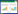The article information
- Happiness V. Ijije, George Z. Chen
- Electrochemical manufacturing of nanocarbons from carbon dioxide in molten alkali metal carbonate salts: roles of alkali metal cations
- Advances in Manufacturing, 2016, 4(1): 23-32
- http://dx.doi.org/10.1007/s40436-015-0125-2
-
Article history
- Received: 13 April 2015
- Accepted: 26 October 2015
- Published online: 9 December 2015
2 Department of Chemical and Environmental Engineering, and Centre for Sustainable Energy Technologies, Faculty of Science and Engineering, University of Nottingham Ningbo China, Ningbo 315100, People's Republic of China
Observed changes in global climate conditions have received unequivocal attention because of the effect on natural and human processes. Reducing the carbon footprint in most processes has become an integral aspect of industrial applications. Attempts to reduce the amount of CO2 emissions from anthropogenic sources have received much attention. One of such methods is the conversion of CO2 to products such as hydrocarbons,methanol and other chemicals and materials. Electro-reduction of CO2 in aqueous solutions is feasible,and the appropriate electrolyte,electrode material,temperature,pressure,pH,catalyst,potential,and operating conditions must be used [1, 2, 3]. On the other hand,electro-reduction of CO2 was also carried out in methanol [4, 5, 6, 7]. Besides aqueous and organic solutions,electro-reduction of CO2 has been reported in ionic liquids [8, 9, 10, 11, 12]. This is a relatively new subject area and the selection of the appropriate ionic liquid is important.
Early researchers established that carbon could be electro-deposited from molten salts containing carbonate ion (CO32-) and lithium ion (Li+) [13, 14, 15]. More recently, a number of authors have suggested that this process could be utilised for the indirect conversion of CO2 to carbon [16, 17, 18, 19, 20, 21]. This conversion route involves the electrochemical reduction of carbonate ions (CO32-) to carbon and oxide ions (O2-) in molten alkali carbonate salts [13]. The produced O2- ions in turn react with CO2 to regenerate the CO32- ions [22, 23]. Several studies have been focused on understanding the chemistry behind molten carbonate electrolysis,albeit with quite different interpretations of the reactions involved [24]. About 90% of the available literature on the subject carried out the electrolysis in the ternary eutectic mixture of Li2CO3-Na2CO3-K2CO3 (molar ratio: 43.5:31.5:25.0),and the reason for this is possibly due to its low eutectic melting temperature (396 ℃). In spite of the existing literature on the subject,there is still a lack of knowledge on the fundamental reactions taking place in the individual carbonates during electrolysis. For example,production of carbon from CO32- ions reportedly only occurred when lithium ions (Li+) were present [13]. Absence of carbon production was reported in mixtures of Na2CO3 and K2CO3 [13, 18, 25]. During the electrolysis in molten Na2CO3-K2CO3,no carbon deposit was obtained. However it was reported that at -2.25 V,alkali metal was liberated to such an extent as to amalgamate with the surface of the gold cathode. Alkali metal was reportedly observed on the surface of the cathode when a potential of -2.7 V was applied during electrolysis in the binary mixture [13]. Besides the investigations in molten Li2CO3 at a gold [13] or molybdenum [26] electrode,studies in the primary alkali metal carbonate mixtures either have received less attention or are simply lacking in the published literature.
Studying these molten alkali carbonates is important to understand their underlying electrochemistry during electrolysis. This work therefore reports cyclic voltammetric investigations at a platinum (Pt) electrode in molten K2CO3,Na2CO3,and Li2CO3. The study investigates the roles of the alkali metal ions (Na+,K+ and Li+ ions) during the electrolysis and how they influence carbon production. Besides cyclic voltammetry (CV) investigations,potentiostatic electrolysis was also carried out in each of the molten salts and electron microscopy was used to characterise the produced carbon.
2 ExperimentalPowders of each of Na2CO3,K2CO3 and Li2CO3 (> 99 pure,Sigma Aldrich) were thermally dried at 140 ℃ under vacuum for up to 14 h to remove moisture. After drying,the salt (250 g) was placed in an alumina crucible (Almath Crucibles Ltd.) before being sealed off in a stainless steel retort where it was heated to the desired temperatures under the CO2 atmosphere. Details of the experimental set have been documented elsewhere [27]. Using a three-electrode cell,CV investigations were carried out. The working and counter electrodes used were a Pt wire (0.25 mm in diameter,0.15 cm2 in contact area with electrolyte) and a stainless steel rod (Grade 304,6.0 mm in diameter,0.45 cm2 area) respectively. Pt was chosen because of its wide potential range and inertness in the electrolyte,and stainless steel as result of its moderate chemical stability and electrical conductivity. The reference electrode used was a stable high temperature Ag/AgCl reference electrode [16, 17, 18, 21]. It was constituted of a silver wire placed in a 50 cm long alumina tube (Multilab Ltd.) containing the molten salt being studied and 10% (molar ratio) AgCl. Cyclic voltammetry (CV) studies were carried out using a PGSTAT30 autolab potentiostat (Ecochemie) which was controlled by a PC using the general purpose electrochemical system (GPES) software. No iR compensation was applied in this work considering that the molten salts have low ionic resistance (<0.1 Ω between working and reference electrodes). Further,the deposition of carbon would increase the surface area of the working electrode, and hence decrease the resistance between the working and reference electrodes. As a result,a pre-set resistance value in the potentiostat for iR compensation by the positive feedback method would cause an over-compensation current that in turn could distort more seriously the recorded CV. Potentiostatic electrolysis was also carried out in the various molten carbonates using similar equipment. The produced carbon was characterised using both scanning and transmission electron microscopy (SEM and TEM).
