文章信息
- 郑浩然, 白璐璐, 王媛媛, 钟佳鑫, 卢金星, 邓慧玲, 谢群, 吴媛.
- Zheng Haoran, Bai Lulu, Wang Yuanyuan, Zhong Jiaxin, Lu Jinxing, Deng Huiling, Xie Qun, Wu Yuan
- 产气荚膜梭菌毒素研究进展
- Progress in research of Clostridium perfringens toxin
- 中华流行病学杂志, 2022, 43(11): 1860-1868
- Chinese Journal of Epidemiology, 2022, 43(11): 1860-1868
- http://dx.doi.org/10.3760/cma.j.cn112338-20220316-00189
-
文章历史
收稿日期: 2022-03-16
2. 中国疾病预防控制中心传染病预防控制所, 北京 102206;
3. 西安市中心医院, 西安 710003;
4. 湘南学院公共卫生学院, 郴州 423000
2. National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3. Xi'an Central Hospital, Xi'an 710003, China;
4. School of Public Health, Xiangnan University, Chenzhou 423000, China
产气荚膜梭菌(Clostridium perfringens)是一种革兰染色阳性且严格厌氧的细菌,能够产生对外界环境具有抗性的芽孢,因此,产气荚膜梭菌能够广泛存在于土壤、污水、食物及动物(包括人类)的正常肠道微生物群中[1]。
产气荚膜梭菌最初根据其产酸产气能力分为产酸型和产气型2种不同的亚型[2]。1933年,Wilsdon通过毒素中和试验,根据菌株产生的α毒素(Clostridium perfringens α-toxin)、β毒素(Clostridium perfringens β-toxin)、ε毒素(Clostridium perfringens ε-toxin)将产气荚膜梭菌分为4种不同的类型(A~D型),这种分型方法自提出后经过4次修改(1933年、1943年、1962-1964年、2018年),被视为产气荚膜梭菌的经典分型方法[1-2]。根据产气荚膜梭菌产生的6种主要毒素(表 1):α毒素、β毒素、ε毒素和ι毒素(Clostridium perfringens ι-toxin)、肠毒素(Clostridium perfringens enterotoxin,CPE)和坏死性肠炎B毒素(Necrotic enteritis B-like toxin,NetB)的组合不同,将其分为A~G型7种类型(2018年以前的分型方案仅有A~E型5种,其中A、C、F型与人类疾病密切相关)。除此之外,不同毒素类型的菌株可能还携带1种或多种不参与分型毒素如β2毒素(Clostridium perfringens β2-toxin)、产气荚膜梭菌溶素O/θ毒素(Perfringens O/Clostridium perfringens θ toxin)、坏死性肠炎F毒素(Necrotic enteritis F-like toxin,NetF)、产气荚膜梭菌二元肠毒素(Binary enterotoxin of Clostridium perfringens,BEC)、产气荚膜梭菌λ毒素(Clostridium perfringens λ-toxin)等,目前尚未发现一种菌株能够携带全部的毒素[2]。
随着现代分子生物学方法与技术的快速发展,基于基因特征分型的方法更有利于对那些引发感染性疾病的菌株进行流行病学调查,与动物毒素中和试验、毒素抗体检测试剂盒相比,分子检测方法能够快速、灵敏、经济地对毒素基因进行检测,以便了解样本菌株的型别,对于临床诊断和治疗具有重要意义。不仅如此,新技术的应用使产气荚膜梭菌毒素的相关研究也日益深入,现就产气荚膜梭菌部分毒素的研究进展进行综述。
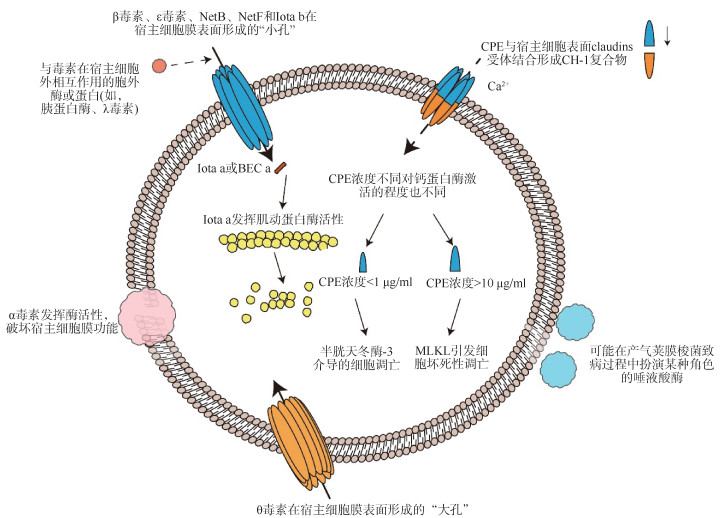
一、产气荚膜梭菌毒素目前已经发现产气荚膜梭菌能够产生20多种细胞毒素和水解酶(表 2),二者在致病的过程中具有相互协同的作用(图 1)。
|
| 注:Iota a & Iota b:产气荚膜梭菌ι毒素;CPE:产气荚膜梭菌肠毒素;NetB:坏死性肠炎B毒素;NetF:坏死性肠炎F毒素;BEC:产气荚膜梭菌二元肠毒素;MLKL:混合连接激酶结构域样蛋白 图 1 产气荚膜梭菌部分毒素、酶和蛋白的作用方式 |
1. 用于产气荚膜梭菌分型的主要毒素:
(1)α毒素:产气荚膜梭菌菌株均携带,是目前被研究最多的一种大小为42.5 kDa的酶,具有磷脂酶C和鞘磷脂酶的生物学活性[23-24],编码基因cpa/plc位于染色体上,大小为1 200 bp(表 2)。α毒素拥有2个结构域,N端锌结合结构域具有催化毒素的作用,C端钙结合结构域负责毒素与细胞膜的结合[25](图 2A)。α毒素可直接发挥酶活性破坏宿主细胞膜的功能,导致细胞溶解和组织坏死(图 1)。除此之外,α毒素还可以同神经节苷脂GM1受体结合激活多种信号通路,诱导受影响的细胞氧化应激和白细胞介素-8的产生[26-27]。有研究表明,α毒素的C端结构域(247~370 aa)可以保护小鼠模型免遭气性坏疽和CPE的侵袭[28-29]。因此认为α毒素的没有毒性的C端结构域对于疫苗的开发是有前景的抗原成分[30]。

|
| 图 2 产气荚膜梭菌结构功能示意图 |
(2)β毒素:由B和C型产气荚膜梭菌产生,是一种大小为35 kDa的蛋白,编码基因cpb位于质粒上,大小为1 011 bp(表 2)。在氨基酸水平上与金黄色葡萄球菌产生的成孔毒素(α毒素)具有28%的相似性,β毒素能在宿主膜表面形成功能性质膜孔(图 1),这种七聚体形式的质膜孔能够导致大量的钙离子、钠离子、氯离子进入细胞,导致细胞肿胀凋亡[4, 31]。
目前认为在不同的细胞类型或不同物种的宿主中,β毒素的受体可能有所不同。Nagahama等[32]研究认为嘌呤受体(P2X7)是β毒素与宿主细胞结合的位点,例如人胚胎肾细胞(HEK-293)本不能与β毒素结合,当转染产生P2X7后,细胞能够结合β毒素并形成寡聚体。然而,根据Bruggisser等[33]最新的研究结果认为血小板内皮细胞黏附分子-1(PECAM-1或CD31)是肠黏膜内皮细胞上的一种β毒素与宿主细胞结合的受体,研究报告显示肠黏膜表面的“细胞屏障”(上皮层)主要是由肠上皮细胞构成的,而这种包含P2X7受体的肠上皮细胞所构成的“细胞屏障”不能与产气荚膜梭菌产生的β毒素相结合。实验显示β毒素可与固有层的血管内皮细胞结合从而导致急性出血性坏死性肠炎的发生,因此P2X7不能用来单独解释β毒素引起坏死性肠炎的致病机制。此外,该研究还发现CD31并不在上皮细胞中表达[33-34]。
(3)ε毒素:由B和D型产气荚膜梭菌菌株产生,编码基因etx位于质粒上,大小为1 119 bp(表 2),需要在肠道蛋白酶水解激活的情况下才能发挥其全部的功能[35]。ε毒素通常以33 kDa的原毒素形式进入到宿主的肠腔中,被细胞外胰蛋白酶、糜蛋白酶以及产气荚膜梭菌中的λ毒素去除部分N末端和C末端的残基将其活化,活化后的ε毒素大小为29 kDa,毒性强度是原毒素的1 000倍[36-37]。通过功能获得和损失实验的组合认为髓鞘和淋巴细胞蛋白可能是ε毒素的候选受体[38]。ε毒素拥有3个结构域(图 2B):结构域1为N端结构域,负责毒素与宿主细胞的受体结合;结构域2为位于N端和C端中间的结构域,包含着一个β发夹结构,在形成孔隙的过程中介导毒素的插入;结构域3为C端结构域,在毒素寡聚化的过程中起作用[39]。
