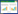The article information
- Lei-Lei Cui, Xiao-Wei Miao, Yu-Feng Song, Wen-Ying Fang, Hong-Bin Zhao, Jian-Hui Fang
- Electrospinning synthesis of novel lithium-rich 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 nanotube and its electrochemical performance as cathode of lithium-ion battery
- Advances in Manufacturing, 2016, 4(1): 79-88
- http://dx.doi.org/10.1007/s40436-016-0133-x
-
Article history
- Received: 4 February 2015
- Accepted: 8 January 2016
- Published online: 29 January 2016
2 Shanghai Key Laboratory of Mechanics in Energy Engineering, Shanghai University, Shanghai 200072, People's Republic of China;
3 Department of Chemical Engineering, University of Waterloo, Waterloo N2L3G1, Canada
Rechargeable lithium-ion batteries have been considered as the key power source for its application in mobile phones and electronic devices due to their high energy storage capacity,excellent cycling performance,and so on [1, 2, 3]. LiCoO2 was the first commercial cathode material introduced into market by Sony in 1991 [4]. LiCoO2 has still been the most widely used as the positive electrode material due to its easy synthesis,relatively good cyclic stability and stable discharge voltage although more than two decades have passed [5]. However,LiCoO2 also has disadvantages,such as its toxicity,high cost and low energy density,which have limited its applications in electrical vehicles (EVs) and plug-in hybrid electric vehicles (PHEVs). After that,many different cathode materials,such as LiFePO4,LiMnPO4,LiMn2O4,and transition metal oxides,are reported as cathodes of lithium batteries and their applications on EVs and PHEVs are evaluated and developed in detail [6, 7, 8]. However,for the electrical vehicles and other power batteries,the higher discharge voltage cathodes with considerable capacity,as well as excellent dynamic performance are required urgently. Among these kinds of batteries,ternary and Li-rich layered materials,such as LiNi1/3Co1/3Mn1/3O2,LiNi0.4Co0.2Mn0.4O2,LiNi0.5Co0.2Mn0.3O2 and xLi2MnO3·(1-x)LiMO2,are attracted by more and more attentions [9, 10, 11].
In recent years,Li-rich layered oxides written as xLi2- MnO3·(1-x)LiMO2(M = Mn,Co,Ni) have attracted great concerns as the next generation cathode materials applied in lithium ion batteries [12] owing to the high specific capacity (≥250 mAh/g),low-cost,environmentally friendly and highvoltage,etc. In this type ofmaterials,C/2 m Li-rich Li2MnO3 phase can be highly integrated with R-3m LiMO2,and Co/Ni doping with a (Co+Ni)/Mn molar ratio of 1/3 or higher is usually applied to improve the structure stability during cycling,but it is electrochemically inactive unless the voltage is up to 4.5 V [13, 14]. However,the disadvantages of this material are the large irreversible capacity loss during the first cycle because of the simultaneous removal of Li+ and O2- which is a net loss of Li2O at the voltage above 4.5 V.Besides,Li-rich layered cathode materials still meet the challenges,such as the poor cycling performance and rate capability because of the drastic activation and structure evolution of the material in the first cycle and the following deterioration upon further cycling [10, 12, 15, 16].To improve the performance,a lot of prepared material methods are conducted,such as coprecipitation [7, 17, 18],sol-gel method [16, 19, 20, 21, 22, 23],combustionmethod [24, 25, 26],ball millingmethod [27],microwave assisted method [28, 29],and so on.
In this paper,electrospinning [30, 31, 32] is used to synthesize the novel micro-nanostructured 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 cathode with a further sintering at 800 ℃ for different heating time. The effects of the heating time on the electrochemical performance and morphologies are investigated. The result shows that the lithium-rich cathode heated at 800 ℃ for 8 h presents the best performance with the initial discharge capacity of 267.7 mAh/g and remains 183.3 mAh/g after 50 cycles. The effects of temperature on the morphology and structure of this novel Li-rich layered material are discussed. Its micro-nanostructure contributes electrochemical stability and fast lithium ions transfer,therefore the high C-rate and long-time performance is expected.