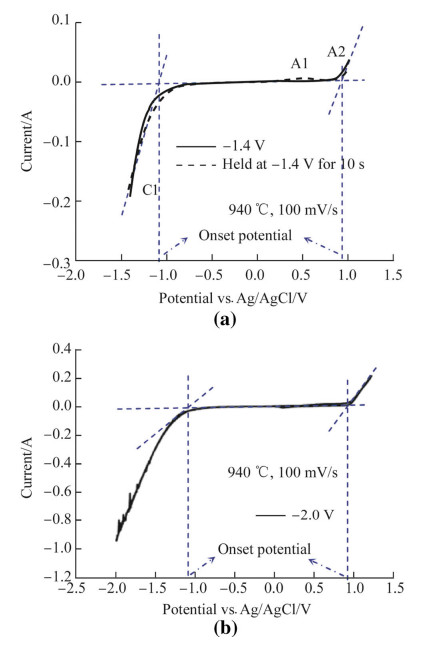
3 Results and discussions 3.1 Molten K2CO3CV was used to investigate the electrode reactions in molten K2CO3 at 940 ℃ at a Pt electrode (the melting temperature of K2CO3 is 891 ℃). In this paper,CV may mean cyclic voltammogram,cyclic voltammetric,and cyclic voltammetry depending on the context. On the CVs,cathodic peaks representing electro-reduction are labelled C while anodic peaks representing electro-oxidation are labelled A. In the CVs displayed in Fig. 1 which were obtained in molten K2CO3,no reduction peak is visible before the cathodic wave C1. This likely indicated that the Pt electrode used was inert in the duration and conditions of the experiment. No obvious reduction in current was observed as the scan duration increased suggesting that the dissolution of Pt in the molten salt was minimal. Possible cathodic reactions in molten alkali carbonate could include alkali metal,carbon and CO formations and the corresponding half-cell reactions are given in reactions (1)-(3) [28]. Using the oxidation of CO32- ions (see reaction (4)) as the reference reaction,the full cell reactions for reactions (1)-(3) can be rewritten (see reactions (5)-(7)).
 |
| Fig. 1 CVs of a 0.25 mm Pt wire in molten K2CO3 at 940 ℃ for negative scan limits of a -1.4 V and held at -1.4 V for 10 s and b -2.0 V; scan rate:100 mV/s; immersion depth of Pt wire: 1.5 cm (The onset potential of a reduction or oxidation current is approximately determined by the crossing point between the two tangent lines on each side of the inflexion on the curve. This method is applied to the rest of the paper.) |
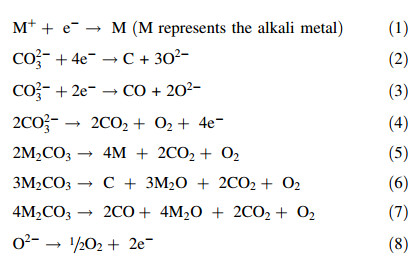
The corresponding standard reduction potentials calculated for the cathodic parts of reactions (5)-(7) at the molten salt temperature (940 ℃) where the alkali metal is potassium (K) are -2.13 V,-2.67 V and -3.10 V vs. CO32-/CO2-O2 respectively (i.e.,for K,carbon and CO formation in molten K2CO3). From these standard potentials,it is highly likely that the formation of K metal via reaction (5) is more favourable than carbon electro-deposition during electrolysis in K2CO3. The electrochemical potential window between the onset potential of peak C1 (-1.1 V vs. Ag/AgCl) and A2 (0.9 V) is about 2.0 V. This can be compared with the calculated standard potential of -2.13 V vs. CO32-/CO2-O2 for K+ ion reduction via reaction (5). Thus,it seems that this reaction dominates during the CV investigation in K2CO3.
When the working electrode potential was made more negative (-2.0 V vs. Ag/AgCl,see Fig. 1b),the cathodic wave (C1) continued to increase and no other cathodic peaks were observed. Because of the high molten salt temperature,the formation of K metal was not physically observed,as this would have completely dissolved in the electrolyte or evaporated considering the boiling temperature of K metal is 764 ℃ [29]. Other cathodic reactions that can occur during electrolysis in K2CO3 as mentioned earlier are the formation of both carbon and CO and the standard potentials for these reactions are -2.67 V and -3.10 V vs. CO32-/CO2-O2 respectively. Under the conditions for the CV investigations,these reactions were probably less favourable because of their more negative potentials. However,the formation of K metal was probably the main cathodic reaction during the electrolysis and hence more favourable than carbon deposition or CO formation because of its less negative potential. In line with the CV results,no carbon deposits were obtained in molten K2CO3 during potentiostatic electrolysis at -2.5 V vs. Ag/ AgCl or during two-electrode electrolysis at 4.0 V.
