their fabrication in combination with dichalogenides [17, 27, 28], attribute charge-transfer and magneto-transport effects [29] for field-effect transistors in radiofrequency (RF) devices [29, 30], and allow for ultra-sensitive functioning of miniature-sized components for frequency detection [10].
Metal and semiconductor-based nanomaterials are additionally critical components in the fields of nanophotonics, because of their plasmonic properties and size-dependent optical properties at the nanoscale, with quantistically tunable properties, such as in quantum dots, quantum wires, and plasmonic nano-antennas [6, 12, 13, 31-33]. A crucial application of nanophotonics using metal nanomaterials is in optically active devices and optoelectric systems, from which new and more efficient solar cell technologies can be developed [34-36], such as in units composed of combinations of metal nanomaterials and silicon materials [37-41], where the nanophotonic and plasmonic properties [42] are the source for energy harvesting functionalities [43, 44].
Nanophotonic applications also include metal-based nanomaterials applied in photon-scanning tunneling microscopy [45-48], where a nano-probe maps the light field above a sample material in order to detect the light signal emitted in the allowed quantum region, as well as the evanescent light occurring in forbidden regions [33]. Metal-based nanomaterials are furthermore critical in nanophotonics for the development and fabrication of nanomaterials via nanoscaling, a process where nanophotonic signals are applicable for canvassing nanomaterials at an ultra-accurate spatial confinement [33].
Metal and semiconductor-based nanomaterials are finally also critical for the novel field of nanomagnetics [49], which applies metal nanomaterials and alloys to manufacture nanomagnets and molecular magnets [50-53]. The fabrication of nanomagnets derives from its own branch, where metal alloys with magnetic properties are engineered and structured through the use of emerging techniques of nanofabrication [54, 55], with applications in biomedical diagnostics [56], as well as manufacturing microtransistors and memory devices [57]. Nanomagnetics relates also to the synthesis of molecular magnets, with applications in quantum computing, where molecular magnets switch spin-identity at a given site during excitation reactions by photonic absorption [53, 58]. Nanomagnetics also involves the synthesis of carbon-based molecular nanowires [52, 59] and is particularly relevant to thin films and alloys with microstructural strength combined with tensile flexibility and high magnetic momentum, which play a pivotal role for high-precision manufacturing of components for research equipment and sensors for the medical field, based on rare-earth metals and rare transition metals [60-62].
Each of the three described areas in this section and their inherent routes of engineering, where synthesis and application of metal and semiconductor-based nanomaterials is the main focus, are hereby reviewed with the respective types of metal-based nanomaterials, alloys, and organometallic materials. Every section for spintronics, nanophotonics, and nanomagnetics contains a state-of-theart review of respective materials.
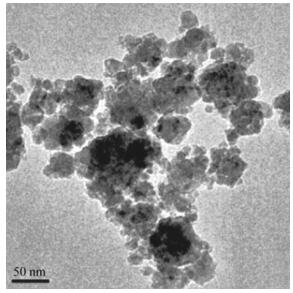
3 Spintronics in metal and semiconductor-based nanoparticlesThe term ''spintronics'' attributes to the ability to modulate the spin of electrons in the electron gas in metals and alloys, organometallic compounds, and in semiconductor materials [28, 63, 64]. Transition metals are widespread in spintronic applications because their high-energy valence electrons [65] and spin-state multiplicity allow for spintorque effects for magnetic and electric field tuning, which are critical for next-stage nanomaterials and nanoarchitectures [66-68]. In combination with organic atoms, such as carbon, spin-tuning and spin-state splitting have been achieved for spintronic organometallic materials. Examples include valleytronic metallorganic compounds, where electrons of the same spin occupy an area in the electron gas, and the electrons of the opposite spin polarize in a separated (often opposite) density volume of the surface of the nanomaterials [69-72]. The term ''valleytronics'', representing a critical part of spintronic theory, is the foundation for isolating pseudospins (total spins of an electron layer or an electron valley) in two or several ways, coupled with the azimuthal momentum of the electrons or directed in the opposite direction of the azimuthal momentum of the electrons (see Fig. 1).
|
|
|
Valleytronics allows for ''localized magnetism'' in the nanostructures, a property that was first observed in the 2D electron gas of a silicon inversion layer by Ohkawa and Uemura [73-75]. From this stage, the first approximations, both theoretical and experimental [28, 76-79], were derived to prepare information storage by attributing the sign of the electron spin in a specific polyatomic ''valley'' of the material, being a conductor or semiconductor. Valleytronics has also been applied in non-metallic materials, such as graphene [29, 64, 80, 81], and has particular advantages in tuning the low-energy dynamics of electrons within an extra wide gap of the semiconducting or conductive material [77, 78], which has direct implications for emerging technologies such as femtosecond electron diffraction and imaging [82, 83] and for medical applications such as cancer treatment [84].
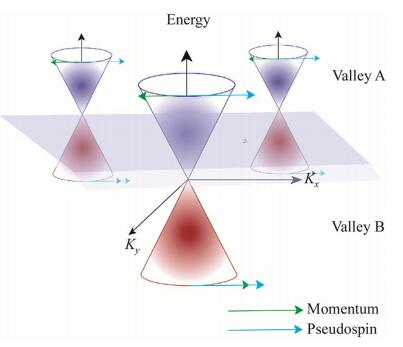
The metallic nanomaterials devised in spintronics have unique properties that make them ideal for spintronics. These properties are the abundance of their occupied and non-occupied d-electron orbitals (see Fig. 2) [85-88], which differ from p-electrons in organic compounds in that they have a higher variation of the magnetic quantum number, resulting in more manifolds than other lower-energy electrons (see Fig. 3) [89, 90].
|
|
|

|
|
|
This offers a higher number of combinations for spinorbit coupling between the electron momentum and the spin state, making transition metals indispensable in spintronic applications; these also form hybridization gaps within their electronic manifolds [87]. Interestingly, some of the most recent studies of spintronics and valleytronics report on breaking the electron-hole symmetry of the electron gas, resulting in a strong magnetic exchange field located in a narrow space in the nanomaterial lattice [77]. New studies also report the application of QHE in silicene and germanium alloys, in the form of the charge-quantum hall effects and spin-QHEs [91-94], which illustrate further how spintronics and its theoretical origin yield a series of nanoelectronic and microelectronic qualities derived by tuning and subdividing the spin-identity populations of electrons in a material, yielding critical advances for information storage and processing basis units (quantum computing and quantum-dot based communications) [17, 95, 96], memory resistors and radio-frequency devices [97, 98], magnetochaloric and inverse magnetochaloric effects [99-101], and other materials for nanooscillation and nanodynamics applications [102-104].
4 Nanophotonics in metal and semiconductor nanoparticlesMetal and semiconductor nanoparticles and nanostructures have exceptional effects when interacting with light and have shown promising applications in recent years. The collective plasmonic oscillations of the electrons in a metal structure, the bandgap, and the position of the energy levels in semiconductors and their size-dependent properties at the nanoscale determine their optical properties and can be used for photon management and manipulation, becoming the fundamental building blocks of the field known as nanophotonics.
The use of metal nanoparticles and their plasmonic properties are dated: the first example of their engineering application is the 4th century Roman glass cage, the Lycurgus Cup, now at the British Museum, which contains gold and silver nanoparticles in colloidal form which give it peculiar optical properties [105]. Similarly, other examples like the shining brilliant reflections of luster pottery and the colors of ancient cathedral windows contain silver, gold and copper nanoclusters. Since then, much progress has been made in the field, both in theoretical and experimental aspects, and many new applications of plasmonic nanoparticles have been developed, spreading from health monitoring and treatment [106, 107] to energy conversion enhancement in solar cells [39, 40].
Concerning biomedical research, plasmonic nanoparticles can be used as highly efficient contrast agents for optical imaging because their light-scattering cross sections greatly exceed the absorption cross section of standard fluorescent dyes [108]. Moreover, the local enhanced electromagnetic field that is generated by plasmonic nanoparticles can excite the vibrational modes of molecules attached or close to their surface, yielding a characteristic response that can be easily recognized. For examples, Sanders et al. [109] demonstrated the efficiency of a gold nano-disc array capable of label-free, sensitive detection of a cancer protein biomarker, free prostate-specific antigen (f-PSA). Silver nanoparticles have been used well by Xu et al. [110] for label-free detection of typical biological proteins: lysozyme, avidin, bovine serum albumin, cytochrome c, and hemoglobin. The silver nanoparticles provide a significant enhancement to boost the Raman signal of proteins, which can be easily detected thanks to surface-enhanced Raman scattering (SERS).
The use of plasmonic nanoparticles as therapeutic actuators has also been a field of intense investigation in recent years. Nanoparticles indeed exhibit passive accumulation at tumor sites through a process known as enhanced permeability and retention (EPR) [111]. This facilitates their potential application in cancer treatment by photothermal therapy (PTT) [112] and light-controlled drug release [113].