2 Experimental 2.1 Materials preparationThe material of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 was prepared by electrospinning method. Polyacrylonitrile (PAN) was added into N,N-dimethylformamide and stirred for 4 h at 75 ℃ to get a stable transparent solution. Stiochiometric amounts of acetate Li(CH3COO)· 2H2O,Ni(CH3COO)2·4H2O,Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O) were dispersed in the solution under the constant stirring for 12 h at 75 ℃. The mixture was switched to the syringe and began to electrospin under a high voltage of 15 kV. The nanofibers were collected on a clean aluminum foil as a mat and calcined at 800 ℃ for different time of 3 h,8 h,12 h in air to eliminate the organic residues. The obtained powders were the composite of lithium-rich layered 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 and used without further treatment.
2.2 Material characterizationThe structure of the material is performed by X-ray diffraction (XRD) using Cu Ka radiation at 40 kV and 250 mA. The data are collected from 10.0°to 90.0°with a step size of 0.02°and courting time of 3.5 s for every step. Rietveld refinement is conducted by general structure analysis system (GSAS). The morphologies and sizes of materials are characterized by scanning electron microscope (SEM,Hitachi-X650 microscope,20 kV) and high resolution transmission electron microscope (HRTEM,JEOL-2010F,200 kV). The specific surface areas of the materials are measured through the Brunauere-Emmette- Teller (BET) procedure from the N2 adsorption desorption isotherms with a micromeritics ASAP 2020+C nitrogen adsorption instrument (micromeritics Inc.,USA) at 77 K. The elemental contents in the materials are analyzed by the inductively coupled plasma optical emission spectrometer) (ICP-OES) (ICAP 6000). The thermal decomposition behavior of the precursors is examined by thermogravimetric analyzer (NETZSCH STA 449 F3 Jupiter).
2.3 Electrochemical testThe electrochemical performances of the samples as the cathode materials are tested using 2016-type coin cells. The working electrodes are prepared by slurry which consists of 80% weight ratio active material,10% weight ratio Super P,and 10% weight ratio polyvinylidene fluoride. The mixture is casted onto Al foil and dried in the vacuum oven at 120 ℃ for 12 h. Cells are assembled in an argon-filled glove box with lithium foil as anode electrode,1 mol/L LiPF6 dissolved in a mixture of ethylene carbonate,dimethyl carbonate and ethyl-methyl carbonate (1:1:1 in volume) as the electrolyte and Celgard 2500 as separator. The galvanostatic charge-discharge performances are tested on a LAND CT2001A battery test system (Wuhan,China) between 2.5 V and 4.8 V (vs. Li+/Li) at the current density from 20 mA/g to 400 mA/g at room temperature. Cyclic voltammetry (CV) tests are measured by electrochemical workstation (CHI660d) in the potential window of 2.5-4.8 V (vs. Li+/Li) at a scan rate of 0.1 mV/s.
3 Results and discussionThe variety component of elements in the Li-rich xLi2MnO3· (1-x)LiNi1/3Co1/3Mn1/3O2 composites affects its electrochemical performance,and we employ ICP-OES to get the elemental composition before we study its structure and morphology. The result of ICP analysis in Table 1 supplies the calculated molar ratio of Li,Ni,Co and Mn is 1.4:0.22:0.22:0.64,which is closed to the stoichiometric result. The small difference of Li content is due to its evaporation when it is heated at high temperature for a long time. The BET specific surface area of the material at different time is comparatively large. The samples calcined at 800 ℃ for 8 h and 12 h obtained almost the same results of 10.28 m2/g and 10.58 m2/g,respectively.
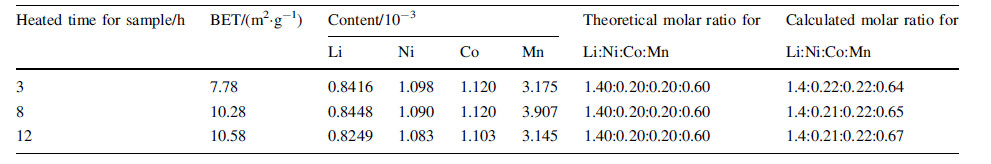 |
To verify the structure and phases of the composites synthesized by electrospinning,XRD patterns are studied and the results are refined in Fig. 1. It is obvious that the samples could not index a known single phase from the database and a complex phase structure seems more possible. The Rietveld refinement of XRD pattern of the composites with different heating time is indexed from slow scan speed diffraction patterns and all the peaks can be indexed to the hexagonal a-NaFeO2 type with R-3m except the supper lattice from 20°to 25°,which is corresponding to the monoclinic Li2MnO3 phase with the space group of C2/m [32]. Distinct splitting (006)/(102) and (018)/(110) peaks can be observed,which results in both good crystallinity and well-formed layered structure [33]. Table 2 shows the result of the refinement. As reported before,the higher ratio value of c/a represents faster Li+ transfer [33] and the sample heated for 8 h shows the highest value. Higher I(003)/I(104) value(>1.2) generally indicates better structure order and low cation mixing [34- 36]. The values of I(003)/I(104) of the samples heated for 3 h,8 h and 12 h are 0.81,0.83 and 0.82,respectively. Therefore,the Li-rich 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 is successfully synthesized by easily electrospinning method.