(4)ι毒素:仅由E型产气荚膜梭菌菌株产生,属于梭状芽孢杆菌二元毒素家族(艰难梭菌CDT毒素、肉毒梭菌C2毒素等)中的一员,由2个独立的成分组成,酶成分和结合成分,分别称为Iota a(Ia)和Iota b(Ib)[40]。Ia和Ib的编码基因位于质粒上,分别被称为iap和ibp,编码基因大小分别为1 365 bp和2 622 bp(表 2)。ι毒素本身没有活性,当蛋白酶或菌株自身携带的λ毒素从Ia和Ib原蛋白的N末端去除部分氨基酸序列时会使它们产生活性[41]。被激活后的Ia包括一个负责与Ib相互作用的N端结构域和一个具有二磷酸腺苷核糖基转移酶活性的C端结构域(图 2C)。活化后的Ib有4个结构域,分别介导与Ia的相互作用、使毒素进入宿主细胞、介导寡聚、与受体(脂解刺激蛋白)结合[42](图 2D)。
ι毒素作用的产生起始于Ib与宿主细胞上的受体结合形成插入到靶细胞质膜中的七聚体孔道[43]。然后,全毒素经内吞作用进入宿主细胞,Ia利用Ib寡聚后形成的孔道迁移到细胞质中,发挥酶活性诱导宿主细胞骨架解体,最终导致细胞凋亡[44-45](图 1)。
(5)CPE:是一种大小为35 kDa的单链多肽,编码基因cpe既可以位于染色体上也可以位于质粒上,大小为957 bp(表 2)。该毒素由所有的F型产气荚膜梭菌产生,也能由部分C、D和E型负责产气荚膜梭菌产生[46]。CPE的晶体结构显示该毒素由一个与宿主细胞受体结合的C末端结构域和一个具有细胞毒性的N末端结构域构成[47-48](图 2E)。
CPE的作用起始于它与宿主细胞的claudin蛋白结合。该受体由4个跨膜区和2个细胞外环路(ECL1和ECL2)构成,对于维持上皮细胞和内皮细胞间紧密连接的结构和功能非常重要[49]。CPE的C末端结构域与受体结合,形成一个大小为90 kDa的复合物,该复合物除CPE本身外还包括claudin-3和/或claudin-4,以及claudin-1蛋白[50]。在37 ℃时,6个小的CPE复合物迅速在宿主细胞膜表面寡聚化形成一个大的前孔复合物,将其命名为CH-1复合物(约540 kDa)。然后,位于前孔复合物中的每个CPE延伸出一个β发夹环,6个β发夹环聚集形成一个β桶状蛋白结构插入宿主细胞膜中,形成一个大小为1.4 nm的通道[51]。由CH-1复合物形成的通道可以渗透钙离子流入细胞质中从而导致宿主细胞死亡,由于CPE的浓度不同,导致流入细胞质中的钙离子浓度不同。因此,不同浓度的CPE对于钙蛋白酶的激活程度不同[52-53]。当CPE浓度较低(1 µg/ml)时,只能引起少量的钙离子内流,钙蛋白酶被轻度激活,触发半胱天冬酶-3介导的细胞凋亡;CPE浓度较高(10 µg/ml)则会引起大量的钙离子内流,钙蛋白酶被强烈地激活,导致混合谱系激酶结构域样蛋白依赖性坏死(图 1)。此外,接近失活的CPE也会导致宿主细胞发生形态学损伤,导致基底外侧细胞膜表面暴露,CPE与另一种紧密连接组分闭合蛋白(occludin)相互作用,形成一个大小约为600 kDa的CPE复合物,称为CH-2复合物。目前尚不清楚CH-2复合物对CPE致病作用的贡献[50]。
(6)NetB:仅由G型产气荚膜梭菌产生,具有物种特异性,可导致禽类动物发生小肠坏死[54]。NetB是一种大小为33 kDa的单链蛋白,编码基因netB位于质粒上,大小为1 371~1 533 bp(表 2)。与该家族的其他毒素相同,NetB与宿主细胞的受体结合后,在细胞表面寡聚,导致细胞病变孔隙(1.6~1.8 nm)的形成[55]。关于NetB与受体结合的位置仍存在着较大争议。之前的研究认为,NetB毒素在寡聚化的过程中需要宿主细胞脂筏当中的胆固醇参与[56]。最近的研究表明,NetB与宿主结合的位置不在脂筏区域,并且在寡聚化的过程中也不需要胆固醇的参与[57]。
2. 不用于产气荚膜梭菌分型的次要毒素:
(1)θ毒素:除了携带染色体cpe基因的F型产气荚膜梭菌和导致急性出血性坏死性肠炎的C型产气荚膜梭菌外,大多数产气荚膜梭菌菌株都可以产生θ毒素[42]。θ毒素是一种大小为54 kDa的蛋白,编码基因位于染色体上,大小为1 500 bp(表 2)。θ毒素拥有4个结构域(图 2G):结构域4负责与宿主细胞的接触,接触后使结构域3中的2个α螺旋结构域改变构象形成2个β发夹插入宿主细胞膜中[1, 42, 58]。这种改变能够使毒素穿透细胞膜并形成15 nm的“大孔”(图 1),这比产气荚膜梭菌中其他成孔毒素形成的致病孔(1.6~1.8 nm)要大得多,但“大孔”破坏宿主细胞渗透压失衡最终导致细胞溶解的作用并不是引发气性坏疽的关键因素[59]。
(2)β2毒素:携带β2毒素的产气荚膜梭菌菌株可以从各种野生和家养动物、人类中分离,也可以从食物、土壤中分离[60]。β2毒素是一种大小为28 kDa的成孔毒素,编码基因cpb2位于质粒上,大小为795 bp(表 2)。虽然名字与β毒素相似,但是二者之间的氨基酸序列没有明显的同一性(< 15%)[61]。尽管该毒素的首次发现是从患有由产气荚膜梭菌导致的坏死性肠炎的仔猪中鉴定得到的,但由于该毒素能够广泛存在于所有类型的菌株当中,β2毒素在菌株致病过程中扮演的角色仍需要进一步的研究[62-63]。
(3)NetF:2015年的研究发现NetF与犬急性出血性胃肠炎和马驹坏死性小肠结肠炎病例中分离出的A型菌株之间存在非常显著的关联[15]。NetF是一种大小为31.7 kDa的单链蛋白,编码基因netF位于质粒上,大小为918 bp,氨基酸序列与NetB有48%的同一性(表 2)。具有成孔能力,孔隙的大小可能为6~8 nm[64]。在最新的分型法则中将该毒素列为潜在的分型毒素,但缺少相关实验(如分子科赫假设)的证实[2]。
(4)BEC:该毒素似乎与日本几次大规模暴发的急性食源性胃肠炎相关,而导致相关疾病的产气荚膜梭菌菌株中并未分离出CPE及其他可能的病原体,随着研究的深入,BEC被发现是导致本次食源性肠胃炎暴发的原因[65]。和ι毒素一样,该毒素也由2个独立的成分组成,即酶效应成分(BEC a)和细胞结合成分(BEC b)分别与ι毒素的单个成分有43%~44%的氨基酸序列同一性(表 2)。目前,经过纯化的BEC a已经被证明具有肌动蛋白二磷酸腺苷核糖基转移酶活性,但目前尚不清楚BEC b结构功能及其与受体结合的位置[66]。一般来说,二元毒素只有当2种成分一起作用时才会对靶细胞产生影响,这些成分作为个体存在时并不发挥生物活性。但是BEC与其他二元毒素不同,在乳鼠试验中即便没有酶成分BEC a的参与,BEC b也具有生物活性,能够导致宿主肠道内液体积聚(图 1)[66-67]。由于目前尚不清楚BEC b对靶细胞的作用模式,因此与NetF相同,BEC也被列为潜在的分型毒素。
二、产气荚膜梭菌引发的主要疾病1. A型产气荚膜梭菌:在气性坏疽发生的过程中,A型产气荚膜梭菌的α毒素能与宿主体内多种细胞和组织产生效应从而发挥重要作用,包括溶血、肌坏死、白细胞生长停滞、血小板聚集、血管收缩和抑制中性粒细胞的分化[68]。此外,菌株中的θ毒素还能与α毒素协同作用,导致宿主组织的外周血管和细胞外基质中的白细胞生长停滞,最终导致肌坏死[1]。A型产气荚膜梭菌除了对于导致人类和动物感染气性坏疽十分重要之外,还被认为是导致牛坏死性出血性肠炎(也称牛肠毒血症)的疑似病原体,虽然已经在牛肠袢模型中得到了证实,但是由于产气荚膜梭菌引发的各种动物的肠道疾病通常是由B~G型所引起的,因此A型产气荚膜梭菌在肠道疾病中的真正作用仍然存在争议[69-70]。
2. B型产气荚膜梭菌:是导致羔羊痢疾的主要原因,但也有少量报道称这种疾病在牛和马中也可出现[71]。这种疾病的主要特征是宿主发生坏死性出血性肠炎,极少数的病例中也出现神经系统症状,如局灶性对称性坏死,以上2种症状分别被认为是由B型产气荚膜梭菌产生的β毒素和ε毒素引起。Rumah等[72]从一名多发性硬化症(MS)患者的粪便中分离出B型产气荚膜梭菌并在该疾病患者中发现ε毒素的血清抗体,但是目前还没有确凿的证据证实B型产气荚膜梭菌与MS之间的真正关系[72-73]。