In the field of energy harvesting, plasmonic nanoparticles can increase solar cell efficiency by at least two different physical principles. Firstly, light can be scattered and trapped into the semiconductor active region of the device by multiple and high-angle scattering, increasing the effective optical path length in the cell. Moreover, a strong localization and enhancement of the near-field intensity in the proximity of the particles can be used as an optical concentrator, increasing the number of electron-hole pairs generated in the semiconductor. A significant amount of numerical simulation papers have been published, considering different nanoparticle materials (Au, Ag, Al), such as that of Catchpole and Polman [114] who investigated the best design for increasing the efficiency of solar cells by depositing metal nano-objects. In particular, they tested different shapes, such as spherical, cylindrical, or hemispherical particles, obtaining a maximum 28-fold increase in efficiency at 800 nm wavelength for 100-nm-diameter Ag hemispheres against Ag full spheres, which showed only a 9-fold increase. The choice of Au nanostructures instead of Ag resulted in a reduction in performance.
An important problem in using metal nanoparticles is the related absorption losses. Indeed, Lim et al. [115] reported that when using 100-nm-diameter Au nanoparticles, the enhancement of the efficiency had a dip in the 400-600 nm spectral regions and a peak near 700 nm. Alternative low-cost metals such as Al were recently investigated by Zhang et al. [116]. They showed that a regular array of 100-nm-diameter Al nanoparticles with a 150 nm period resulted in an increase of the cell absorption up to 40%. It is worth observing that all the described studies deal with numerical simulations. Experimental tests were reported by Zhang et al. [116], who demonstrated a significant photocurrent enhancement at specific wavelengths (16-fold at 1 050 nm) and an overall 30% efficiency increase for the cell by using Ag nano-islands (large 120-350 nm) obtained by depositing a thin Ag coating by thermal evaporation and annealing at 200 ℃ in nitrogen.
Concerning semiconductor materials, Martin-Rodriguez et al. [117] have recently synthesized quantum dots composed of cadmium and selenium doped with ytterbium ions, which induce photo-functionalization of the CdSe lattice by electronic interaction with the rare-earth metal f-electrons, forming luminescent quantum dots. A similar approach for functionalizing nanocrystals for photonic purposes was published in Ref. [118], which attributed luminescence to ZnS nanocrystals by post-synthetically adding europium and terbium ions to Ln(NO3)3·xH2O salt solutions of ZnS and CdS nanoparticles. The quantum yield of the particles was registered for various formulae of the salt solutions of the ZnS nanoparticles. Quantum yield defines the number of times a specific radiation-induced event occurs per photon absorbed by the system, thus increasing with higher luminescent properties of the materials. The Zn(Tb)S/Tb solutions displayed the highest quantum yield of value of 2.5 ± 0.3, a feature that is attributed to the energy transfer resulting from the multicoordinating ability of the rare earth Tb3+ of zinc and sulfur atoms. In both CdSe and CdS/ZnS nanoclusters, the rare earths localize first on the surface and then take place internally in the nanocrystals, forming the energy transferring network with the other elements [117, 118]. This coordination is visible in the excitonic emission spectra, which show that Yb3+ reduces CdSe excitonic emission, yielding an upper limit of energy transfer probability of about 80% from the nanocrystal to the rare earth [117].
5 Nanomagnetics: functional metal and semiconductor nanoparticlesThe aforementioned particles, particularly for the spintronics section, are also relevant to the field of nanomagnetics as components for computing and logic [10] and nanomagnetics for biotechnology [119]. Nanomagnetics is defined by the manufacturing of nanoparticles with exceptional magnetic properties, often defined by advanced spin-state orders of the metal and alloys. Such spin-state properties can be achieved by considering ferrofluids as a starting point. Ferrofluids are organic polymers mixed with ferromagnetic nanoparticles from Fe2+, Fe3+, Mn2+, or other transition metals precipitated with oxides [120]. Hematite-based (Fe2O3) nanomagnetic polymers have been synthesized by polymerizing N-isopropylacrylamide (NIPAM) as the main monomer, with methylene-bisacrylamide as the cross-linker and potassium persulfate as the initiator of the chain reaction [120]. Particles generated by this method remained discrete with a mean diameter of 12 nm, and magnetic measurements revealed that the particles showed superparamagnetic properties only with a decrease of magnetism after binding with the polymer because of the increase in surface spin disorientation. This feature is of particular interest because it indicates that the nanomagnetic materials of Fe2O3 yield a sharing of electrons with the polymeric framework of organic molecules, providing the ability to delocalize the magnetic momentum from the ferrous molecules to their surroundings. Polymerization was carried out for 6 h, where mercaptoethanol was used as the chain transfer reagent. The mercapto group donates a sulfur atom which forms a covalent bond to the Fe2O3 units, yielding coated nanoparticles with polymeric chains [120].
A similar approach was used by Shamin et al. [121] in a second study, where nanoparticles were prepared in the same approach as mentioned previously and used for attaching and adsorbing lysozyme. This approach of devising Fe2O3 particles as carriers of layers of sorptive organic compounds introduces a novel approach for fabrication of biotechnology-oriented applications, such as ELISA, protein separation, and other methods for biotechnology. Horng et al. [122] also devised a similar rationale, in order to perform immunoassays with nanomagnetic Fe2O3 particles, coated with a biotin probe, synthesized also through a co-precipitation process to probe the bio-target (avidin). The published method showed that the same particles used by Shamin et al. [121] could be synthesized in hydrodynamic diameter from 30 nm to 90 nm. Parekh and Upadhyay [123] reported magnetic Mn0.5Zn0.5Fe2O4 nanoparticles with dimension of 0.82 nm. The Mn-Zn coated ferrite nanoparticles were synthesized using a thermal deposition technique with acetic acid solutions of the metals, in combination with 1, 2-hexadecanediol, oleic acid, oleyamine, and dioctyl ether. The nanoparticles show zero remanence and coercivity at room temperature, whereas at 5 K coercivity was registered to 1.934 × 104 A/m. The nanoparticles are superparamagnetic above 100 K and experience finite magnetic coercivity below the freezing temperature. Interestingly, this combination of Mn, Zn, and Fe in nanoparticles exhibits a non-saturizing behavior at 5 K, which suggests a core structure in the magnetic system, where the core is ordered in terms of spin populations, and the surface results in disordered spins due to the high surface energy. This registered pattern of spin-population forms the basis for a possible quantum-tunneling effect in the Mn-Zn ferrite nanoparticles, which has also been reported for a frozen fluid for Mn-Zn [124]. The tunneling effect in magnetic nanoparticles can be explained by geometrical phase effects [125] and indicates the formation of an energy cavity in the tunneling path of the magnetic energy potential of the lattice. This provides an effect that can be measured in a qualitative analysis of the energy structure of the dominant tunneling paths in the phase space [125].
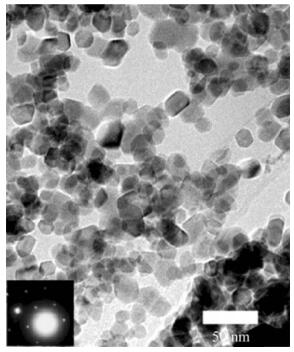
The coordination of magnetic nanoparticles and arrangement to one another is also an interesting aspect of nanomagnetics, and can be used for memory-processing functions in computational units. Chakraverty et al. [126] prepared nanoparticles of Ni0.35Zn0.65Fe2O4 by mechanical alloying processes utilizing Fritsch Planetary Mono Mill Pulverisette 6, giving a mean particle diameter of 27 nm, with sizes up to 100 nm (see Fig. 4). According to the rationale of the study, the use of the dispersion pattern of the particles forms the foundation for devising these particles in memory processing units. The polydispersity of the particle volumes leads to a wide distribution of blocking temperatures, where the memory effects are a consequence of the polydispersed state of the system at a given temperature, which lies between the blocking temperatures of different-sized particles [126]. From this point, a sudden increase in the externally applied magnetic field can also be stored in the system as long as the field is not too high [126]. The results from this study show that a nanomagnetic particle system can be used to encode a sudden increase or decrease in magnetic field while the system is being cooled, forming a logical switch for information processing. This can in turn be decoded by measuring the magnetic response curve generated during heating [126].
|
|
|
Many different approaches have been followed and developed in recent years for the controlled synthesis and engineering of metal/semiconductor nanoparticles. In general these can be divided into chemical and/or physical methods and combinations of the two methods.
6.1 Chemical methodsEngineering nanoparticles for special applications as described above requires tuning the nanoparticles' dimensions and shapes, because both the chemical reactivity and the physical properties of the nanoparticles are directly related to the lattice symmetries, geometries, and nanoparticle shapes and forms [127-130]. The processes for engineering nanoparticles comprise several methods, where thermal methods by microwave heating are conventional [131, 132]. Microwave heating has been used to prepare metal nanoparticles very rapidly to produce metal nanopowders, and has been a central part of microwaveassisted chemistry [133]. The approach of using microwaves takes advantage of the formation of electric fields and charges in the phase where nanoparticles are formed, which are nano-confined regions where temperatures as high as 10 000 ℃ and pressures up to 1 GPa are generated [133]. The metal nanoparticles are formed using reactions with reductive organic compounds, often applied as polyols [133-135]. Polyols have a low viscosity, which contributes to the reaction environment by forming a gel-like medium that forms localized spots where chemical reactions and nanoparticle formation take place [133]. Microwave-assisted reactions have a higher kinetic rate of reaction than non-microwave methods, and contribute to the synthetic process by forming more novel phases [136] than chemical reactions, which makes microwave methods a critical component for synthesizing dispersions and powders of even and uneven shapes [133, 137]. During this synthesis processes, the metal nanoparticles are formed by catalytic reduction of their metalhydroxide form M(OH)x, which accepts electrons from the polyol liquid [133]. This generates an oxidized form of the polyol that remains in liquid solution, where platinum nanoparticles deposit into particles of 5-25 nm diameter within 10-15 min [133, 137]. The approach is applied to various transition metals with the potential of modifying the shape and dimensions by modulating the pH value of the metal hydroxide solutions [133].