During the anodic sweep,the only two visible peaks on the CVs displayed in Fig. 1 are peaks A1 and the anodic limit A2. The reaction at the anodic limit A2 is likely the oxidation of CO32- ions to CO2 and O2 according to reaction (4) [18, 28, 30, 31, 32]. This attribution is reasonable because the molten salt contains mostly CO32- ions. Considering the working electrode area and the immersion depth,the current density for peak A2 is>300 mA/cm2 and from literature,during electrolysis in alkali metals carbonates,the oxidation of CO32- ions to CO2 was reportedly more favourable at higher current densities [33]. Anodic peak A1 on the other hand is probably the oxidation of O2- ions to O2 (see reactions (8)). The presence of a small amount of O2- ions in molten K2CO3 is expected in accordance with the equilibrium of CO32- = CO2 + O2- (see reaction (4)). The peak current of A1 did not increase when the working electrode potential was held at -1.4 V for 10 s and neither did it increase when the electrode potential was made more negative.
3.2 Molten Na2CO3CV studies were also carried in molten Na2CO3 (melting temperature 851 ℃) and the CVs obtained at a Pt electrode in the molten salt at 870 ℃ are displayed in Fig. 2. Unlike molten K2CO3 which has limited interaction with the Pt electrode,it appears that Pt oxidation occurs faster in Na2CO3 and the reduction peak C2 beginning at -0.57 V in Fig. 2a observed during the early stages of the CV studies is attributed to the reduction of PtO32- ions. In the literature,a similar peak was observed during CV investigations in the ternary alkali carbonate mixture [34].
 |
| Fig. 2 CVs of a 0.25 mm Pt wire working electrode in molten Na2CO3 at 870 ℃ for negative scan limits of a -1.4, -1.6 and -1.8 V, and b -2.4 V |
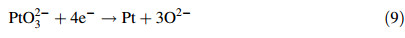
The standard potential for the reduction of Na+ ions to Na metal at 870 ℃ is -2.18 V vs. CO32-/CO2-O2. In Fig. 2a,the electrochemical potential window between the onset of peak C3 (-1.1 V vs. Ag/AgCl) and A2 (0.4 V vs. Ag/AgCl) is about 1.50 V,which is narrower than that calculated for the standard potential for Na formation. It is also possible that Na-Pt alloy might have formed as this can occur at less negative potentials than formation of Na metal. When the electrode potential was made more negative (i.e.,-2.4 V vs. Ag/AgCl,Fig. 2b),cathodic peak C4 was observed. This peak is probably due to sodium carbide formation according to reaction (10) [35]. The reduction of CO32- ions to carbon and O2- ions is also possible and occurs at a standard potential of -2.29 V vs. CO32-/CO2- O2. This potential is very close to that for the formation of Na metal. Reduction of CO32- ions to CO on the other hand has a more negative standard potential (-2.63 V vs. CO32-/CO2-O2). Therefore,during electrolysis in molten Na2CO3,the main cathodic reaction is likely the reduction of Na+ ions to Na metal and probably dominates over other reactions.
As mentioned earlier,the standard potential for the reduction of CO32- to carbon in this molten salt is very close to that for Na metal formation. This does likely suggest the formation of carbon. However,any carbon formed at the electrode probably reacted with Na metal to form carbide compounds via reaction (10).
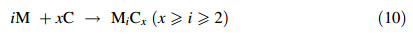
As the electrode potential was made more negative, peaks A1 (-0.3 V vs. Ag/AgCl),A3 (-1.5 V) and A4 (-1.2 V) were observed on the CVs. Holding the working electrode potential at -2.4 V for 10 s also increased the peak currents for A3 and A4. These peaks are likely due to the re-oxidation of cathodic products on the electrode surface. This observation is quite interesting as besides peak A1,no other oxidation peaks were observed during CV investigations in K2CO3. In Na2CO3,the cathodic products remained longer on the electrode surface. Peaks A3 and A4 are likely associated with the re-oxidation of carbide compounds,and anodic stripping of the Na-Pt alloy and/or oxidation of Na metal respectively,while peaks A1 and A2 are for the oxidation of O2- ions to O2 and of CO32- ions to CO2 and O2.
Potentiostatic electrolysis was carried out at -3.0 V vs. Ag/AgCl in the molten Na2CO3 electrolyte. The working electrode material was a 5 mm diameter mild steel rod while both the counter and reference electrodes remained the same as those used in the CV investigations. After 30 min electrolysis,black deposit-like masses were present on the working electrode surface (see Fig. 3). From physical observations,the mass remaining attached to the surface of the electrode after it was pulled out from the molten salt was time dependent,i.e.,pulling the working electrode out quickly means that more products remains on the surface. The masses on the electrode surface were likely a mixture of solidified salt,carbide compounds,and Na metal formed during the electrolysis. The boiling point for Na metal is 883 ℃ [29],which is very close to the molten Na2CO3 temperature of 870 ℃. This possibly explains why the product on the electrode surface was very unstable as Na metal is near its boiling temperature and would therefore more likely dissolve in the electrolyte. Therefore the masses on the working electrodes explain anodic peaks A3 and A4 during the CV scan. Upon insertion in a 2.3 mol/L HCl,the masses on the electrode dissolved and the acid solution turned green,so the masses were not carbon.