3. C型产气荚膜梭菌:引发的疾病通常是急性的且具有高度的致死性。C型产气荚膜梭菌是引发人患急性坏死性出血性肠炎和其他哺乳动物发生坏死性肠炎的主要原因。这种疾病通常发生在患者长期食用富含大量胰蛋白酶抑制剂的食物后,又食用了一些被C型产气荚膜梭菌污染的食物(如未加工熟的猪肉)。此外,在糖尿病患者和其他胰腺功能减退的患者中也有感染C型产气荚膜梭菌的病例报告[74]。新出生的动物对该菌株具有较强的易感性,新出生动物因食用富含大量蛋白酶抑制剂的初乳导致患病风险增加[75]。这些证据说明胰蛋白酶是抵抗C型产气荚膜梭菌感染的重要因素。
4. D型产气荚膜梭菌:引发的疾病根据严重程度被分为超急性、急性、亚急性、慢性4种不同的类型[76]。超急性症状表现为,当大量的毒素进入血液循环当中,会导致绵羊发生没有任何征兆的猝死。D型产气荚膜梭菌引发的急性中毒会导致绵羊抽搐、昏迷最终死亡。慢性症状通常发生于ε毒素浓度较低或接种过疫苗后感染D型产气荚膜梭菌的绵羊中,被感染的宿主通常会表现出临床病程较长的神经系统症状,包括失明、共济失调等。在部分亚急性、慢性中毒的山羊当中会出现坏死性出血性结肠炎或小肠结肠炎,伴或不伴有肠毒素血症,而在绵羊当中并没有发现类似的肠道损伤[77]。虽然D型产气荚膜梭菌在动物中可引发严重的肠毒素血症,但目前尚无文献报道其可以引发人类的中毒。
5. E型产气荚膜梭菌:存在于许多健康的动物和人类的肠道中,因此E型产气荚膜梭菌的致病作用目前尚未阐述清楚。不过在2种动物(山羊和新出生的小牛)和人类中已经描述了少数E型产气荚膜梭菌引发相关疾病的病例[78-79]。
6. F型产气荚膜梭菌:F型产气荚膜梭菌中编码CPE的基因位置不同,导致的疾病也不同,编码基因位于染色体上的F型产气荚膜梭菌(FC)通常导致食源性疾病(如食物中毒);编码基因位于质粒上的F型产气荚膜梭菌(FP)通常导致非食源性肠道疾病[如抗生素相关性腹泻(AAD)、散发性腹泻]。
(1)食源性疾病:食物中毒引发的症状通常包括腹泻和腹部痉挛,12~24 h内发展,24 h后恢复,症状较轻常呈自限性[80]。然而,由FC型产气荚膜梭菌引发的食物中毒可导致身体虚弱的人群、老年人以及在精神病院接受治疗的年轻患者死亡[81-82]。造成死亡的原因是胃肠道功能下降或因患有其他疾病服用药物导致的胃肠道相关副作用[1, 83]。当感染了FC型产气荚膜梭菌后,患者不会出现腹泻的症状,肠道内的CPE与肠道之间的长时间接触促进了毒素进入血液循环,损伤肝、肾等内脏器官从而导致了致命的肠毒素血症。
(2)非食源性胃肠道疾病:在所有非食源性胃肠道疾病中,FP型产气荚膜梭菌引起的病例约占5%~15%[84]。与该菌株引发的食源性疾病相比,FP型产气荚膜梭菌引发的胃肠道疾病通常持续时间更长(长达数周)且病情更为严重[85]。在医院环境中,接受抗生素治疗的患者(尤其是老年人)容易被医院环境中的FP型产气荚膜梭菌感染,随后这些菌株在宿主体内产生CPE从而引发AAD。
7. G型产气荚膜梭菌:可引发禽类物种的坏死性肠炎,是全球范围内家禽养殖业常见的疾病,给该产业每年造成了约2亿~60亿美元的损失,目前尚未发现G型菌株在非禽类物种中的病例描述[86]。
三、总结与展望本综述介绍了产气荚膜梭菌部分毒素以及不同型别菌株引发的疾病。产气荚膜梭菌引发的动物疾病目前已被国家规定为二类动物疫病[87]。近些年来,相关领域的科研工作者们正在加快疫苗的研发以减轻该菌所带来的产业经济损失。产气荚膜梭菌菌株产生的ε毒素的毒性强度仅次于肉毒杆菌毒素、破伤风梭菌毒素[88]。ε毒素已被美国CDC列为B类生物恐怖战剂[89]。产气荚膜梭菌还是一种能够引起人类或动物患上包括气性坏疽、坏死性肠炎、食物中毒和非食源性肠道疾病的重要病原微生物,且被美国、日本及欧盟部分国家列为细菌性食源性疾病暴发的主要原因[90-92]。与其他国家相比,我国由该菌引发的细菌性食源性疾病的病例报道却较为少见。在非食源性肠道疾病中,尽管艰难梭菌被认为是引发该病的关键因素,占所有病例的10%~33%,产气荚膜梭菌在AAD的发病过程中也扮演着关键的角色。来自伊朗对于医院内艰难梭菌进行常规监测的研究中发现由产气荚膜梭菌引发AAD的比例为22.4%(68/303)且50岁以上的患者出现由产气荚膜梭菌引发AAD的风险远远高于年轻患者[93]。文献Meta分析显示,从2001-2021年全球范围内的住院患者中,由艰难梭菌、产气荚膜梭菌引发的AAD占比分别为19.6%、14.9%[94]。在我国关于产气荚膜梭菌在医院中引发抗生素性腹泻的流行情况缺乏相关的系统研究。不过,在我国河南省的一家医院对于艰难梭菌进行常规检测的过程中发现由产气荚膜梭菌引发的AAD的比例为4.3%(48/1 108),该研究同时指出在该医院年龄超过50岁以及患有癌症、肝胆系统疾病的患者更容易感染FP型和A型产气荚膜梭菌[95]。除此之外,本实验室在对于艰难梭菌的常规监测过程中,发现产气荚膜梭菌引发AAD的比例甚至超过了艰难梭菌。随着我国人口老龄化的趋势不断加速,未来在医院中会有更多老年住院患者,产气荚膜梭菌引起AAD的风险也会显著增加,因此,医疗机构和CDC应加强或开展与产气荚膜梭菌相关疾病的检测,尤其是不同毒素型产气荚膜梭菌的精准快速鉴别。
许多参与产气荚膜梭菌分型的主要毒素的致病机制尚未明确,比如α毒素对于引发人类和动物胃肠道疾病是否发挥作用、ε毒素与受体结合的真正位置等。除了参与分型的主要毒素之外,产气荚膜梭菌产生的其他毒力因素如唾液酸酶(NanI、NanJ、NanH)的研究也在进行当中,但目前仍处于起步阶段,需要更多的研究来证实这些因素在菌株致病过程中的真正作用。
利益冲突 所有作者声明无利益冲突
| [1] |
Uzal FA, Freedman JC, Shrestha A, et al. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease[J]. Fut Microbiol, 2014, 9(3): 361-377. DOI:10.2217/fmb.13.168 |
| [2] |
Rood JI, Adams V, Lacey J, et al. Expansion of the Clostridium perfringens toxin-based typing scheme[J]. Anaerobe, 2018, 53: 5-10. DOI:10.1016/j.anaerobe.2018.04.011 |
| [3] |
Justin N, Walker N, Bullifent HL, et al. The first strain of Clostridium perfringens isolated from an avian source has an alpha-toxin with divergent structural and kinetic properties[J]. Biochemistry, 2002, 41(20): 6253-6262. DOI:10.1021/bi012015v |
| [4] |
Theoret JR, Uzal FA, Mcclane BA. Identification and characterization of Clostridium perfringens beta toxin variants with differing trypsin sensitivity and in vitro cytotoxicity activity[J]. Infect Immun, 2015, 83(4): 1477-1486. DOI:10.1128/iai.02864-14 |
| [5] |