Other chemical methods for nanofabrication include electrochemical reduction of ionic liquids. Ionic liquids are based on metal ions in ionic state with an organic compound of opposite charge. Ionic liquids are highly applicable as reagents for nanomaterial engineering and have been used to prepare cadmium nanoparticles [138] and gold nanoparticles from the imidazolium cation [139], and to provide for accuracy in shaping and controlling the size of metal nanoparticles [140]. Using this method, applying polymers in the solution assists furthermore in shaping and altering the properties of the formed nanoparticles, which can also lead to generation of other shapes and sizes [141]. Electrochemical synthesis has also been combined with multi-walled carbon nanotubes (MWCNTs) as support material to further alter the properties of the nanoparticles, as performed by Guo and Li [142] in the synthesis of palladium nanoparticles. The surfaces of MWCNTs have super-aromatic properties [143], which have a particular ability to trap positively charged ions, particularly metal ions, given the partial negative charge that emerges across the center of the aromatic ring of the hexagonal moieties of MWCNTs [144]. Electrochemical methods of synthesis provide the smallest nanoparticle diameters, among all chemical methods, and can achieve particles as small as 2-7 nm in diameter [145]. The disadvantage of electrochemical synthesis of nanoparticles is the reduced level of particle growth speed, because electric charges and currents can be challenging to tune for intensity, towards specific ionic concentrations, sample volumes, and other components in the chemical reaction. However, the inclusion of scaffolds and support materials can increase the accuracy and reproducibility of nanoparticle synthesis when using electrochemical impulses, where for instance encapsulation in single-walled nanotubes [52] can be particularly efficient for synthesizing nanoparticles with uniform dimensions.
Functionalization of nanoparticles is an additional chemical process that enhances the electronic qualities of the nanomaterial, required for spintronic applications or other avenues; it has also been applied to provide ferromagnetic properties in diamagnetic materials after combination of ZnO nanorods with Al2O3 shells [146]. The ferromagnetic effect results at the interface between the two materials, after annealing the ZnO/Al2O3 core-shell nanowires and activating the formation of the ZnAl2O4 phase. The magnetic functionality of the nanoparticles was found to be stable up to 750 K [146]. The study showed that fully diamagnetic materials (ZnO and Al2O3) could impart intriguing ferromagnetic properties in combination, which otherwise would be more challenging to achieve perse. Another group recently achieved ferroelectric and magnetocapacitive properties in scandium-doped BiFeO3 nanocubes [147], where scandium increased significantly the electric polarization in the nanoparticles, attributing magnetoelectric effects to the nanocubes. This effect results from the ten unoccupied d-orbitals of the scandium ion (Sc3+) (see previous section on d-orbitals), which can take part in forming a new electronic framework in the nanocubes. This functionalization approach contributes in establishing a new magnetoelectric coupling in the nanocubes where the magnetic moment is primarily given by the Fe2+/Fe3+ ions in the BiFeO3 nanostructures (see Fig. 5) [147]. Interestingly, the re-coordination of the nanocubes after Sc-doping induced changes in the structural geometry, which shows that the Sc ions compete with the existing frameworks between Fe3+/Fe2+ and the bismuth and oxygen ions, forming potentially a completely new lattice unit and thus altering the physical qualities of the material. Such events at the nanoscale during functionalization have been reported previously [148, 149]. One of these studies [148] showed that the rare earth element lanthanum in the lanthanide form altered the ground structure, size, and shape of alkaline-earth fluoride nanocrystals, which resulted in a tuning approach of the emission spectra and paramagnetism of the given nanocrystals. A similar mechanism was reported for ZnO nanocrystals after doping with Mg2+ ions [148], with the induction of new shapes and geometries from tetrapods of ZnO. This approach was also used to tune the optoelectronic properties of ZnO, thus yielding new functionalities at various dopant concentrations [146]. Nanoparticles can also be synthesized with accurate and uniform dimensions by using metal-organic frameworks (MOFs) [150], where the metal nanoparticles are embedded in the carbon matrix by the controlled thermolysis of the MOF. Nanoparticles synthesized by this method include CuO, Co3O4, ZnO, MgO, CdO, and CdS [150]. In this context, a study published by Pal et al. [151] shows that simple thermolysis of MOFs in inert (N2) and partial oxygen (air) environments leads to the formation of single phase crystalline metal and metal oxide nanoparticles, which are passivated in the carbon matrix. The approach of in situ synthesis of Cu and Co nanoparticles [150], embedded in a porous carbon matrix, uses [Cu2(hfbba)2(3-mepy)2]·(DMF)2(3-mepy) (F-MOF-4) and [Co2(hfbba)2(3-mepy)2]·(DMF)3 (Co-HFM OF-D), which are synthesized according to the reported synthesis protocol [152], where the synthesized blue-colored crystals of F-MOF-4 are placed on a silica substrate and heated in a tube furnace to 900 ℃ under N2 atmosphere, with a heating rate of 10 ℃/min to thermolyze the organic species to yield pure Cu/Co nanoparticles. The material is cooled to room temperature and analyzed for purity. The catalytic carbonization process is another method of nanoparticle production of most recent origin [153], which uses a water-soluble sodium chloride (NaCl) solution as a supporting material, and encapsulates cobalt nanoparticles in carbon cages with a productivity of about 100%. This method allows for production of smaller quantities than other methods, such as the arc-discharge method; however its products can be fully separated from the supporting materials by a simple washing process and the method itself requires less energy. The method of catalytic carbonization also allows for tuning the morphologies of the products, by altering the salts used in the supporting material. For instance, when NaCl, NaF, and Al2O3 were used as supporting materials [153], the morphologies of the carbon products changed from carbonencapsulated magnetic nanoparticles (CEMNs) to an intermediate state (quasi-nanocages) between CEMNs and carbon nanotubes (CNTs), and further to CNTs, respectively. The catalytic carbonization method allows for a more predictable morphology-tuning of embedded metal nanoparticles, compared to other methods such as temperature annealing, because the ionic compounds used at specific concentrations yield a better predictability of the resulting charge-to-charge steering of the forming nanoparticles.
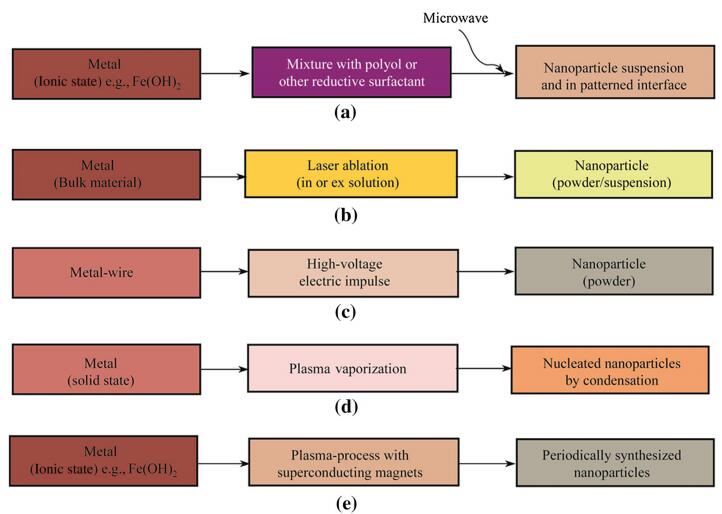
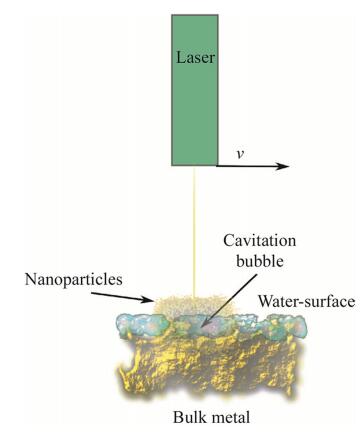
Nanoparticle synthesis by physical methods is mainly based on thermal impulse generation as in electro-explosion, plasma formation by superconducting magnets, laser ablation, and plasma-vaporization (see Fig. 6) [154-164]. The first of these methods, electro-explosion, devises a metallic wire as a source for nanoparticle generation, and applies a high-voltage impulse on the wire in electroshocks, which form nanoparticles rapidly as volatile nanopowders [158, 161, 165]. The laser-ablation method (see Fig. 7) is a frequently applied alternative to the abovementioned method and offers higher precision, and can be used for gram-quantity synthesis of metallic or ceramic nanoparticles [166, 167]. A laser pulse at femtosecond and millisecond intervals is directed on the bulk metal material, and often offers particle production without generating excessively high temperatures. Production rates of nanomaterials are 100-300 lg/s with the results of 1 000-1 500 mg/h of nanoparticles [168]. Recent advances in laser ablation include modifications of the pulse intensity, pulse overlap [169], and pulse frequency amplification. Additionally, the water layer used to cool the material during metal ablation plays a critical role for the productivity of nanoparticles, and is inversely proportional to the amount of nanoparticles generated [167]. The scan speed of the laser pulse is also a critical property for nanoparticle yield during the ablation process. For instance, with a 100 mm/s laser pulse movement across the metal surface, the nanoparticle productivity is increased by 300% over smaller laser scan speeds, which increases linearly in the range of 50-500 mm/s in scan speed [167]. The laser pulse repetition furthermore affects the yield of nanoparticles constructively by low repetition frequencies (4-6 kHz) [167]; in other words, the frequency of the laser pulse is directly related to particle yield.