 |
| Fig. 1 Rietveld refinement of XRD pattern of the 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 heating at 800 ℃ for 3 h a, 8 h b and 12 c in the air atmosphere |
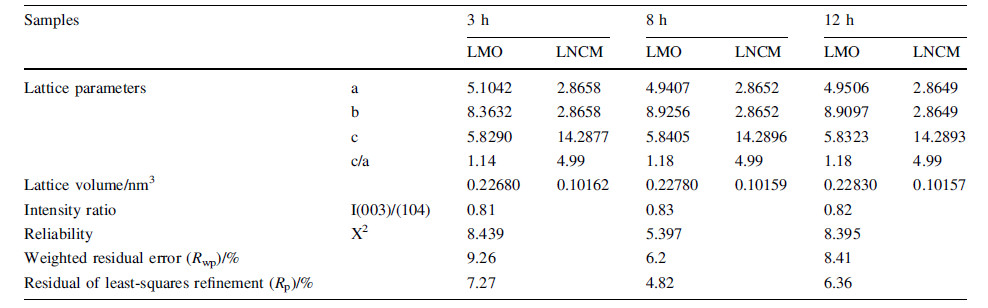 |
Figure 2 shows the TG-DSC of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 precursor with PAN in the range of 25-800 ℃ to detect the chemical reaction of the precursor and the calcined temperature of the product [37]. As can be observed from Fig. 2b,the decomposition process of the material is supposed to be divided into two stages. The mass-loss from room temperature to 250 ℃ can be indexed to the loss of DMF and the crystal water of acetates and the weight loss is about 2.5% occurred in this process companying a endothermic reaction. Another serious endothermic pyrolysis with mass-loss of about 6.5% occurred at 250 ℃ to 390 ℃. The DSC curve indicates that the mass-loss at about 390 ℃ corresponded to an exothermic peak of the decomposition of the PAN polymer main chain and the formation of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 [37]. Below 700 ℃,there is no obvious mass-loss and endothermic peak,confirming that the ideal heat-treatment temperature is higher than 700 ℃. To prevent the lithium evaporation at high temperature and get well crystallized Li-rich composite,800 ℃ as calcination temperature is selected.
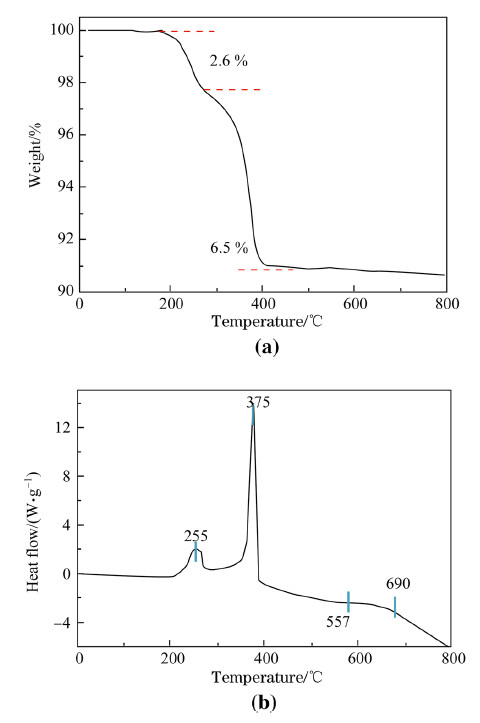 |
| Fig. 2 a Thermogravimetric (TG) and b differential scanning calorimetric (DSC) analysis of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 precursors with PAN |
To study the morphology of the obtained Li-rich composites and evaluate the relationship between morphology and electrochemical performances,we take the SEM images of obtained 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 samples (see Fig. 3). As shown in Fig. 3,the morphologies of the Li-rich composites are different when sintered for different time. The obtained raw wires of precursor (see Fig. 3d,d') are smooth surface and homogenous wire with the diameter of 1 lm and dozens micrometers in length. All of these three samples undergone heat-treatment are almost hollow tubes assembling with different diameter of nanoparticles loosely. With the heat-treatment time increasing,the nanoparticles grow up from about 50 nm to 150 nm and the surface becomes much rougher. In addition,the diameter of hollow tubes decreases from dozens micrometers to one micrometer when the heat-treatment time delays from 3 h to 12 h. Differently,the tubes heated