Harkness JM, Li JH, McClane BA. Identification of a lambda toxin-negative Clostridium perfringens strain that processes and activates epsilon prototoxin intracellularly[J]. Anaerobe, 2012, 18(5): 546-552. DOI:10.1016/j.anaerobe.2012.09.001 |
| [6] |
Miyamoto K, Yumine N, Mimura K, et al. Identification of novel Clostridium perfringens type E strains that carry an iota toxin plasmid with a functional enterotoxin gene[J]. PLoS One, 2011, 6(5): e20376. DOI:10.1371/journal.pone.0020376 |
| [7] |
Miyamoto K, Fisher DJ, Li JH, et al. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates[J]. J Bacteriol, 2006, 188(4): 1585-1598. DOI:10.1128/JB.188.4.1585-1598.2006 |
| [8] |
Keyburn AL, Bannam TL, Moore RJ, et al. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens[J]. Toxins, 2010, 2(7): 1913-1927. DOI:10.3390/toxins2071913 |
| [9] |
Jaakkola K, Virtanen K, Lahti P, et al. Comparative genome analysis and spore heat resistance assay reveal a new component to population structure and genome epidemiology within Clostridium perfringens enterotoxin-carrying isolates[J]. Front Microbiol, 2021, 12: 717176. DOI:10.3389/fmicb.2021.717176 |
| [10] |
Luo RR, Yang QL, Huang XY, et al. Clostridium perfringens beta2 toxin induced in vitro oxidative damage and its toxic assessment in porcine small intestinal epithelial cell lines[J]. Gene, 2020, 759: 144999. DOI:10.1016/j.gene.2020.144999 |
| [11] |
Gohari IM, Kropinski AM, Weese SJ, et al. Plasmid characterization and chromosome analysis of two netF+ Clostridium perfringens isolates associated with foal and canine necrotizing enteritis[J]. PLoS One, 2016, 11(2): e0148344. DOI:10.1371/journal.pone.0148344 |
| [12] |
Ueda K, Kawahara K, Kimoto N, et al. Analysis of the complete genome sequences of Clostridium perfringens strains harbouring the binary enterotoxin BEC gene and comparative genomics of pCP13-like family plasmids[J]. BMC Genomics, 2022, 23(1): 226. DOI:10.1186/s12864-022-08453-4 |
| [13] |
Kawahara K, Yonogi S, Munetomo R, et al. Crystal structure of the ADP-ribosylating component of BEC, the binary enterotoxin of Clostridium perfringens[J]. Biochem Biophys Res Commun, 2016, 480(2): 261-267. DOI:10.1016/j.bbrc.2016.10.042 |
| [14] |
Manich M, Knapp O, Gibert M, et al. Clostridium perfringens delta toxin is sequence related to beta toxin, NetB, and Staphylococcus pore-forming toxins, but shows functional differences[J]. PLoS One, 2008, 3(11): e3764. DOI:10.1371/journal.pone.0003764 |
| [15] |
Gohari IM, Parreira VR, Nowell VJ, et al. A novel pore-forming toxin in type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis[J]. PLoS One, 2015, 10(4): e0122684. DOI:10.1371/journal.pone.0122684 |
| [16] |
Jin F, Matsushita O, Katayama S, et al. Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease[J]. Infect Immun, 1996, 64(1): 230-237. DOI:10.1128/iai.64.1.230-237.1996 |
| [17] |
Li JH, Mcclane BA. The sialidases of Clostridium perfringens type D strain CN3718 differ in their properties and sensitivities to inhibitors[J]. Appl Environ Microbiol, 2014, 80(5): 1701-1709. DOI:10.1128/aem.03440-13 |
| [18] |
Chiarezza M, Lyras D, Pidot SJ, et al. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence[J]. Infect Immun, 2009, 77(10): 4421-4428. DOI:10.1128/iai.00548-09 |
| [19] |
Myers GSA, Rasko DA, Cheung JK, et al. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens[J]. Genome Res, 2006, 16(8): 1031-1040. DOI:10.1101/gr.5238106 |