|
|
|
|
|
|
Plasma vaporization is a conventionally applied method for nanoparticle production. Plasma vaporization, initially introduced in 1999 by Bica [162], melts steel or a metal alloy for subsequent sputtering through an argon plasma jet. The first tests gave the production of iron-pentacarbonyl, Fe(Co)5 in plasma, with mean particle diameters of 12.2 nm. Smaller diameters down to 6 nm were obtained by thermal decomposition of Fe 2-etilhexamaleath, which gave amorphous and crystalline graphite particles with applicability for conductive magnetic liquids. Furthermore, the plasma vaporization in either argon plasma or in helium plasma yields nanoparticles of α-Fe and Fe3O4 with diameters in the range of 3-90 nm. In recent years, plasma vaporization has been widely used in nanomaterial engineering, and has been combined with encapsulation in carbon cage structures [170]. Plasma vaporization is particularly suitable for production of carbon-coated nanoparticles; however, other techniques are equally compatible, such as arc discharge [171], RF plasma torch [172, 173], combustion [174], magnetron and ion beam cosputtering [175], and high-temperature annealing [176]. The RF plasma torch method is of particular interest, because it deposits thin films with exact chemical compositions on a substrate, of quite considerable molecular weight (Co50Pt15C35), with a grain size ranging from 5 nm to 15 nm [175]. The role of the carbon cages in the synthesis by the RF plasma torch process has induced particularly interesting chemical and physical conditions, which lead to a hexagonal close-packed phase separated by graphite-like carbon boundaries. The role of carbon in the synthesis process is highly valuable, and can affect both the symmetry and chemical formula of the produced films. Other studies, which have mapped the formation of crystals in carbon cages, have shown that carbon cages affect the morphology of the packing [177]. Indeed, the method of devising carbon cages in the RF plasma synthesis process has given a higher coercivity of the synthesized film by 9.55 × 105 A/m, compared to the regular case of cobaltcarbon. Figure 6 illustrates further the amplitude of the physical methods.
6.3 Biological methodsPlants can also be suitable reactors to synthesize nanoparticles, as recent studies have shown [178]. Algae, fungi, plants, bacteria, yeast, and actinomycetes are organisms that can be used to synthesize nanoparticles in reactors. The mode of synthesis follows the reduction of ions to solid nanometals, by the activity of metallo-enzymes, which are regularly used for detoxification and transformation purposes of toxic metal ions. Gold nanoparticles in the range of 20-40 nm have been synthesized using sterilized geranium leaves (P. graveolens), after exposure to chloroaurate ions [179]. The time for reduction was registered to 60 min, where nanoparticles of decahedral and icosahedral shapes were synthesized. Similar rates were found for palladium nanoparticles, produced to 55-80 nm in diameter [180]. Cinnamomum camphora (C. camphora) leaves have also been used to synthesize nanoparticles of silver with the additional use of reducing agents. The produced silver nanoparticles ranged from 5 nm to 40 nm [181]. The method of production by Huang et al. [181] was described as follows: silver nitrate (AgNO3) was mixed with an extract of sun-dried C. camphora leaf, with 50 mL of deionized water, which were vigorously shaken. The mixtures in the flasks were then centrifuged and precipitates removed. The procedure then followed an experimental setup designed to carry out continuous-flow biosynthesis of silver nanoparticles, where two helical tubular microreactors were employed in the setup. Combined with another tubular microreactor, the mixture, the resulting lixivium and aqueous AgNO3 were continuously fed into the reactors by a peristaltic pump, resulting in a silver hydrosol effluent containing silver nanoparticles.
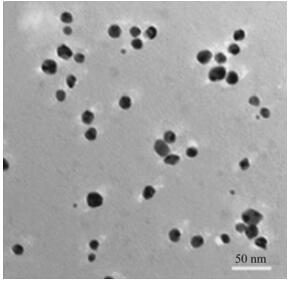
Algae have also been used successfully to synthesize gold nanoparticles [182], via the reduction of an aqueous chloroauric acid solution with dried biomass of a freshwater epilithic green alga, Prasiola crispa. The particles formed were of face-centered cubic structure of gold with an average crystallite diameter of 9.8 nm and smallest diameter of 5 nm (see Fig. 8). However, more diverse methods have been used in producing gold nanoparticles recently [183], where the flower Lantana camara (L. camara) was used as a vehicle for nanoparticle formation. The shape and size of gold nanoparticles were successfully controlled by introducing small amounts of L. camara flower extract to an ionic solution of gold, where spherical nanoparticles of average size (10.6 ± 2.9) nm were produced. Paul et al. [184] used leaf extracts of Pogestemon benghalensis to produce gold nanoparticles in a similar fashion as the studies mentioned previously. The nanoparticles had a mean crystalline size of 13 nm, and the nanoparticles arranged predominantly in spherical and triangular shapes with sizes from 10 nm to 50 nm. Catalytic activity was registered for these particles, as it was for the same particles produced in L. camara. In general, the amount of nanoparticles and their purity are lower by biological methods compared to chemical and physical methods. The biological methods can be optimized by blending chemical reactions with the biological components, because the majority of the biological approaches are reduction reactions with biological components such as enzymes or other types of cell components.
|
|
|
In conclusion, the future of nanoparticle production can be centered particularly on physical methods, where the production rates are higher and defined by more approaches that are predictable. However, the costs and energy requirements for physical methods can be a deficiency of this type of nanoparticle production, and require method optimization by developing better magnetic and high-energy alloys to exert rapid and more energy-and cost-efficient paths of nanoparticle production. Chemical and biological methods are equally important, particularly the former. However, the generation of large quantities with a high degree of uniformity can be a challenge, particularly with the latter method.
7 ConclusionsWe have here surveyed the literature on spintronics, nanophotonics, and nanomagnetics, and derived a comprehensive study on the recent and emerging avenues of development in these fields of applied nanosciences. Our study shows that the most valuable materials are still alloys of transition metals and oxides. However, carbon nanomaterials can form the basis for future emerging magnetic and spintronic materials once large-scale methods of production arise with the development of nanotechnologies for electronic, diagnostic, and computational applications. It is a noteworthy approach to study the effects that occur between materials in either nanoparticle form, crystals, dots, or nanowires, particularly after post-synthetical modification [118], encapsulation of metals in nanotubes [52, 59], and most importantly in the interface between metal oxides, rare earth oxides, and transition metals, because it is here that the true potential of nanomaterials emerges. Interface properties, re-coordination of elements in nanocrystals, and growth of functional materials can give valuable future approaches for engineering functional nanomaterials based on ubiquitous and rather common substrates, because the ferromagnetic and magnetic properties are critical for applications in spintronics, nanophotonics, and nanomagnetics.