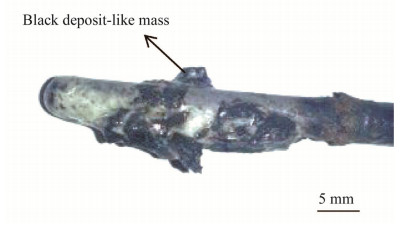 |
| Fig. 3 Image of deposit obtained after electrolysis in molten Na2CO3 at 870 ℃ (working electrode: 5.0 mm mild steel, electrode potential: -3.0 V vs. Ag/AgCl) |
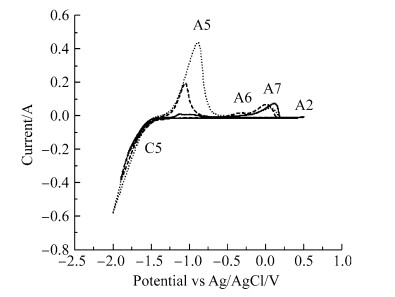
CV investigations in molten Li2CO3 (melting temperature: 723 ℃) at 740 ℃ were carried out and the obtained CVs are displayed in Fig. 4. During the cathodic sweep,similar to the CVs obtained in molten K2CO3,there were no peaks before the cathodic wave C5 suggesting the Pt working electrode was also inert in the molten salt. The electrochemical potential window between the current onset potential of peaks C5 (-1.35 V vs. Ag/AgCl) and A2 (0.4 V vs. Ag/AgCl) is about 1.75 V in Fig. 4. The potential window is fairly close to the standard potential of -1.57 V vs. CO32-/CO2-O2 calculated for the reduction of CO32- ions to carbon (see reaction (5)) in molten Li2CO3 at 740 ℃. Cathodic wave C5 is thus likely due to the reduction of CO32- ions to carbon and O2- ions. In molten Li2CO3,CO32- ions can also be reduced to CO. The standard potential however,for Li metal formation at 740 ℃ is -2.76 V vs. CO32-/CO2-O2,which is more negative than that for carbon deposition. Based on the standard potentials,carbon deposition at the working electrode surface can occur first. Nevertheless,it is worth mentioning that the overpotential for alkali metal ion reduction on a bare Pt electrode is low (i.e.,Li formation at an activity lower than one possibly occurs at less negative potential than -2.76 V vs. CO32-/CO2-O2). It is also possible to form Pt-Li alloys,which may shift positively the potential for the reduction of Li+ ions.
 |
| Fig. 4 CVs of a 0.25 mm Pt wire working electrode in molten Li2CO3 for negative scan limits of -1.8 V (solid line), –1.9 V (dashed line) and -2.0 V (dotted line) at 740 ℃. Scan rate: 100 mV s–1 |
Although mentioned,once the electrode surface is covered by carbon,the overpotential for carbon deposition onto carbon will be lower than that required to deposit carbon on a bare Pt electrode. The reduction of CO32- ions to CO is also possible and the standard potential for this reaction is -1.73 V vs. CO32-/CO2-O2 at 740 ℃. This potential is more negative than the standard potential for carbon deposition (-1.57 V),which indicates that carbon deposition is more likely to occur first.
The formation of lithium carbide (Li2C2) via reaction (11) (full reaction (12)) is also possible and the corresponding standard potential for the reaction is -1.76 V vs. CO32-/CO2-O2 at the molten salt temperature. From these standard reduction potentials,the formation of CO and Li2C2 likely competed with carbon deposition during electrolysis in Li2CO3. During potentiostatic electrolysis in molten Li2CO3 at 740 ℃,carbon was electro-deposited at -1.70 V vs. Ag/AgCl. Figure 5 shows images of carbon electro-deposited on a mild steel rod during two-electrode electrolysis at cell voltages from 2 V to 4 V.
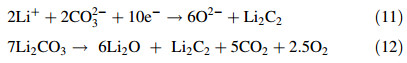
The currents of anodic peaks A5 and A6 increased,as the electrode potential was made more negative (from -1.8 V to -2.0 V),as shown in Fig. 4. Peak A5 starting at a current wave of around -1.3 V is likely due to the reoxidation of the deposited carbon with O2- ions according to reaction (13) and the O2- ions are those generated during the reduction and likely remain in the immediate vicinity of the working electrode. Peak A6 lying around -0.5 V can be attributed to the oxidation of carbon via reaction (14) (carbon re-oxidation with CO32- ions) [28]. To support these attributions,the standard potentials of reactions (13) and (14) at 740 ℃ are -1.39 V and -1.03 V vs. CO32-/CO2-O2 respectively and are close to the onset of the peak waves in the CVs (see Fig. 4). Oxidation peak A7 at -0.2 V could be due to the oxidation of O2- ions or a different form of carbon. The anodic limit A2 on the other hand remains the oxidation of CO32- ions to CO2 and O2.
During electrolysis therefore in a eutectic mixture of molten Li2CO3-Na2CO3-K2CO3 at 450 ℃,because the standard potentials for CO32- ions reduction to carbon and O2- ions in K2CO3 (-3.25 V) and Na2CO3 (-2.70 V) are more negative than that in Li2CO3 (-1.88 V vs. CO32-/ CO2-O2),carbon electro-deposition is facilitated by the presence of Li+ ions. Similarly,the corresponding potentials for CO formation in Li2CO3,Na2CO3,and K2CO3 are -2.28 V,-3.38 V and -4.11 V vs. CO32-/CO2-O2 respectively. In other words,both carbon production and CO formation are more favourable in carbonate salts containing Li+ ions. During electrolysis therefore,once carbon starts to deposit on the electrode,the reaction is dominant to the point where it suppresses the occurrence of other cathodic reactions (the electrode surface is covered with carbon).