| [20] |
Canard B, Garnier T, Saint-Joanis B, et al. Molecular genetic analysis of the nagH gene encoding a hyaluronidase of Clostridium perfringens[J]. Mol Gen Genet, 1994, 243(2): 215-224. DOI:10.1007/bf00280319 |
| [21] |
Jiang YF, Kulkarni RR, Parreira VR, et al. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins[J]. Avian Dis, 2009, 53(3): 409-415. DOI:10.1637/8656-021109-Reg.1 |
| [22] |
Ohtani K, Bando M, Swe T, et al. Collagenase gene (colA) is located in the 3'-flanking region of the perfringolysin O (pfoA) locus in Clostridium perfringens[J]. FEMS Microbiol Lett, 1997, 146(1): 155-159. DOI:10.1111/j.1574-6968.1997.tb10186.x |
| [23] |
Sakurai J, Nagahama M, Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action[J]. J Biochem, 2004, 136(5): 569-574. DOI:10.1093/jb/mvh161 |
| [24] |
Ferreira MRA, Moreira GMSG, Cunha CEPD, et al. Recombinant alpha, beta, and epsilon toxins of Clostridium perfringens: production strategies and applications as veterinary vaccines[J]. Toxins (Basel), 2016, 8(11): 340. DOI:10.3390/toxins8110340 |
| [25] |
Flores-Díaz M, Thelestam M, Clark GC, et al. Effects of Clostridium perfringens phospholipase C in mammalian cells[J]. Anaerobe, 2004, 10(2): 115-123. DOI:10.1016/j.anaerobe.2003.11.002 |
| [26] |
Oda M, Terao Y, Sakurai J, et al. Membrane-binding mechanism of Clostridium perfringens alpha-toxin[J]. Toxins (Basel), 2015, 7(12): 5268-5275. DOI:10.3390/toxins7124880 |
| [27] |
Oda M, Kabura M, Takagishi T, et al. Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex[J]. J Biol Chem, 2012, 287(39): 33070-33079. DOI:10.1074/jbc.M112.393801 |
| [28] |
Williamson ED, Titball RW. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene[J]. Vaccine, 1993, 11(12): 1253-1258. DOI:10.1016/0264-410x(93)90051-x |
| [29] |
Shreya D, Uppalapati SR, Kingston JJ, et al. Immunization with recombinant bivalent chimera r-Cpae confers protection against alpha toxin and enterotoxin of Clostridium perfringens type A in murine model[J]. Mol Immunol, 2015, 65(1): 51-57. DOI:10.1016/j.molimm.2015.01.005 |
| [30] |
Goossens E, Verherstraeten S, Valgaeren BR, et al. The C-terminal domain of Clostridium perfringens alpha toxin as a vaccine candidate against bovine necrohemorrhagic enteritis[J]. Vet Res, 2016, 47(1): 52. DOI:10.1186/s13567-016-0336-y |
| [31] |
Richard OK, Springer S, Finzel J, et al. Application of an endothelial cell culture assay for the detection of neutralizing anti-clostridium perfringens beta-toxin antibodies in a porcine vaccination trial[J]. Toxins (Basel), 2019, 11(4): 225. DOI:10.3390/toxins11040225 |
| [32] |
Nagahama M, Seike S, Shirai H, et al. Role of P2X7 receptor in Clostridium perfringens beta-toxin-mediated cellular injury[J]. Biochim Biophys Acta (BBA) General Sub, 2015, 1850(11): 2159-2167. DOI:10.1016/j.bbagen.2015.08.011 |
| [33] |
Bruggisser J, Tarek B, Wyder M, et al. CD31 (PECAM-1) serves as the endothelial cell-specific receptor of Clostridium perfringens β-toxin[J]. Cell Host Microbe, 2020, 28(1): 69-78.e6. DOI:10.1016/j.chom.2020.05.003 |
| [34] |
Lencer WI. Everything illuminated-Clostridium perfringens β-toxin[J]. Cell Host Microbe, 2020, 28(1): 5-6. DOI:10.1016/j.chom.2020.06.006 |
| [35] |
Navarro MA, Mcclane BA, Uzal FA. Mechanisms of action and cell death associated with Clostridium perfringens toxins[J]. Toxins (Basel), 2018, 10(5): 212. DOI:10.3390/toxins10050212 |