for 3 h (see Figs. 3a,a') are long and the wall is thin which indicate the bad crystallinity of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 or incompletely deposition of PAN. When the heating time increases to 8 h,the tubes become a little shorter and the walls become thicker (see Figs. 3b,b'),thus enhancing the electrochemical cycling performance. In case of the composite heat-treated for 12 h,the hollow tubes-like is loosely assembled by agglomeration of particles (see Figs. 3c,c'),meaning good crystallinity while longer lithium transfer channel which is not desirable. TG/DSC patterns also confirm that when increasing heattreatment time,the precursors go through three different processes,deposition of polymer,the formation of new phases and the crystallization. In the heat-treatment process,PAN decomposes as carbon oxides and nitrogen oxides and leaves enough space. These empty spaces decrease the aggregation and growth of nanoparticles. The precursor salts dispersed in PAN decompose and form new phases with a successively grown up to nanoparticles. The shape of raw wires is kept totally,while the surface becomes rough and new wall formed with aggregation nanoparticles of Li-rich composite. This micro-nanostructured hollow tube is expected to improve the dynamic behaviour,as well as the cycling performance.
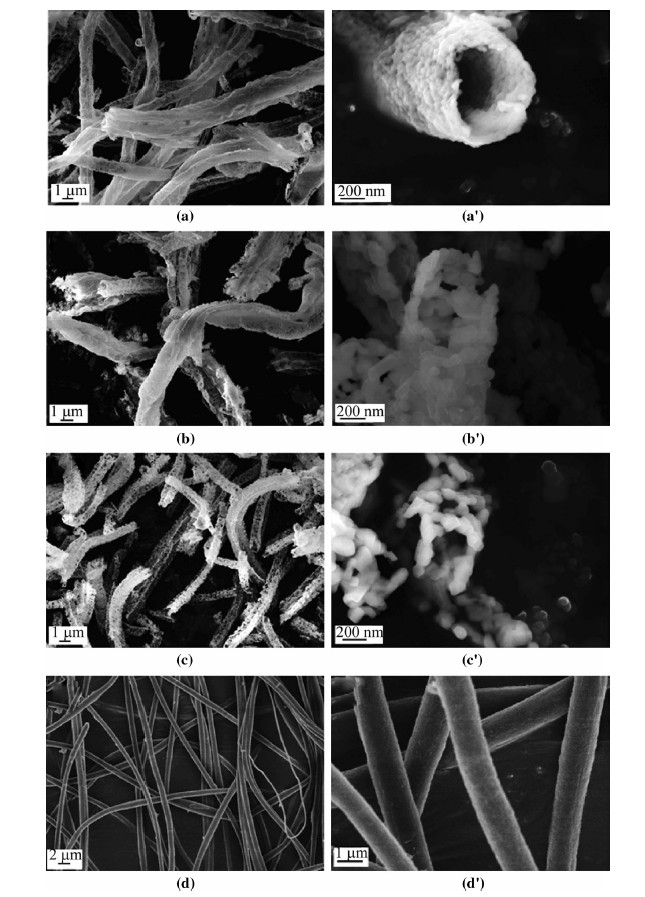 |
| Fig. 3 SEM images of the material 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 heating at 800 ℃ for a,a' 3 h, b, b' 8 h and c, c' 12 h in the air atmosphere with different magnification, d, d' the raw wire got by electrospinning |
Figure 4 shows the HRTEM images of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 for different heating time. The crystal d-spacing between the neighboring lattice fringes is measured of 43.4 nm,47.1 nm and 45.7 nm,respectively. The sample of 8 h shows the closest value to the (0 0 3) plane of the hexagonal layered phase of 47 nm and exhibits the clearest lattice fringe. Therefore,the sample calinated at 800℃ for 8 h has more ideal hexagonal layered phase indicating more stable structure for lithiation/ delithiation process. The novel micro-nanostructured assembling Li-rich composite indicates good electrochemical reversible performance and high C-rate performance because of its small particle size,which is benefit for lithium transfer. The stability for its assembled micrometer tube in length prevents the volume expansion and the aggregation of particles in the charge/discharge process.