| 1. | Trauzettel B, Bulaev DV, Loss D, et al(2007)Spin qubits in graphene quantum dots.Nat Phys 3, 192-196 doi:10.1038/nphys544 |
| 2. | Zhu S, Zhang J, Qiao C, et al(2011)Strongly green-photoluminescent graphene quantum dots for bioimaging applications.Chem Commun 47, 6858-6860 doi:10.1039/c1cc11122a |
| 3. | Pradhan A, Holloway T, Mundle R, et al(2012)Energy harvesting in semiconductor-insulator-semiconductor junctions through excitation of surface plasmon polaritons.Appl Phys Lett 100-061127 |
| 4. | Park K, Lee M, Liu Y, et al(2012)Flexible nanocomposite generator made of BaTiO3 nanoparticles and graphitic carbons.Adv Mater 24, 2999-3004 doi:10.1002/adma.v24.22 |
| 5. | Gittins DI, Bethell D, Schiffrin DJ, et al(2000)A nanometrescale electronic switch consisting of a metal cluster and redoxaddressable groups.Nature 408, 67-69 doi:10.1038/35040518 |
| 6. | Huang Y, Duan X, Lieber CM(2005)Nanowires for integrated multicolor nanophotonics.Small 1, 142-147 |
| 7. | Brongersma ML, Kik PG(2007)Surface plasmon nanophotonics.Springer, Netherlands |
| 8. | Wolf SA, Lu J, Stan MR, et al(2010)The promise of nanomagnetics and spintronics for future logic and universal memory.Proc IEEE 98, 2155-2168 doi:10.1109/JPROC.2010.2064150 |
| 9. | Awschalom DD, Flatté ME(2007)Challenges for semiconductor spintronics.Nat Phys 3, 153-159 doi:10.1038/nphys551 |
| 10. | Wolf S, Awschalom D, Buhrman R, et al(2001)Spintronics: a spin-based electronics vision for the future.Science 294, 1488-1495 doi:10.1126/science.1065389 |
| 11. | Mourachkine A, Yazyev O, Ducati C, et al(2008)Template nanowires for spintronics applications: nanomagnet microwave resonators functioning in zero applied magnetic field.Nano Lett 8, 3683-3687 doi:10.1021/nl801820h |
| 12. | Ohtsu M, Kobayashi K, Kawazoe T, et al(2002)Nanophotonics: design, fabrication, and operation of nanometric devices using optical near fields.IEEE J Sel Top Quantum Electron 8, 839-862 doi:10.1109/JSTQE.2002.801738 |
| 13. | Qian F, Li Y, Gradecak S, et al(2004)Gallium nitride-based nanowire radial heterostructures for nanophotonics.Nano Lett 4(10), 1975-1979 doi:10.1021/nl0487774 |
| 14. | Žutić I, Fabian J, Sarma SD(2004)Spintronics: fundamentals and applications.Rev Mod Phys 76, 323 doi:10.1103/RevModPhys.76.323 |
| 15. | Ling X, Zhou X, Shu W, et al(2013)Realization of tunable photonic spin hall effect by tailoring the Pancharatnam-Berry phase.Sci Rep 5, 5557 |
| 16. | Thibeault SA, Kang JH, Sauti G, et al(2015)Nanomaterials for radiation shielding.MRS Bull 40, 836-841 doi:10.1557/mrs.2015.225 |
| 17. | Xu X, Yao W, Xiao D, et al(2014)Spin and pseudospins in layered transition metal dichalcogenides.Nat Phys 10, 343-350 doi:10.1038/nphys2942 |
| 18. | McAlister S(1978)The hall effect in spin glasses.J Appl Phys 49, 1616-1621 doi:10.1063/1.324923 |
| 19. | Senthil T, Marston J, Fisher MP(1999)Spin quantum hall effect in unconventional superconductors.Phys Rev B 60(6), 4245-4254 doi:10.1103/PhysRevB.60.4245 |
| 20. | Hirsch JE(1999)Spin hall effect.Phys Rev Lett 83(9), 1834-1837 doi:10.1103/PhysRevLett.83.1834 |
| 21. | Dyakonov M, Perel V(1971)Possibility of orienting electron spins with current.Sov J Exp Theor Phys Lett 13, 467-469 |
| 22. | Girvin SM (1999) The quantum hall effect: novel excitations and broken symmetries. In: Comtet A, Jolicoeur T, Ouvry S et al (eds) Topological aspects of low dimensional systems. Springer, Berlin, pp 53-175 |
| 23. | Laughlin RB(1983)Anomalous quantum hall effect: an incompressible quantum fluid with fractionally charged excitations.Phys Rev Lett 50, 1395-1398 doi:10.1103/PhysRevLett.50.1395 |
| 24. | Burr GW, Kurdi BN, Scott JC, et al(2008)Overview of candidate device technologies for storage-class memory.IBM J Res Dev 52, 449-464 doi:10.1147/rd.524.0449 |
| 25. | Wang KL, Alzate JG, Amiri PK(2013)Low-power non-volatile spintronic memory: STT-RAM and beyond.J Phys Appl Phys 46(7), 074003 doi:10.1088/0022-3727/46/7/074003 |
| 26. | Wang X, Keshtbod P, Wang Z, et al(2015)Spin-orbitronics memory device with matching and self-reference functionality.IEEE Trans Magn 51, 1-4 |
| 27. | Jiang Z, Zhang Y, Tan YW, et al(2007)Quantum hall effect in graphene.Solid State Commun 143(1-2), 14-19 doi:10.1016/j.ssc.2007.02.046 |
| 28. | Zibouche N, Philipsen P, Kuc A, et al(2014)Transition-metal dichalcogenide bilayers: switching materials for spintronic and valleytronic applications.Phys Rev B 90, 125440 doi:10.1103/PhysRevB.90.125440 |
| 29. | Chua C, Connolly M, Lartsev A, et al(2014)Quantum hall effect and quantum point contact in bilayer-patched epitaxial graphene.Nano Lett 14, 3369-3373 doi:10.1021/nl5008757 |
| 30. | Klitzing KV(1995)Physics and application of the quantum hall effect.Phys B Condens Matter 204(1-4), 111-116 doi:10.1016/0921-4526(94)00250-Y |
| 31. | Kirchain R, Kimerling L(2007)A roadmap for nanophotonics.Nat Photonics 1, 303-305 doi:10.1038/nphoton.2007.84 |
| 32. | Cortes C, Newman W, Molesky S, et al(2012)Quantum nanophotonics using hyperbolic metamaterials.J Opt 14(6), 063001 doi:10.1088/2040-8978/14/6/063001 |
| 33. | Shen Y, Friend CS, Jiang Y, et al(2000)Nanophotonics: interactions, materials, and applications.J Phys Chem B 104, 7577-7587 doi:10.1021/jp0016131 |
| 34. | Callahan DM, Munday JN, Atwater HA(2012)Solar cell light trapping beyond the ray optic limit.Nano Lett 12, 214-218 doi:10.1021/nl203351k |
| 35. | Yu Z, Raman A, Fan S(2010)Fundamental limit of nanophotonic light trapping in solar cells.Proc Natl Acad Sci 107, 17491-17496 doi:10.1073/pnas.1008296107 |
| 36. | Mokkapati S, Catchpole K(2012)Nanophotonic light trapping in solar cells.J Appl Phys 112, 101101 doi:10.1063/1.4747795 |
| 37. | Teperik TV, De Abajo FG, Borisov A, et al(2008)Omnidirectional absorption in nanostructured metal surfaces.Nat Photonics 2, 299-301 doi:10.1038/nphoton.2008.76 |
| 38. | Podolskiy VA, Sarychev AK, Shalaev VM(2002)Plasmon modes in metal nanowires and left-handed materials.J Nonlinear Opt Phys Mater 11, 65-74 doi:10.1142/S0218863502000833 |
| 39. | Polman A(2008)Plasmonics applied.Science 322, 868-869 doi:10.1126/science.1163959 |
| 40. | Atwater HA, Polman A(2010)Plasmonics for improved photovoltaic devices.Nat Mater 9, 205-213 doi:10.1038/nmat2629 |
| 41. | Green MA, Pillai S(2012)Harnessing plasmonics for solar cells.Nat Photonics 6, 130-132 doi:10.1038/nphoton.2012.30 |
| 42. | Delacour C, Blaize S, Grosse P, et al(2010)Efficient directional coupling between silicon and copper plasmonic nanoslot waveguides: toward metal-oxide-silicon nanophotonics.Nano Lett 10, 2922-2926 doi:10.1021/nl101065q |
| 43. | Tsakalakos L, Balch J, Fronheiser J, et al(2007)Silicon nanowire solar cells.Appl Phys Lett 91, 233117 doi:10.1063/1.2821113 |
| 44. | Kim HS, Lee CR, Im JH et al (2012) Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci Rep 2(8): 591 |
| 45. | Ferrell T, Sharp S, Warmack R(1992)Progress in photon scanning tunneling microscopy (PSTM).Ultramicroscopy 42, 408-415 |
| 46. | Paesler M, Moyer P, Jahncke C, et al(1990)Analytical photon scanning tunneling microscopy.Phys Rev B 42, 6750 doi:10.1103/PhysRevB.42.6750 |
| 47. | Bourillot E, Fornel FD, Goudonnet JP, et al(1995)Imaging of test quartz gratings with a photon scanning tunneling microscope: experiment and theory.J Opt Soc Am A 12(8), 1749-1764 doi:10.1364/JOSAA.12.001749 |
| 48. | Carminati R, Greffet JJ (1995) Two-dimensional numerical simulation of the photon scanning tunneling microscope. Concept of transfer function. Opt Commun 116: 316-321 |
| 49. | Skomski R(2003)Nanomagnetics.J Phys Condens Matter 15, R841 doi:10.1088/0953-8984/15/20/202 |