| [36] |
Popoff MR. Epsilon toxin: a fascinating pore-forming toxin[J]. FEBS J, 2011, 278(23): 4602-4615. DOI:10.1111/j.1742-4658.2011.08145.x |
| [37] |
Freedman JC, Li JH, Uzal FA, et al. Proteolytic processing and activation of Clostridium perfringens epsilon toxin by caprine small intestinal contents[J]. mBio, 2014, 5(5): e01994-14. DOI:10.1128/mBio.01994-14 |
| [38] |
Rumah KR, Ma YH, Linden JR, et al. The myelin and lymphocyte protein MAl is required for binding and activity of Clostridium perfringens ε-toxin[J]. PLoS Pathog, 2015, 11(5): e1004896. DOI:10.1371/journal.ppat.1004896 |
| [39] |
Ivie SE, Mcclain MS. Identification of amino acids important for binding of Clostridium perfringens epsilon toxin to host cells and to HAVCR1[J]. Biochemistry, 2012, 51(38): 7588-7595. DOI:10.1021/bi300690a |
| [40] |
Sakurai J, Nagahama M, Oda M, et al. Clostridium perfringens iota-toxin: structure and function[J]. Toxins (Basel), 2009, 1(2): 208-228. DOI:10.3390/toxins1020208 |
| [41] |
Gibert M, Petit L, Raffestin S, et al. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity[J]. Infect Immun, 2000, 68(7): 3848-3853. DOI:10.1128/iai.68.7.3848-3853.2000 |
| [42] |
Li JH, Adams V, Bannam TL, et al. Toxin plasmids of Clostridium perfringens[J]. Microbiol Mol Biol Rev, 2013, 77(2): 208-233. DOI:10.1128/mmbr.00062-12 |
| [43] |
Nagahama M, Takehara M, Kobayashi K. Interaction of Clostridium perfringens iota toxin and lipolysis-stimulated lipoprotein receptor (LSR)[J]. Toxins (Basel), 2018, 10(10): 405. DOI:10.3390/toxins10100405 |
| [44] |
Takehara M, Takagishi T, Seike S, et al. Cellular entry of Clostridium perfringens Iota-Toxin and Clostridium botulinum C2 toxin[J]. Toxins (Basel), 2017, 9(8): 247. DOI:10.3390/toxins9080247 |
| [45] |
Richard JF, Mainguy G, Gibert M, et al. Transcytosis of iota-toxin across polarized CaCo-2 cells[J]. Mol Microbiol, 2002, 43(4): 907-917. DOI:10.1046/j.1365-2958.2002.02806.x |
| [46] |
Yanagimoto K, Uematsu K, Yamagami T, et al. The circulation of Type F Clostridium perfringens among humans, sewage, and Ruditapes philippinarum (Asari Clams)[J]. Pathogens, 2020, 9(8): 669. DOI:10.3390/pathogens9080669 |
| [47] |
Briggs DC, Naylor CE, Smedley JG, et al. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins[J]. J Mol Biol, 2011, 413(1): 138-149. DOI:10.1016/j.jmb.2011.07.066 |
| [48] |
Saitoh Y, Suzuki H, Tani K, et al. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin[J]. Science, 2015, 347(6223): 775-778. DOI:10.1126/science.1261833 |
| [49] |
Kondoh M. Claudin as a novel target for drug delivery system[J]. Yakugaku Zassh, 2006, 126(9): 711-721. DOI:10.1248/yakushi.126.711 |
| [50] |
Shrestha A, Uzal FA, Mcclane BA. The interaction of Clostridium perfringens enterotoxin with receptor claudins[J]. Anaerobe, 2016, 41: 18-26. DOI:10.1016/j.anaerobe.2016.04.011 |
| [51] |
Mitchell LA, Koval M. Specificity of interaction between Clostridium perfringens enterotoxin and claudin-family tight junction proteins[J]. Toxins (Basel), 2010, 2(7): 1595-1611. DOI:10.3390/toxins2071595 |
| [52] |
Mcclane B, Shrestha A. Using more Than 1 (Path) way to kill a host cell: lessons from Clostridium perfringens enterotoxin[J]. Microbiol Insights, 2020, 13: 1178636120931518. DOI:10.1177/1178636120931518 |
| [53] |