3.4 Structure of electro-deposited carbonThe deposited carbon can be readily removed from the cathode by immersion in dilute acid. To further remove solidified carbonate salts and other metal oxide based impurities,washing in more concentrated acid,e.g., 2.3-7.8 mol/L HCl is effective [27]. After further washing in water,filtration and drying at 60-120 ℃ in air or vacuum,the sample is ready for further use and analysis. SEM and TEM images of carbon deposited in molten Li2CO3 at 4 V are displayed in Fig. 6. Under SEM,the deposited carbon was observed to consist of micrometre sized and irregularly shaped particles and flakes (see Fig. 6a).
 |
| Fig. 5 5.0 mm diameter mild steel rod electrodes coated with deposited carbon after electrolysis in molten Li2CO3 at 740 ℃ and 2.0, 2.5, 3.0, and 4.0 V |
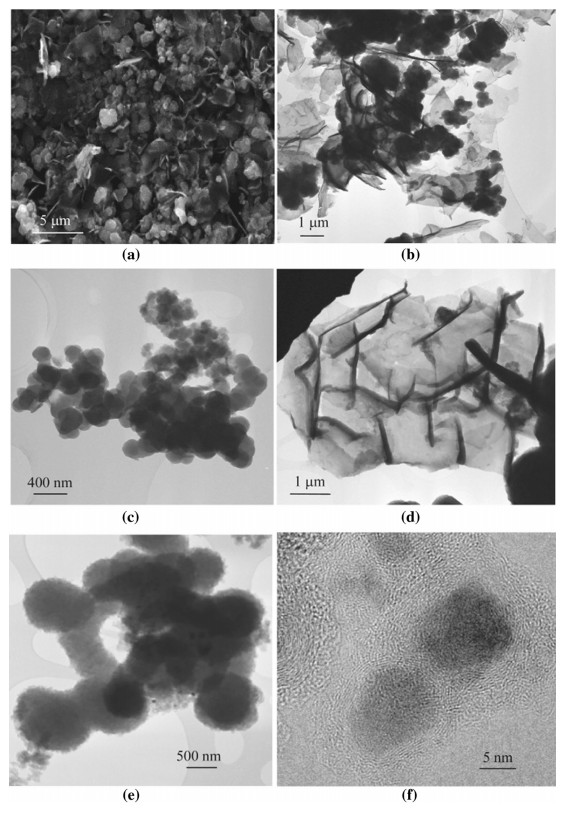 |
| Fig. 6 SEM (a) and TEM (b–d) images of carbon deposited at 4.0 V in molten Li2CO3 at 740 ℃ under the CO2 atmosphere (the TEM (e) and HRTEM (f) images of the carbon deposited from the binary mixture of Li2CO3-K2CO3(mole ratio: 62:38) under CO2 at 700 ℃ and 4.0 V (e), and 540 ℃ and 5.0 V (f) [27]) |
At higher magnifications under TEM,flakes of micrometres long (see Fig. 6b) and sub-micrometre sized quasi-spherical particles (see Fig. 6c) were also the dominant features in the carbon. Some of the flakes could be several tens of square micrometres in area and contained sub-structures separated by the dark lines (see Fig. 6d). Interestingly,the diameters of the quasi-spherical particles were smaller compared to those deposited in Li2CO3- K2CO3 at lower temperatures (see Fig. 6e) [27]. Also,the ring-like structures observed previously in carbon produced from the binary mixture was absent in that deposited in Li2CO3. These differences further emphasise the effect of molten salt composition and temperature on the carbon morphology and structure.
Finally,the deposited carbon was found to contain 83%- 92% weight ratio carbon and 5%-14% weight ratio oxygen with the remaining being impurities that could be tracked down to the stainless steel used in the electrolyser [27]. Figure 6f is an HRTEM image of the carbon produced in the binary Li2CO3-K2CO3 mixture,showing the impurity core enclosed by multiple carbon layers [27]. Based on these electron microscopic findings,it is clear that the produced carbon is made up of both amorphous and graphitic structures. In literature,both amorphous and graphitic carbons have been produced in the ternary mixture of carbonate salts,which agrees with the observations in Refs. [19, 28, 36, 37].
3.5 Further discussionIn this work,onset potential for a reduction or oxidation current on the CV has been estimated for the convenience of comparison and discussion. It was found that the way of estimation as illustrated in Fig. 1 was relatively simple and convenient in most cases,except for the cathodic current related with reduction of alkali metal ions,e.g.,C1 in Fig. 1 and C3 in Fig. 2. A possible reason is that the alkali metal may escape from the electrode surface as liquid,and has a certain level of solubility in its own molten salt,and may boil at the working temperature,which is evident for K by the gas bubbling caused current fluctuation at potentials more negative than -1.65 V on the CV in Fig. 1b. In these cases,the alkali metal would have an activity at the electrode surface lower than unity compared with a deposited pure alkali metal layer. This in turn would lead to a positive shift of the potential for reduction of the alkali metal cation in the molten salt. Such a consideration is in agreement with the measured electrochemical potential window of molten K2CO3 (2 V) being smaller than the theoretical value (2.17 V),as shown in Fig. 1. A similar case is shown in Fig. 2 for molten Na2CO3.
In relation with formation of alkali metal in molten carbonate under CO2,the following reactions may proceed either in the molten salt or in the gas phase.