| 50. | Saywell A, Magnano G, Satterley CJ, et al(2010)Self-assembled aggregates formed by single-molecule magnets on a gold surface.Nat Commun 1, 75 |
| 51. | del Carmen Giménez-López M, Moro F, La Torre A, et al(2011)Encapsulation of single-molecule magnets in carbon nanotubes.Nat Commun 2, 407 doi:10.1038/ncomms1415 |
| 52. | Manzetti S(2013)Molecular and crystal assembly inside the carbon nanotube: encapsulation and manufacturing approaches.Adv Manuf 1(3), 198-210 doi:10.1007/s40436-013-0030-5 |
| 53. | Leuenberger MN, Loss D(2001)Quantum computing in molecular magnets.Nature 410, 789-793 doi:10.1038/35071024 |
| 54. | Haynes CL, Van Duyne RP(2001)Nanosphere lithography: a versatile nanofabrication tool for studies of size-dependent nanoparticle optics.J Phys Chem B 105, 5599-5611 doi:10.1021/jp010657m |
| 55. | Rokhvarger AE, Chigirinsky LA(2004)Design and nanofabrication of superconductor ceramic strands and customized leads.Int J Appl Ceram Technol 1, 129-139 |
| 56. | Krishnan KM(2010)Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy.IEEE Trans Magn 46, 2523-2558 doi:10.1109/TMAG.2010.2046907 |
| 57. | Welser J, Wolf SA, Avouris P et al (2011) Applications: nanoelectronics and nanomagnetics. In: Nanotechnol. Res. Dir. Soc. Needs 2020. Springer, Berlin, pp 375-415 |
| 58. | Bogani L, Wernsdorfer W(2008)Molecular spintronics using single-molecule magnets.Nat Mater 7, 179-186 doi:10.1038/nmat2133 |
| 59. | Manzetti S, Lu T(2013)Alternant conjugated oligomers with tunable and narrow HOMO-LUMO gaps as sustainable nanowires.RSC Adv 3, 25881-25890 doi:10.1039/c3ra41572d |
| 60. | Li C, Lin J(2010)Rare earth fluoride nano-/microcrystals: synthesis, surface modification and application.J Mater Chem 20, 6831-6847 doi:10.1039/c0jm00031k |
| 61. | Vetrone F, Naccache R, Zamarron A, et al(2010)Temperature sensing using fluorescent nanothermometers.ACS Nano 4, 3254-3258 doi:10.1021/nn100244a |
| 62. | Bünzli JCG, Comby S, Chauvin AS, et al(2007)New opportunities for lanthanide luminescence.J Rare Earths 25, 257-274 doi:10.1016/S1002-0721(07)60420-7 |
| 63. | Bloss W, Sham L, Vinter V(1979)Interaction-induced transition at low densities in silicon inversion layer.Phys Rev Lett 43, 1529 doi:10.1103/PhysRevLett.43.1529 |
| 64. | Cserti J, Dávid G(2006)Unified description of Zitterbewegung for spintronic, graphene, and superconducting systems.Phys Rev B 74, 172305 doi:10.1103/PhysRevB.74.172305 |
| 65. | Manzetti S, Patek M(2016)The accurate wavefunction of the active space of the rhenium dimer resolved using the ab initio Brueckner coupled-cluster method.Struct Chem 27(4), 1071-1080 doi:10.1007/s11224-015-0726-1 |
| 66. | Tulapurkar A, Suzuki Y, Fukushima A, et al(2005)Spin-torque diode effect in magnetic tunnel junctions.Nature 438, 339-342 doi:10.1038/nature04207 |
| 67. | Ohno H(2010)A window on the future of spintronics.Nat Mater 9, 952-954 doi:10.1038/nmat2913 |
| 68. | Locatelli N, Cros V, Grollier J(2014)Spin-torque building blocks.Nat Mater 13, 11-20 |
| 69. | Mai C, Barrette A, Yu Y, et al(2013)Many-body effects in valleytronics: direct measurement of valley lifetimes in singlelayer MoS2.Nano Lett 14, 202-206 |
| 70. | Zeng M, Feng Y, Liang G(2011)Graphene-based spin caloritronics.Nano Lett 11, 1369-1373 doi:10.1021/nl2000049 |
| 71. | Myoung N, Seo K, Lee SJ, et al(2013)Large current modulation and spin-dependent tunneling of vertical graphene/MoS2 heterostructures.ACS Nano 7, 7021-7027 doi:10.1021/nn402919d |
| 72. | Cheng Y, Zhu Z, Tahir M, et al(2013)Spin-orbit-induced spin splittings in polar transition metal dichalcogenide monolayers.EPL Europhys Lett 102, 57001 doi:10.1209/0295-5075/102/57001 |
| 73. | Ohkawa FJ, Uemura Y(1977)Theory of valley splitting in an N-channel (100) inversion layer of Si I: formulation by extended zone effective mass theory.J Phys Soc Jpn 43, 907-916 doi:10.1143/JPSJ.43.907 |
| 74. | Ohkawa FJ, Uemura Y(1977)Theory of valley splitting in an N-channel (100) inversion layer of Si Ⅱ: electric break through.J Phys Soc Jpn 43, 917-924 doi:10.1143/JPSJ.43.917 |
| 75. | Ohkawa FJ, Uemura Y(1977)Theory of valley splitting in an N-channel (100) inversion layer of Si Ⅲ: enhancement of splittings by many-body effects.J Phys Soc Jpn 43, 925-932 doi:10.1143/JPSJ.43.925 |
| 76. | Behnia K(2012)Condensed-matter physics: polarized light boosts valleytronics.Nat Nanotechnol 7, 488-489 doi:10.1038/nnano.2012.117 |
| 77. | Ezawa M(2013)Spin valleytronics in silicene: quantum spin hall-quantum anomalous hall insulators and single-valley semimetals.Phys Rev B 87, 155415 doi:10.1103/PhysRevB.87.155415 |
| 78. | Ezawa M(2014)Valleytronics on the surface of a topological crystalline insulator: elliptic dichroism and valley-selective optical pumping.Phys Rev B 89, 195413 doi:10.1103/PhysRevB.89.195413 |
| 79. | Nebel CE(2013)Valleytronics: electrons dance in diamond.Nat Mater 12, 690-691 doi:10.1038/nmat3724 |
| 80. | Maassen J, Ji W, Guo H(2010)Graphene spintronics: the role of ferromagnetic electrodes.Nano Lett 11, 151-155 |
| 81. | Novoselov K, Blake P, Katsnelson M(2001)Graphene: electronic properties.Encycl Mater Sci Technol 244, 1-6 |
| 82. | Pronschinske A, Pedevilla P, Murphy CJ, et al(2015)Enhancement of low-energy electron emission in 2D radioactive films.Nat Mater 14, 904-907 doi:10.1038/nmat4323 |
| 83. | Sundaram SK, Mazur E(2002)Inducing and probing nonthermal transitions in semiconductors using femtosecond laser pulses.Nat Mater 1, 217-224 doi:10.1038/nmat767 |
| 84. | Sanche L(2015)Cancer treatment: low-energy electron therapy.Nat Mater 14, 861-863 doi:10.1038/nmat4333 |
| 85. | Mattheiss LF(1973)Energy bands for 2H-Nb Se2 and 2H-Mo S2.Phys Rev Lett 30, 784-787 doi:10.1103/PhysRevLett.30.784 |
| 86. | Mattheiss LF(1966)Band structure and Fermi surface for rhenium.Phys Rev 151, 450-464 doi:10.1103/PhysRev.151.450 |
| 87. | Mattheiss LF(1973)Band structures of transition-metaldichalcogenide layer compounds.Phys Rev B 8, 3719-3740 doi:10.1103/PhysRevB.8.3719 |
| 88. | Te Velde G, Bickelhaupt FM, Baerends EJ, et al(2001)Chemistry with ADF.J Comput Chem 22, 931-967 doi:10.1002/(ISSN)1096-987X |
| 89. | Schrödinger E(1926)An undulatory theory of the mechanics of atoms and molecules.Phys Rev 28, 1049-1070 doi:10.1103/PhysRev.28.1049 |
| 90. | Schrödinger E(1940)A method of determining quantum-mechanical eigenvalues and eigenfunctions.Proceedings of the Royal Irish Academy, pp, 9-16 |
| 91. | Tahir M, Schwingenschlögl U(2013)Valley polarized quantum hall effect and topological insulator phase transitions in silicene.Sci Rep 3, 1075 doi:10.1038/srep01075 |
| 92. | Kaloni TP, Singh N, Schwingenschlögl U(2014)Prediction of a quantum anomalous hall state in Co-decorated silicene.Phys Rev B 89(3), 208-220 |
| 93. | Liu CC, Feng W, Yao Y(2011)Quantum spin hall effect in silicene and two-dimensional germanium.Phys Rev Lett 107(7), 2989-2996 |
| 94. | Zhang XL, Liu LF, Liu WM(2013)Quantum anomalous hall effect and tunable topological states in 3D transition metals doped silicene.Sci Rep 3, 2908 doi:10.1038/srep02908 |
| 95. | Wu G, Lue NY, Chang L(2011)Graphene quantum dots for valley-based quantum computing: a feasibility study.Phys Rev B 84, 195463 doi:10.1103/PhysRevB.84.195463 |
| 96. | Lee MK, Lue NY, Wen CK, et al(2012)Valley-based fieldeffect transistors in graphene.Phys Rev B 86, 165411 doi:10.1103/PhysRevB.86.165411 |
| 97. | Macià F, Kent AD, Hoppensteadt FC(2011)Spin-wave interference patterns created by spin-torque nano-oscillators for memory and computation.Nanotechnology 22, 95301 doi:10.1088/0957-4484/22/9/095301 |
| 98. | Wang X, Chen Y, Xi H, et al(2009)Spintronic memristor through spin-torque-induced magnetization motion.IEEE Electron Device Lett 30, 294-297 doi:10.1109/LED.2008.2012270 |