Shrestha A, Gohari IM, Mcclane BA. RIP1, RIP3, and MLKL contribute to cell death caused by Clostridium perfringens enterotoxin[J]. mBio, 2019, 10(6): e02985-19. DOI:10.1128/mBio.02985-19 |
| [54] |
Keyburn AL, Boyce JD, Vaz P, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens[J]. PLoS Pathog, 2008, 4(2): e26. DOI:10.1371/journal.ppat.0040026 |
| [55] |
Yan XX, Porter CJ, Hardy SP, et al. Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens[J]. mBio, 2013, 4(1): e00019-13. DOI:10.1128/mBio.00019-13 |
| [56] |
Savva CG, Da Costa SPF, Bokori-Brown M, et al. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens[J]. J Biol Chem, 2013, 288(5): 3512-3522. DOI:10.1074/jbc.M112.430223 |
| [57] |
Islam AA, Nakatani M, Nakajima T, et al. The cytotoxicity and molecular mechanisms of the Clostridium perfringens NetB toxin[J]. J Vet Med Sci, 2021, 83(2): 187-194. DOI:10.1292/jvms.20-0623 |
| [58] |
Rossjohn J, Feil SC, Mckinstry WJ, et al. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form[J]. Cell, 1997, 89(5): 685-692. DOI:10.1016/s0092-8674(00)80251-2 |
| [59] |
Harris RW, Sims PJ, Tweten RK. Evidence that Clostridium perfringens theta-toxin induces colloid-osmotic lysis of erythrocytes[J]. Infect Immun, 1991, 59(7): 2499-2501. DOI:10.1128/iai.59.7.2499-2501.1991 |
| [60] |
Zeng J, Song FY, Yang Y, et al. The generation and characterization of recombinant protein and antibodies of Clostridium perfringens Beta2 toxin[J]. J Immunol Res, 2016, 2016, 5708468. DOI:10.1155/2016/5708468 |
| [61] |
Gibert M, Jolivet-Renaud C, Popoff MR. Beta2 toxin, a novel toxin produced by Clostridium perfringens[J]. Gene, 1997, 203(1): 65-73. DOI:10.1016/s0378-1119(97)00493-9 |
| [62] |
Zhang JL, Yang QL, Yang JJ, et al. Comprehensive analysis of transcriptome-wide m6A methylome upon Clostridium perfringens Beta2 toxin exposure in porcine intestinal epithelial cells by m6A sequencing[J]. Front Genet, 2021, 12: 689748. DOI:10.3389/fgene.2021.689748 |
| [63] |
Gao XL, Huang XY, Yang QL, et al. MicroRNA-21-5p targets PDCD4 to modulate apoptosis and inflammatory response to Clostridium perfringens beta2 toxin infection in IPEC-J2 cells[J]. Dev Comp Immunol, 2021, 114: 103849. DOI:10.1016/j.dci.2020.103849 |
| [64] |
Mehdizadeh Gohari I, Unterer S, Whitehead AE, et al. NetF-producing Clostridium perfringens and its associated diseases in dogs and foals[J]. J Vet Diagn Invest, 2020, 32(2): 230-238. DOI:10.1177/1040638720904714 |
| [65] |
Kiu R, Sim K, Shaw A, et al. Genomic analysis of Clostridium perfringens BEC/CPILE-positive, toxinotype D and E strains isolated from healthy children[J]. Toxins (Basel), 2019, 11(9): 543. DOI:10.3390/toxins11090543 |
| [66] |
Yonogi S, Matsuda S, Kawai T, et al. BEC, a novel enterotoxin of Clostridium perfringens found in human clinical isolates from acute gastroenteritis outbreaks[J]. Infect Immun, 2014, 82(6): 2390-2399. DOI:10.1128/iai.01759-14 |
| [67] |
Stiles BG, Pradhan K, Fleming JM, et al. Clostridium and Bacillus binary enterotoxins: bad for the bowels, and eukaryotic being[J]. Toxins (Basel), 2014, 6(9): 2626-2656. DOI:10.3390/toxins6092626 |
| [68] |
Kennedy CL, Lyras D, Cheung JK, et al. Cross-complementation of Clostridium perfringens PLC and Clostridium septicum α-toxin mutants reveals PLC is sufficient to mediate gas gangrene[J]. Microbes Infect, 2009, 11(3): 413-418. DOI:10.1016/j.micinf.2009.01.002 |