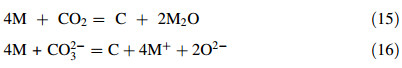
According to thermodynamic data,both reactions are favourable for Li in molten Li2CO3,but only reaction (15) may proceed in K2CO3 and Na2CO3 at sufficiently low temperatures (<850 ℃ for K and <910 ℃ for Na). In this work,the electrode potential was not sufficiently negative to reduce Li+ to Li,and both reactions (15) and (16) should have not occurred. For K2CO3,the working temperature (940 ℃) was too high to enable reaction (15),but it may occur in molten Na2CO4 at 870 ℃. However,if it did happen,reaction (15) should have made little effect on electrolysis because no carbon was collected at the cathode.
Note that only metals were used to make electrodes in this work,although carbon was a popular electrode material for molten salt electrolysis. The main reason is that a carbon cathode will erode via intercalation of alkali metals to produce various nanomaterials of carbon [35]. Carbon anodes are also unstable due to reaction with oxygen to form CO and CO2 gases [38].
Another related issue is the onset potential of A2 (0.90 V vs. Ag/AgCl) in Fig. 1,being more positive than that (about 0.40 V vs. Ag/AgCl) in Figs. 2 and 4. As we have observed experimentally in Refs. [16, 17, 18, 38],the stability of the Ag/AgCl reference electrode in each molten carbonate salt used in this work is very high and can be used repeatedly for long durations without considerable shifts in potential. On the other hand,the working temperatures were different in these CV experiments,which must have affected the potential of A2. However,a more important factor contributing to this difference is that the Ag/AgCl reference electrode used in each of the molten salts contains a different internal electrolyte. Namely,in molten K2CO3,the internal electrolyte was a mixture of K2CO3 and AgCl (10% molar ratio),in molten Li2CO3,it was Li2CO3 mixed with AgCl (10% molar ratio),and in molten Na2CO3,it was Na2CO3 and AgCl (10% molar ratio). These different internal electrolytes affect the potential of the reference electrode,giving rise to the observed difference in the A2 potential. By comparing the potentials of A2 and A1 for the three molten salts,it can be seen that both A1 and A2 are more positive in K2CO3 than those in Na2CO3 and Li2CO3. In other words,the potential of the Ag/AgCl reference electrode in molten K2CO3 is about 0.5 V more negative than that in Li2CO3 or Na2CO3.
A detailed study on the origin of this difference is beyond the scope of this work,but it may be worthy of a qualitative speculation. It may be related to the competition between an alkali metal ion,M+ (= K+,Na+ or Li+),and the Ag+ ion for association or pairing with either the Cl- or CO32- ion. Formation of ion pairs between the Ag+ ion and the anion would decrease the activity of free Ag+ ions, and hence negatively shift the potential of the Ag/AgCl reference electrode. According to the ‘‘hard and soft (Lewis) acids and bases’’ (HSAB) theory,the alkali cations can be ranked in an order of Li+> Na+> K+ ~Ag+ in hardness,which is the same as the polarisation power of each of the cations,whilst Cl- is harder than CO32-. Thus, the Li+-Cl- and Na+-Cl- pairs are stronger than the K+- Cl- pair,whilst the Ag+-Cl- pair is stronger than the Ag+- CO32- pair. In turn,it can be derived that the free Ag+ ion activity would be higher in the mixture of AgCl with Li2- CO3 or Na2CO3 than with K2CO3,leading to a more positive potential for the Ag/AgCl reference electrode in molten Li2CO3 or Na2CO3 than in molten K2CO3.
Finally,formation of alkali metal carbide is considered in this work to account for CVs and the cathode deposit obtained in molten Na2CO3. In fact,reaction (10) is thermodynamically feasible for formation of Li2C2 (ΔG0 = -44.476 kJ at 740 ℃),but not for Na2C2 (ΔG0 = 60.701 kJ at 870 ℃) or K2C2 (K boils at 774 ℃) at the working temperatures in this work. However,the fairly small DG0 values for both Li2C2 and Na2C2 imply possible kinetic alterations. Because of the large difference in deposition potential between Li metal and carbon,it is unlikely that formation of Li2C2 could dominate the cathodic reactions. In molten Na2CO3,because the deposition potentials of Na and carbon are very close,and also considering that freshly and simultaneously formed Na and carbon atoms can be highly reactive to each other,the formation of Na2C2 or another form of sodium carbide is kinetically feasible.
This hypothesis was studied by SEM,EDX and XRD analyses of the cathodic deposit from molten Na2CO3. The deposit sample was collected as powder from the cathode by gentle scraping and kept in a sealed bottle. Typical results are presented in Fig. 7. The SEM images (see Figs. 7a,b) and EDX spectra (see Figs. 7c,d) were both Na2C2 and Na2CO3,which agrees very well with the EDX spectra,showing the deposit differing from Na2CO3 by an obviously lower amount of oxygen. Figure 7e compares the XRD pattern of the deposit sample with the Na2C2 standard. The pattern of the deposit matched well the standard of Na2CO3 (not shown),although the presence of Na2C2 cannot be excluded according to Fig. 7e. It is possible that the Na2C2 in the deposit may not be highly crystalline,and hence not sensitive to XRD analysis.