| 99. | Kainuma R, Imano Y, Ito W, et al(2006)Magnetic-field-induced shape recovery by reverse phase transformation.Nature 439, 957-960 doi:10.1038/nature04493 |
| 100. | Mañosa L, González-Alonso D, Planes A, et al(2010)Giant solid-state barocaloric effect in the Ni-Mn-In magnetic shapememory alloy.Nat Mater 9, 478-481 doi:10.1038/nmat2731 |
| 101. | Krenke T, Duman E, Acet M, et al(2005)Inverse magnetocaloric effect in ferromagnetic Ni-Mn-Sn alloys.Nat Mater 4, 450-454 doi:10.1038/nmat1395 |
| 102. | Khalsa G, Stiles MD, Grollier J(2015)Critical current and linewidth reduction in spin-torque nano-oscillators by delayed self-injection.Appl Phys Lett 106, 242402 doi:10.1063/1.4922740 |
| 103. | Locatelli N, Mizrahi A, Accioly A, et al(2014)Noise-enhanced synchronization of stochastic magnetic oscillators.Phys Rev Appl 2, 034009 doi:10.1103/PhysRevApplied.2.034009 |
| 104. | Keatley P, Gangmei P, Dvornik M, et al(2013)Isolating the dynamic dipolar interaction between a pair of nanoscale ferromagnetic disks.Phys Rev Lett 110, 187202 doi:10.1103/PhysRevLett.110.187202 |
| 105. | Barber D, Freestone I(1990)An investigation of the origin of the colour of the Lycurgus cup by analytical transmission electron microscopy.Archaeometry 32, 33-45 doi:10.1111/arch.1990.32.issue-1 |
| 106. | Webb JA, Bardhan R(2014)Emerging advances in nanomedicine with engineered gold nanostructures.Nanoscale 6, 2502-2530 doi:10.1039/c3nr05112a |
| 107. | Anker JN, Hall WP, Lyandres O, et al(2008)Biosensing with plasmonic nanosensors.Nat Mater 7, 442-453 doi:10.1038/nmat2162 |
| 108. | Hellebust A, Richards-Kortum R(2012)Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics.Nanomed 7, 429-445 doi:10.2217/nnm.12.12 |
| 109. | Sanders M, Lin Y, Wei J, et al(2014)An enhanced LSPR fiberoptic nanoprobe for ultrasensitive detection of protein biomarkers.Biosens Bioelectron 61, 95-101 doi:10.1016/j.bios.2014.05.009 |
| 110. | Xu LJ, Zong C, Zheng XS, et al(2014)Label-free detection of native proteins by surface-enhanced Raman spectroscopy using iodide-modified nanoparticles.Anal Chem 86, 2238-2245 doi:10.1021/ac403974n |
| 111. | Yu MK, Park J, Jon S(2012)Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy.Theranostics 2, 3 doi:10.7150/thno.3463 |
| 112. | Huang X, El-Sayed MA(2011)Plasmonic photo-thermal therapy (PPTT).Alex J Med 47, 1-9 doi:10.1016/j.ajme.2011.01.001 |
| 113. | Carregal-Romero S, Ochs M, Rivera-Gil P, et al(2012)NIRlight triggered delivery of macromolecules into the cytosol.J Controll Release 159, 120-127 doi:10.1016/j.jconrel.2011.12.013 |
| 114. | Catchpole KR, Polman A(2008)Design principles for particle plasmon enhanced solar cells.Appl Phys Lett 93, 191113 doi:10.1063/1.3021072 |
| 115. | Lim S, Mar W, Matheu P, et al(2007)Photocurrent spectroscopy of optical absorption enhancement in silicon photodiodes via scattering from surface plasmon polaritons in gold nanoparticles.J Appl Phys 101, 104309 doi:10.1063/1.2733649 |
| 116. | Zhang D, Yang X, Hong X, et al(2015)Aluminum nanoparticles enhanced light absorption in silicon solar cell by surface plasmon resonance.Opt Quantum Electron 47, 1421-1427 doi:10.1007/s11082-014-0103-0 |
| 117. | Martín-Rodríguez R, Geitenbeek R, Meijerink A(2013)Incorporation and luminescence of Yb3+ in CdSe nanocrystals.J Am Chem Soc 135, 13668-13671 doi:10.1021/ja4077414 |
| 118. | Mukherjee P, Sloan RF, Shade CM, et al(2013)A postsynthetic modification of Ⅱ-Ⅵ semiconductor nanoparticles to create Tb3+ and Eu3+ luminophores.J Phys Chem C 117, 14451-14460 doi:10.1021/jp404947x |
| 119. | Chen CJ, Haik Y, Chatterjee J (2004) Nanomagnetics in biotechnology. In: Proceedings of the international workshop on materials analysis and processing in magnetic fields, Tallahassee, Florida, 17-19 March 2004 |
| 120. | Shamim N, Hong L, Hidajat K, et al(2007)Thermosensitive polymer (N-isopropylacrylamide) coated nanomagnetic particles: preparation and characterization.Colloids Surf B Biointerfaces 55, 51-58 doi:10.1016/j.colsurfb.2006.11.007 |
| 121. | Shamim N, Liang H, Hidajat K, et al(2008)Adsorption, desorption, and conformational changes of lysozyme from thermosensitive nanomagnetic particles.J Colloid Interface Sci 320, 15-21 doi:10.1016/j.jcis.2007.08.012 |
| 122. | Horng HE, Yang SY, Huang Y, et al(2005)Nanomagnetic particles for SQUID-based magnetically labeled immunoassay.IEEE Trans Appl Supercond 15, 668-671 doi:10.1109/TASC.2005.849995 |
| 123. | Parekh K(2010)Upadhyay R Static and dynamic magnetic properties of monodispersed Mn0.5Zn0.5Fe2O4 nanomagnetic particles.J Appl Phys 107, 053907 doi:10.1063/1.3310807 |
| 124. | Taketomi S(1998)Spin-glass-like complex susceptibility of frozen magnetic fluids.Phys Rev E 57, 3073 |
| 125. | Yoo SK, Lee SY(2000)Geometrical phase effects in biaxial nanomagnetic particles.Phys Rev B 62, 5713-5718 doi:10.1103/PhysRevB.62.5713 |
| 126. | Chakraverty S, Ghosh B, Kumar S, et al(2006)Magnetic coding in systems of nanomagnetic particles.Appl Phys Lett 88, 042501 doi:10.1063/1.2166203 |
| 127. | Miller J, Kropf A, Zha Y, et al(2006)The effect of gold particle size on Au-Au bond length and reactivity toward oxygen in supported catalysts.J Catal 240, 222-234 doi:10.1016/j.jcat.2006.04.004 |
| 128. | Carlson C, Hussain SM, Schrand AM, et al(2008)Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species.J Phys Chem B 112, 13608-13619 doi:10.1021/jp712087m |
| 129. | El-Sayed MA(2001)Some interesting properties of metals confined in time and nanometer space of different shapes.Acc Chem Res 34, 257-264 doi:10.1021/ar960016n |
| 130. | Nikoobakht B, El-Sayed MA(2003)Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method.Chem Mater 15, 1957-1962 doi:10.1021/cm020732l |
| 131. | Sreeprasad T, Nguyen P, Kim N, et al(2013)Controlled, defectguided, metal-nanoparticle incorporation onto MoS2 via chemical and microwave routes: electrical, thermal, and structural properties.Nano Lett 13, 4434-4441 doi:10.1021/nl402278y |
| 132. | Gawande MB, Shelke SN, Zboril R, et al(2014)Microwaveassisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics.Acc Chem Res 47, 1338-1348 doi:10.1021/ar400309b |
| 133. | Komarneni S, Li D, Newalkar B, et al(2002)Microwave-polyol process for Pt and Ag nanoparticles.Langmuir 18, 5959-5962 doi:10.1021/la025741n |
| 134. | Zhao Y, Zhu J, Hong J, et al(2004)Microwave-induced polyol-process synthesis of copper and copper oxide nanocrystals with controllable morphology.Eur J Inorg Chem 2004, 4072-4080 doi:10.1002/(ISSN)1099-0682 |
| 135. | Cheng W, Cheng HW(2009)Synthesis and characterization of cobalt nano-particles through microwave polyol process.AIChE J 55, 1383-1389 doi:10.1002/aic.v55:6 |
| 136. | Komarneni S, Roy R, Li Q(1992)Microwave-hydrothermal synthesis of ceramic powders.Mater Res Bull 27, 1393-1405 doi:10.1016/0025-5408(92)90004-J |
| 137. | Gao F, Lu Q, Komarneni S(2005)Interface reaction for the selfassembly of silver nanocrystals under microwave-assisted solvothermal conditions.Chem Mater 17, 856-860 doi:10.1021/cm048663t |
| 138. | Manzetti S (2017) NANOGEL: Synthesis of cadmium nanoparticles from a carefully selected ionic liquid of Cd2+ and benzoic acid. www. fjordforsk. no/nanogel. php |
| 139. | Itoh H, Naka K, Chujo Y(2004)Synthesis of gold nanoparticles modified with ionic liquid based on the imidazolium cation.J Am Chem Soc 126, 3026-3027 doi:10.1021/ja039895g |
| 140. | Grzelczak M, Pérez-Juste J, Mulvaney P, et al(2008)Shape control in gold nanoparticle synthesis.Chem Soc Rev 37, 1783-1791 doi:10.1039/b711490g |
| 141. | Yin B, Ma H, Wang S, et al(2003)Electrochemical synthesis of silver nanoparticles under protection of poly (N-vinylpyrrolidone).J Phys Chem B 107, 8898-8904 doi:10.1021/jp0349031 |