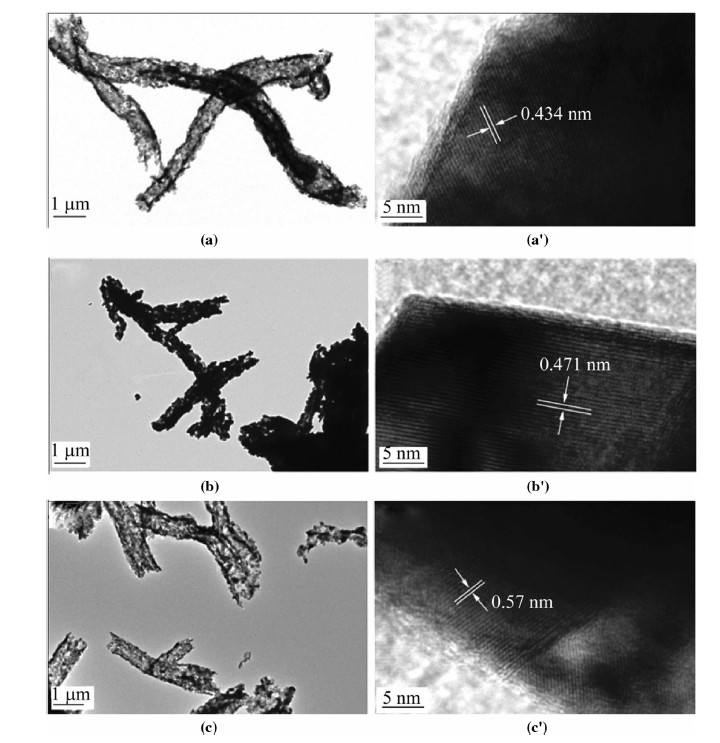 |
| Fig. 4 HRTEM images of the material 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 heating at 800 ℃ for a,a' 3 h, b, b' 8 h and c, c' 12 h in the air atmosphere |
The electrochemical performance and lithium storage mechanism of the Li-rich 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 cathode are evaluated by constant current charge/discharge and coulometric differentiation method. Figure 5 shows the first two cycles of charge/discharge curves and the differential capacity vs. voltage of the samples for different heating time at a current density of 20 mA/g (1C = 200 mA/g) within the voltage of 2.5-4.8 V (vs. Li+/Li). The sample heated for 8 h delivers the highest charge/discharge capacity of 321.8mAh/g/ 267.7 mAh/g with a high initial coulomb efficiency of 83.2%. The initial charge curve has two plateaus at 3.9 V and 4.5 V,respectively. The first plateau is corresponding to the Li+ ions extracting from the space group of R-3m accompanying with the oxidation of Ni2+/Ni4+ and Co3+/Co4+. Another plateau is attributed to the releasing of Li+ and O2-,considered as Li2O,from the layered Li2MnO3 which only happens during the first cycle and this process is irreversible because of the rearrangement of ions [38]. It can be observed from Fig. 5b that there are two processes during the first charge,corresponding to the two cathodic peaks at 3.9 Vand 4.6 V,which is agreement with that of charge/discharge profiles. The anodic peak at around 4.0 V is more electrochemical reversible. All the cathodes have two obvious charge/discharge plateaus around 3.9 V and the wide and float integrated Q from 3.2 V to 3.8 V means continue lithiation process with wide potential window and contributes relative high capacity.