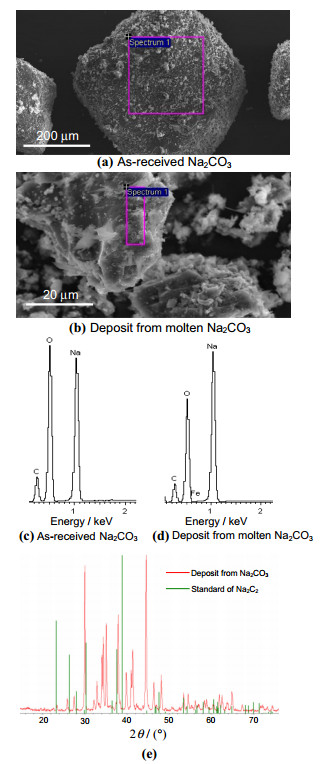 |
| Fig. 7 a, b SEM images, c, d EDX spectra of the boxed areas in a, b, and e XRD patterns of as-received Na2CO3 a, c, potentiostatic deposit in molten Na2CO3 b, d, and e, red line, see Fig. 3 and the Na2C2 standard e, green line |
In this study,the role of alkali metal cation during electrolysis in alkali metal carbonates under CO2 was investigated. The main cathodic reaction during CV investigations in molten Na2CO3 and K2CO3 was likely the formation of alkali metal (i.e.,Na and K metals) as the potential required for this reaction is less negative compared to that for carbon electro-deposition on a bare Pt electrode. Carbon production did not occur in both molten salts,however in Na2CO3,very unstable products were formed on the electrode surface. A number of anodic peaks were observed during CV investigations,which were likely due to the re-oxidation of Na metal,and carbide related compounds. Studies by dissolution in acid,SEM,EDX and XRD confirmed the cathode deposit from molten Na2CO3 to be non-carbon. While the SEM/EDX analysis implied presence of carbide in the deposit,XRD pattern showed no clear evidence for crystalline Na2C2. In molten K2CO3,no anodic peak relating to possible re-oxidation of any cathodic product was observed. This is probably due to the K metal evaporating because of the high molten salt temperature or K metal simply dissolving into the bulk of the electrolyte.
During investigation in Li2CO3,the main cathodic reaction was probably the reduction of CO32- ions to carbon and O2- ions. Standard reduction potentials and obtained CVs are in agreement as the potential for carbon deposition is more positive compared to that for other possible cathodic reactions including CO,Li2C2 and alkali Li metal formation. Making the working electrode potential more negative resulted in an increase in cathodic products and corresponding anodic peak currents. Carbon was electro-deposited on a Pt electrode at -1.7 V in Li2CO3 at 740 ℃ and the two main anodic peaks on the CVs are associated with carbon re-oxidation. Characterization of the produced carbon using both SEM and TEM revealed ring,flake and sheet-like structures,and quasispherical particles. The morphology of the carbon contained both amorphous and graphitic structures and varied with molten salt composition,temperature,and electrolysis voltage.
Acknowledgments This research received partial funding from the University of Nottingham (Dean of Engineering Scholarship for H.V.I. in 2011),the EPSRC (EP/J000582/1),and the Ningbo Municipal Government (3315 Plan and IAMET Special Fund, 2014A35001-1). The authors thank Nicola Weston for TEM studies.| 1. | Aresta M, Dibenedetto A, Angelini A (2013) The use of solar energy can enhance the conversion of carbon dioxide into energyrich products: stepping towards artificial photosynthesis. Philos Trans R Soc A 371:20120111 |
| 2. | Whipple DT, Kenis PJA (2010) Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J Phys Chem Lett 1:3451-3458 |
| 3. | Mikkelsen M, Jørgensen M, Krebs FC (2010) The teraton challenge. a review of fixation and transformation of carbon dioxide. Energy Environ Sci 3:43-81 |
| 4. | Kaneco S, Iiba K, Katsumata Het al (2007) Effect of sodium cation on the electrochemical reduction of CO2 at a copper electrode in methanol. J Solid State Electrochem 11:490-495 |
| 5. | Kaneco S, Iiba K, Suzuki SK et al (1999) Electrochemical reduction of carbon dioxide to hydrocarbons with high faradaic efficiency in LiOH/Methanol. J Phys Chem B 103:7456-7460 |
| 6. | Saeki T, Hashimoto K, Kimura N et al (1995) Electrochemical reduction of CO2 with high current density in a CO2 + methanol medium II. CO formation promoted by tetrabutylammonium cation. J Electroanal Chem 390:77-82 |
| 7. | Fisher BJ, Eisenberg R (1980) Electrocatalytic reduction of carbon dioxide by using macrocycles of nickel and cobalt. J Am Chem Soc 102:7361-7363 |
| 8. | Zhou F, Liu S, Yang B et al (2014) Highly selective electrocatalytic reduction of carbon dioxide to carbon monoxide on silver electrode with aqueous ionic liquids. Electrochem Commun 46:103-106 |
| 9. | Carlesi C, Carvajal D, Vasquez D et al (2014) Analysis of carbon dioxide-to-methanol direct electrochemical conversion mediated by an ionic liquid. Chem Eng Process 85:48-56 |
| 10. | Rosen BA, Salehi-Khojin A, Thorson MR et al (2011) Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334:643-644 |
| 11. | Snuffin LL, Whaley LW, Yu L (2011) Catalytic electrochemical reduction of CO2 in ionic liquid EMIMBF3Cl. J Electrochem Soc 158:F155-F158 |