| 142. | Guo D, Li H(2004)Electrochemical synthesis of Pd nanoparticles on functional MWNT surfaces.Electrochem Commun 6, 999-1003 doi:10.1016/j.elecom.2004.07.014 |
| 143. | Manzetti S, Andersen O, Garcia C, et al(2016)Molecular simulation of carbon nanotubes as sorptive materials: sorption effects towards retene, perylene and cholesterol to 100 degrees Celsius and above.Mol Simul 141, 1-10 |
| 144. | Manzetti S(2012)Chemical and electronic properties of polycyclic aromatic hydrocarbons: a review.Handb Polycycl Aromat Hydrocarb Chem Occur Health Issues, 309-330 |
| 145. | Rodriguez-Sanchez L, Blanco M, Lopez-Quintela M(2000)Electrochemical synthesis of silver nanoparticles.J Phys Chem B 104, 9683-9688 doi:10.1021/jp001761r |
| 146. | Xing G, Wang D, Cheng CJ, et al(2013)Emergent ferromagnetism in ZnO/Al2O3 core-shell nanowires: towards oxide spinterfaces.Appl Phys Lett 103, 022402 doi:10.1063/1.4813217 |
| 147. | Dutta DP, Mandal BP, Naik R, et al(2013)Magnetic, ferroelectric, and magnetocapacitive properties of sonochemically synthesized Sc-doped BiFeO3 nanoparticles.J Phys Chem C 117, 2382-2389 doi:10.1021/jp310710p |
| 148. | Ghosh S, Yang R, Kaumeyer M, et al(2014)Fabrication of electrically conductive metal patterns at the surface of polymer films by microplasma-based direct writing.ACS Appl Mater Interfaces 6, 3099-3104 doi:10.1021/am406005a |
| 149. | Chen D, Yu Y, Huang F, et al(2010)Modifying the size and shape of monodisperse bifunctional alkaline-earth fluoride nanocrystals through lanthanide doping.J Am Chem Soc 132, 9976-9978 doi:10.1021/ja1036429 |
| 150. | Yang Y, Jin Y, He H, et al(2010)Dopant-induced shape evolution of colloidal nanocrystals: the case of zinc oxide.J Am Chem Soc 132, 13381-13394 doi:10.1021/ja103956p |
| 151. | Pal S, Bhunia A, Jana PP, et al(2015)Microporous La-metal-organic framework (MOF) with large surface area.Chem Eur J 21, 2789-2792 doi:10.1002/chem.201405168 |
| 152. | Dey R, Bhattacharya B, Pachfule P, et al(2014)Flexible dicarboxylate based pillar-layer metal organic frameworks: differences in structure and porosity by tuning the pyridyl based N, N' linkers.Cryst Eng Commun 16, 2305-2316 doi:10.1039/c3ce42028k |
| 153. | Liu BH, Ding J, Zhong Z, et al(2002)Large-scale preparation of carbon-encapsulated cobalt nanoparticles by the catalytic method.Chem Phys Lett 358, 96-102 doi:10.1016/S0009-2614(02)00592-4 |
| 154. | Lowndes DH, Rouleau CM, Thundat T, et al(1998)Silicon and zinc telluride nanoparticles synthesized by pulsed laser ablation: size distributions and nanoscale structure.Appl Surf Sci 127, 355-361 |
| 155. | Mafuné F, Kohno J, Takeda Y, et al(2000)Formation and size control of silver nanoparticles by laser ablation in aqueous solution.J Phys Chem B 104, 9111-9117 doi:10.1021/jp001336y |
| 156. | Mafuné F, Kohno J, Takeda Y, et al(2000)Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation.J Phys Chem B 104, 8333-8337 doi:10.1021/jp001803b |
| 157. | Becker MF, Brock JR, Cai H, et al(1998)Nanoparticles generated by laser ablation.Conf Lasers Electro-Opt 10(5), 151-152 |
| 158. | Sen P, Ghosh J, Abdullah A, et al(2003)Preparation of Cu, Ag, Fe and Al nanoparticles by the exploding wire technique.J Chem Sci 115, 499-508 doi:10.1007/BF02708241 |
| 159. | Andrievski R(2003)Modern nanoparticle research in Russia.J Nanoparticle Res 5, 415-418 doi:10.1023/B:NANO.0000006092.41059.82 |
| 160. | Goswami N, Sen P(2004)Water-induced stabilization of ZnS nanoparticles.Solid State Commun 132, 791-794 doi:10.1016/j.ssc.2004.09.022 |
| 161. | Phillips J, Perry WL, Kroenke WJ (2004) Method for producing metallic nanoparticles. U. S. Patent No. 6, 689, 192, 10 February 2004 |
| 162. | Bica I(1999)Nanoparticle production by plasma.Mater Sci Eng B 68, 5-9 doi:10.1016/S0921-5107(99)00422-5 |
| 163. | Swihart MT(2003)Vapor-phase synthesis of nanoparticles.Curr Opin Colloid Interface Sci 8, 127-133 doi:10.1016/S1359-0294(03)00007-4 |
| 164. | Kaneko T, Hatakeyama R, Takahashi S (2013) Plasma process on ionic liquid substrate for morphology controlled nanoparticles. INTECH Open Access Publisher. Chapter 24 |
| 165. | Graneau P(1983)First indication of Ampere tension in solid electric conductors.Phys Lett A 97, 253-255 doi:10.1016/0375-9601(83)90760-0 |
| 166. | Amendola V, Meneghetti M(2009)Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles.Phys Chem Chem Phys 11, 3805-3821 doi:10.1039/b900654k |
| 167. | Sajti CL, Sattari R, Chichkov BN, et al(2010)Gram scale synthesis of pure ceramic nanoparticles by laser ablation in liquid.J Phys Chem C 114, 2421-2427 doi:10.1021/jp906960g |
| 168. | Abdolvand A, Khan SZ, Yuan Y, et al(2008)Generation of titanium-oxide nanoparticles in liquid using a high-power, highbrightness continuous-wave fiber laser.Appl Phys A 91, 365-368 doi:10.1007/s00339-008-4448-8 |
| 169. | Wang X, Shephard JD, Dear FC, et al(2008)Optimized nanosecond pulsed laser micromachining of Y-TZP ceramics.J Am Ceram Soc 91, 391-397 doi:10.1111/jace.2008.91.issue-2 |
| 170. | Borysiuk J, Grabias A, Szczytko J, et al(2008)Structure and magnetic properties of carbon encapsulated Fe nanoparticles obtained by arc plasma and combustion synthesis.Carbon 46, 1693-1701 doi:10.1016/j.carbon.2008.07.011 |
| 171. | Scott JHJ, Majetich SA(1995)Morphology, structure, and growth of nanoparticles produced in a carbon arc.Phys Rev B 52, 12564-12571 doi:10.1103/PhysRevB.52.12564 |
| 172. | Delaunay JJ, Hayashi T, Tomita M, et al(1997)CoPt-C nanogranular magnetic thin films.Appl Phys Lett 71, 3427-3429 doi:10.1063/1.120356 |
| 173. | Li T, Yan H, Wang H, et al(2005)CoPt/C nanogranular magnetic thin film.Int J Mod Phys B 19, 2261-2271 doi:10.1142/S0217979205029687 |
| 174. | Lu Y, Zhu Z, Liu Z(2005)Carbon-encapsulated Fe nanoparticles from detonation-induced pyrolysis of ferrocene.Carbon 43, 369-374 doi:10.1016/j.carbon.2004.09.020 |
| 175. | Hayashi T, Hirono S, Tomita M, et al(1997)Magnetic thin films of cobalt nanocrystals encapsulated in graphite-like carbon.Cambridge University Press, Cambridge, p 33 |
| 176. | Harris P, Tsang S(1998)A simple technique for the synthesis of filled carbon nanoparticles.Chem Phys Lett 293, 53-58 doi:10.1016/S0009-2614(98)00770-2 |
| 177. | Britz DA, Khlobystov AN(2006)Noncovalent interactions of molecules with single walled carbon nanotubes.Chem Soc Rev 35, 637-659 doi:10.1039/b507451g |
| 178. | Iravani S(2011)Green synthesis of metal nanoparticles using plants.Green Chem 13, 2638-2650 doi:10.1039/c1gc15386b |
| 179. | Shankar SS, Ahmad A, Pasricha R, et al(2003)Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes.J Mater Chem 13, 1822-1826 doi:10.1039/b303808b |
| 180. | Yang X, Li Q, Wang H, et al(2010)Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf.J Nanoparticle Res 12, 1589-1598 doi:10.1007/s11051-009-9675-1 |
| 181. | Huang J, Lin L, Li Q, et al(2008)Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried Cinnamomum camphora leaf in tubular microreactors.Ind Eng Chem Res 47, 6081-6090 doi:10.1021/ie701698e |
| 182. | Sharma B, Purkayastha DD, Hazra S, et al(2014)Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa.Mater Lett 116, 94-97 doi:10.1016/j.matlet.2013.10.107 |
| 183. | Kumar B, Smita K, Cumbal L(2016)Biofabrication of nanogold from the flower extracts of Lantana camara.IET Nanobiotechnol 10, 154-157 doi:10.1049/iet-nbt.2015.0035 |
| 184. | Paul B, Bhuyan B, Purkayastha DD et al (2015) Green synthesis of gold nanoparticles using Pogestemon benghalensis (B) O. Ktz. leaf extract and studies of their photocatalytic activity in degradation of methylene blue. Mater Lett 148: 37-40 |
 2017, Vol. 5
2017, Vol. 5