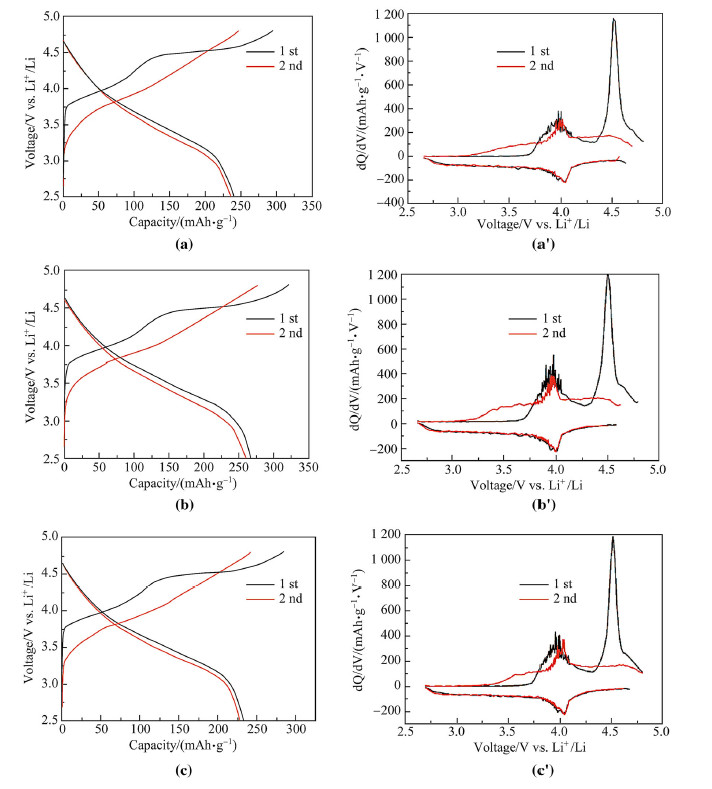 |
| Fig. 5 The first and second charge–discharge curves and differential capacity vs. voltage of the sample 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 heating at 800 ℃ for a,a' 3 h, b, b' 8 h and c, c' 12 h in the air atmosphere in the voltage range between 2.5 V and 4.8 V (vs. Li+/Li) at a current density of 20 mA/g |
Figure 6 shows the cyclic performance and the rate capability of the sample 0.4Li2MnO3·0.6LiNi1/3Co1/3 Mn1/3O2 synthesized by different heating time of 3 h,8 h and 12 h between 2.5 V and 4.8 V. The sample heated for 8 h shows the best cycling performance with the initial discharge capacity of 267.7 mAh/g and remains 183.3 mAh/g after 50 cycles. The cells of 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 synthesized by different heating time of 3 h,8 h and 12 h are charged with 0.1C and discharged with different current densities from 0.1C to1C and back to 0.1C,as shown in Figs. 6a,b. As the current densities increasing,the discharge capacity decreases and the sample heated for 8 h has the best rate capacity of 263.2 mAh/g,215.8 mAh/g,167.4 mAh/g and 109.6 mAh/g and it still recovers to high capacity of 215 mAh/g when back to 0.1C. The excellent high C-rate performance and well recovery ability are attributed to its micro-nanostructured hollow tube and thin tube wall assembled by the small nanoparticles. The electrospining technique effectively controls the nanostructure with stable electrochemical performance. The micro-nanostructured morphology of 0.4Li2MnO3· 0.6LiNi1/3Co1/3Mn1/3O2 meets the requirement of the dynamic design that shortens lithium ion transfer channel and more stable structure adapting for large volume expansion/contraction.
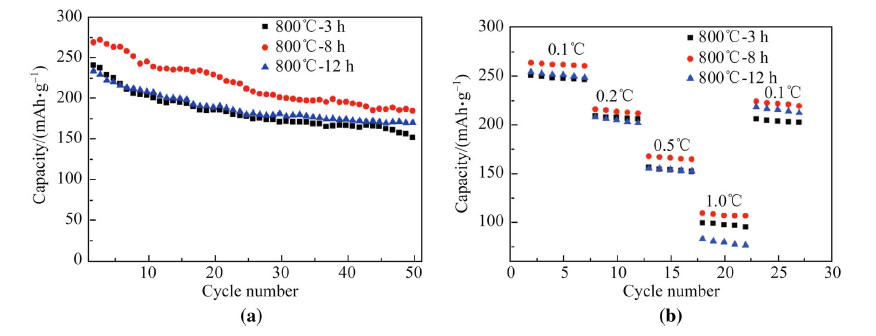 |
| Fig. 6 The cyclic performance a and the rate capability b of the sample 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 heating at 800 ℃ for different calcination time in the charge/discharge window between 2.5 V and 4.8 V(vs. Li+/Li) |
The novel 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 nanotube cathode material is synthesized via electrospinning and sintered treatment. Different heating time is important to the morphology and electrochemical performance which are all investigated in the paper. The sample synthesized by heating at 800 ℃ for 8 h displays the desirable crystallinity and small particle size of 80-150 nm. It also shows good cycle performance which delivers the high discharge capacity of 267.7 mAh/g at the first cycle and remains 183.3 mAh/g after 50 cycles for its micro-nanostructure with stable morphology during the charge/discharge process. But the C-rate capacity is not as high as the reference reported [39, 40]. More works need to be done to further improve the performance of this Li-rich material. For the facile electrospining synthesis method,it can be regarded as a potential method to prepare other micro-nanostructured cathode material of Li-ion battery.