| 12. | Barrosse-Antle LE, Compton RG (2009) Reduction of carbon dioxide in 1-butyl-3-methylimidazolium acetate. Chem Commun 3744-3746 |
| 13. | Ingram MD, Baron B, Janz GJ (1966) The electrolytic deposition of carbon from fused carbonates. Electrochim Acta 11:1629-1639 |
| 14. | Delimarskii YK, Gordis'kii OV, Grishchenko VF (1964) Cathode liberation of carbon from molten carbonates. Dokl Akad Nauk SSSR 156:650-651 |
| 15. | DelimarskiiI YK, Shapova VI, Vasilenko VA et al (1975) Electrolytic production of pure carbon. Otkrytiya Izobret Prom Obraztsy Tovarnye Znaki 52:176-177 |
| 16. | Siambun NJ, Mohamed H, Hu D et al (2011) Utilisation of carbon dioxide for electro-carburisation of mild steel in molten carbonate salts. J Electrochem Soc 158:H1117-H1124 |
| 17. | Siambun NJ (2011) Electrolysis of molten carbonate salts and its applications. Dissertation, University of Nottingham, Nottingham |
| 18. | Lawrence RC (2013) Carbon from carbon dioxide via molten carbonate electrolysis: fundamental investigations. Dissertation, University of Nottingham, Nottingham |
| 19. | Yin H, Mao X, Tang D et al (2013) Capture and electrochemical conversion of CO2 to value-added carbon and oxygen by molten salt electrolysis. Energy Environ Sci 6:1538-1545 |
| 20. | Tang D, Yin H, Mao X et al (2013) Effects of applied voltage and temperature on the electrochemical production of carbon powders from CO2 in molten salt with an inert anode. Electrochim Acta 114:567-573 |
| 21. | Ijije HV, Lawrence RC, Siambun NJ et al (2014) Electro-deposition and re-oxidation of carbon in carbonate-containing molten salts. Faraday Discuss 172:105-116 |
| 22. | Kawamura H, Ito Y (2000) Electrodeposition of cohesive carbon films on aluminum in a LiCl-KCl-K2CO3 melt. J Appl Electrochem 30:571-574 |
| 23. | Massot L, Chamelot P, Bouyer F et al (2002) Electrodeposition of carbon films from molten alkaline fluoride media. Electrochim Acta 47:1949-1957 |
| 24. | Ijije HV, Lawrence RC, Chen GZ (2014) Carbon electrodeposition in molten salts: electrode reactions and applications. RSC Adv 4:35808-35817 |
| 25. | DelimarskiiI YK, Shapoval VI, Grishchenko VF et al (1970) Electrolysis of fused carbonates of alkali metals under pressure. Zh Prikl Khim 43:2634-2638 |
| 26. | Dimitrov AT (2009) Study of molten Li2CO3 electrolysis as a method for production of carbon nanotubes. Maced Source 28:111-118 |
| 27. | Ijije HV, Sun C, Chen GZ (2014) Indirect electrochemical reduction of carbon dioxide to carbon nanopowders in molten alkali carbonates: process variables and product properties. Carbon 73:163-174 |
| 28. | Le Van K, Groult H, Lantelme F et al (2009) Electrochemical formation of carbon nano-powders with various porosities in molten alkali carbonates. Electrochim Acta 54:4566-4573 |
| 29. | Mallard WG, Lindstrom PJ (ed) (1969) NIST chemistry webbook, NIST standard reference. NIST chemistry webbook, NIST standard reference database, National Institute of standards and technology, Gaithersburg |
| 30. | Novoselova IA, Oliinyk NF, Volkov SV et al (2008) Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization. Physica E 40:2231-2237 |
| 31. | Delimarskii YK, Gordis'kii OV, Grishchenko VF (1965) Reactions taking place during electrolysis of fused carbonates. Ukr Khim Zh 31:32-37 |
| 32. | Bartlett HE, Johnson KE (1967) Electrochemical studies in molten Li2CO3-Na2CO3. J Electrochem Soc 114:457-461 |
| 33. | Lorenz PK, Janz GJ (1970) Electrolysis of molten carbonates: anodic and cathodic gas evolving reactions. Electrochim Acta 15:1025-1035 |
| 34. | Lantelme F, Kaplan B, Groult H et al (1999) Mechanism for elemental carbon formation in molecular ionic liquids. J Mol Liq 83:255-269 |
| 35. | Chen GZ, Kinloch I, Shaffer MSP et al (1998) Electrochemical investigation of the formation of carbon nanotubes in molten salts. High Temp Mater Processes (New York) 2:459-469 |
| 36. | Bartlett HE, Johnson KE (1967) Electrochemical studies in molten Li2CO3-Na2CO3. J Electrochem Soc 114:457-461 |
| 37. | Ito Y, Shimada T, Kawamura H (1992) Electrochemical formation of thin carbon film from molten chloride system. Proc Electrochem Soc 16:574-585 |
| 38. | Wang H, Siambun NJ, Yu L et al (2012) A robust alumina membrane reference electrode for high temperature molten salts. J Electrochem Soc 159(9):H740-H746 |
| 39. | Chen HL, Jin XB, Yu LP et al (2014) Influences of graphite anode area on electrolysis of solid metal oxides in molten salts. J Solid State Electrochem 18(12):3317-3325 |
 2016, Vol. 4
2016, Vol. 4