Acknowledgments The project is funded by the 085 Project of Shanghai Education Commission,Science and Technology Commission of Shanghai Municipality (Grant No. 15ZR1415100),the China Scholarship Council (Grant No. 201406895017) and Shanghai University International Cooperation and Exchange Fund.We also thank the Analysis and Research Center of Shanghai University for sample characterization.| 1. | Armstrong AR, Bruce PG (1996) Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 381:499-500 |
| 2. | Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359-367 |
| 3. | Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271-4302 |
| 4. | Ozawa Kazunori (1994) Lithium-ion rechargeable batteries with LiCoO2 and carbon electrodes: the LiCoO2/C system. Solid State Ion 69:212-221 |
| 5. | Ozan T, Hatice AK, Li Y et al (2013) Synthesis and characterization of xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries. J Power Sources 241:522-528 |
| 6. | Jang YI, Huang B, Wang H et al (1999) LiAlyCo1-yO2((R-3m) intercalation cathode for rechargeable lithium batteries. J Electrochem Soc 146:862-868 |
| 7. | Yuan LX, Wang ZH, Zhang WX et al (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4:269-284 |
| 8. | Shaju KM, Rao GVS, Chowdari BVR (2003) Performance of layered Li Ni1/3Co1/3Mn1/3O2 as cathode for Li-ion batteries. Electrochim Acta 48:145-151 |
| 9. | Makimura Y, Ohzuku T (2003) Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J Power Sources 119:156-160 |
| 10. | Thackeray MM, Kang SH, Johnson CS et al (2006) Comments on the structural complexity of lithium-rich Li1+xM1-xO2 electrodes (M = Mn, Ni, Co) for lithium batteries. Electrochem Commun 81:531-1538 |
| 11. | Yabuuchi N, Yoshii K, Myung ST et al (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404-4419 |
| 12. | Ito A, Li DC, Sato Y et al (2010) Cyclic deterioration and its improvement for Li-rich layered cathode material Li [Ni0.17Li0.2Co0.007Mn0.56]O2. J Power Sources 195:567-573 |
| 13. | Ye DL, Wang LZ (2014) Li2MnO3 based Li-rich cathode materials: towards a better tomorrow of high energy lithium ion batteries. Adv Funct Mater 29:A59-A69 |
| 14. | Kang SH, Thackeray MM (2009) Enhancing the rate capability of high capacity xLi2MnO3·(1-x)LiMO2 (M = Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem Commun 11:748-751 |
| 15. | Lu Z, Dahn JR (2002) Structure and electrochemistry of layered Li [CrxLi(1/3-x/3)Mn(2/3-2x/3)]O2. J Electrochem Soc 149:A1454- A1459 |
| 16. | Kang SH, Kempgens P, Greenbaum S et al (2007) Interpreting the structural and electrochemical complexity of 0.5Li2 MnO3·0.5LiMO2 electrodes for lithium batteries (M = Mn0.5-xNi0.5-xCo2x, 0 ≤ x ≤ 0.5). J Mater Chem 17:2069-2077 |
| 17. | Shi SJ, Tu JP, Zhang YJ et al (2013) Effect of Sm2O3 modification on Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathode material for lithium ion batteries. Electrochim Acta 108:441-448 |
| 18. | Yuan LX, Wang ZH, Zhang WX et al (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4:269-284 |
| 19. | Jin SJ, Park KS, Cho MH et al (2006) Effect of composition change of metals in transition metal sites on electrochemical behavior of layered Li [Co1-2x(Li1/3Mn2/3)x(Ni1/2Mn1/2)x]O2 solid solutions. Solid State Ion 177:105-112 |
| 20. | Kang SH, Amine K (2005) Layered Li (Li0.2Ni0.15+0.5z Co0.10Mn0.55-0.5z)O2-zFz cathode materials for Li-ion secondary batteries. J Power Sources 146:654-657 |
| 21. | Zheng JM, Wu XB, Yang Y (2011) A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim Acta 56:3071-3078 |
| 22. | Sivaprakash S, Majumder SB (2010) Spectroscopic analyses of 0.5Li [Ni0.8Co0.15Zr2]O2-0.5 Li[Li1/3Mn2/3]O2 composite cathodes for lithium rechargeable batteries. Solid State Ion 181:730-739 |
| 23. | Wei YJ, Nikolowski K, Zhan SY et al (2009) Electrochemical kinetics and cycling performance of nano Li[Li0.23Co0.3Mn0.47]O2 cathode material for lithium ion batteries. Electrochem Commun 11:2008-2011 |
| 24. | Amalraj F, Kovacheva D, Talianker M et al (2010) Synthesis of integrated cathode materials xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and studies of their electrochemical behavior. J Electrochem Soc 157:A1121-A1130 |
| 25. | Kim GY, Yi SB, Park YJ et al (2008) Electrochemical behaviors of Li[Li(1-x)/3Mn(2-x)/3Nix/3Cox/3]O2 cathode series (0< x< 1) synthesized by sucrose combustion process for high capacity lithium ion batteries. Mater Res Bull 43:3543-3552 |
| 26. | Shi SJ, Tu JP, Tang YY et al (2013) Combustion synthesis and electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with improved rate capability. J Power Sources 228:14-23 |
| 27. | West WC, Soler J, Ratnakumar BV (2012) Preparation of high quality layered-layered composite Li2MnO3-LiMO2 (M = Ni, Mn, Co) Li-ion cathodes by a ball milling-annealing process. J Power Sources 204:200-204 |
| 28. | Peng QW, Tang ZY, Zhang LQ et al (2009) Synthesis of layered Li1.2+x[Ni0.25Mn0.75]0.8-xO2 materials (0 ≤ x≤ 4/55) via a new simple microwave heating method and their electrochemical properties. Mater Res Bull 44:2147-2151 |
| 29. | Miao XW, Yan Y, Wang CG et al (2014) Optimal microwaveassisted hydrothermal synthesis of nanosized xLi2MnO3·(1-x) LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium ion battery. J Power Sources 247:219-227 |
| 30. | Hosono E, Wang YG, Kida N et al (2010) Synthesis of triaxial LiFePO4 nanowire with a VGCF core column and a carbon shell through the electrospinning method. Appl Mater Interfaces 2:212 |
| 31. | Mizuno Y, Hosono E, Saito T et al (2012) Electrospinning synthesis of wire-structured LiCoO2 for electrode materials of highpower Li-ion batteries. J Phys Chem C 116:10774-10780 |
| 32. | Eiji H, Tatsuya S, Junichi H et al (2013) Synthesis of LiNi0.5Mn1.5O4 and 0.5Li2MnO3-0.5LiNi1/3Co1/3Mn1/3O2 hollow nanowires by electrospinning. Cryst Eng Comm 15:2592-2597 |
| 33. | Shi SJ, Tu JP, Zhang YD et al (2013) Morphology and electrochemical performance of Li [Li0.2Mn0.56Ni0.16Co0.08]O2 cathode materials prepared with different metal sources. Electrochim Acta 109:828-834 |
| 34. | Hwang B, Santhanam R, Chen C (2003) Effect of synthesis conditions on electrochemical properties of LiNi1-yCoyO2 cathode for lithium rechargeable batteries. J Power Sources 114:244 |
| 35. | Alcantara R, Lavela P, Tirado J et al (1998) Changes in structure and cathode performance with composition and preparation temperature of lithium cobalt nickel oxide. J Electrochem Soc 145:730 |
| 36. | Chang Z, Chen Z, Wu F et al (2008) Synthesis and characterization of high-density non-spherical Li(Ni1/3Co1/3Mn1/3)O2 cathode material for lithium ion batteries by two-step drying method. Electrochim Acta 53:5927 |
| 37. | Min JW, Yim CJ, Im WB (2014) Preparation and electrochemical characterization of flower-like Li1.2Ni0.17Co0.17Mn0.5O2 microstructure cathode by electrospinning. Ceram Int 40:2029 |
| 38. | Min JW, Kalathil AK, Yim CJ et al (2014) Morphological effects on the electrochemical performance of lithium-rich layered oxide cathodes, prepared by electrospinning technique, for lithium-ion battery applications. Mater Charact 92:118 |
| 39. | Johnson CS, Li N, Lefief C et al (2008) Characterization and electrochemistry of lithium battery electrodes: xLi2MnO3·(1-x) LiMn0.333Ni0.333Co0.333O2 (0 ≤x ≤ 0.7). Chem Mater 20:6095 |
| 40. | Min JW, Yim CJ, Im WB (2013) Facile synthesis of electrospun Li1.2Ni0.17Co0.17Mn0.5O2 nanofiber and its enhanced high-rate performance for lithium-ion battery applications. ACS Appl Mater Interfaces 5:7765-7769 |
 2016, Vol. 4
2016, Vol. 4