| [69] |
Morris WE, Dunleavy MV, Diodati J, et al. Effects of Clostridium perfringens alpha and epsilon toxins in the bovine gut[J]. Anaerobe, 2012, 18(1): 143-147. DOI:10.1016/j.anaerobe.2011.12.003 |
| [70] |
Goossens E, Valgaeren BR, Pardon B, et al. Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: a review on bovine necro-haemorrhagic enteritis[J]. Vet Res, 2017, 48(1): 9. DOI:10.1186/s13567-017-0413-x |
| [71] |
Uzal FA. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats[J]. Anaerobe, 2004, 10(2): 135-143. DOI:10.1016/j.anaerobe.2003.08.005 |
| [72] |
Rumah KR, Linden J, Fischetti VA, et al. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease[J]. PLoS One, 2013, 8(10): e76359. DOI:10.1371/journal.pone.0076359 |
| [73] |
Wagley S, Bokori-Brown M, Morcrette H, et al. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis[J]. Mult Scler J, 2019, 25(5): 653-660. DOI:10.1177/1352458518767327 |
| [74] |
Uzal FA, Mcclane BA. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections[J]. Vet Microbiol, 2011, 153(1/2): 37-43. DOI:10.1016/j.vetmic.2011.02.048 |
| [75] |
Nagahama M, Ochi S, Oda M, et al. Recent insights into Clostridium perfringens beta-toxin[J]. Toxins (Basel), 2015, 7(2): 396-406. DOI:10.3390/toxins7020396 |
| [76] |
Finnie JW, Navarro MA, Uzal FA. Pathogenesis and diagnostic features of brain and ophthalmic damage produced by Clostridium perfringens type D epsilon toxin[J]. J Vet Diagn Invest, 2020, 32(2): 282-286. DOI:10.1177/1040638719900190 |
| [77] |
Stiles BG, Barth G, Barth H, et al. Clostridium perfringens epsilon toxin: a malevolent molecule for animals and man?[J]. Toxins (Basel), 2013, 5(11): 2138-2160. DOI:10.3390/toxins5112138 |
| [78] |
Kim HY, Byun JW, Roh IS, et al. First isolation of Clostridium perfringens type E from a goat with diarrhea[J]. Anaerobe, 2013, 22: 141-143. DOI:10.1016/j.anaerobe.2013.06.009 |
| [79] |
Diancourt L, Sautereau J, Criscuolo A, et al. Two Clostridium perfringens type E isolates in France[J]. Toxins (Basel), 2019, 11(3): 138. DOI:10.3390/toxins11030138 |
| [80] |
Mcclane BA, Robertson SL, Li J. Clostridium perfringens[M]. Food Microbiol, 2012: 465-489. DOI:10.1128/9781555818463.ch18
|
| [81] |
Bamford C, Milligan P, Kaliski S. Dangers of Clostridium perfringens food poisoning in psychiatric patients[J]. S Afr J Psychiatr, 2019, 25: 1339. DOI:10.4102/sajpsychiatry.v25i0.1339 |
| [82] |
Bos J, Smithee L, Mcclane B, et al. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection[J]. Clin Infect Dis, 2005, 40(10): e78-83. DOI:10.1086/429829 |
| [83] |
Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year Bi-national pharmacovigilance study of serious or fatal 'Slow Gut' reactions, and comparison with international drug safety advice[J]. CNS Drugs, 2017, 31(8): 699-709. DOI:10.1007/s40263-017-0448-6 |
| [84] |
Mehdizadeh Gohari I, Navarro MA, Li JH, et al. Pathogenicity and virulence of Clostridium perfringens[J]. Virulence, 2021, 12(1): 723-753. DOI:10.1080/21505594.2021.1886777 |
| [85] |
Navarro MA, Li JH, Beingesser J, et al. Nani sialidase enhances the action of Clostridium perfringens enterotoxin in the presence of Mucus[J]. mSphere, 2021, 6(6): e0084821. DOI:10.1128/mSphere.00848-21 |
| [86] |
Lee KW, Lillehoj HS, Kim W, et al. Research Note: First report on the detection of necrotic enteritis (NE) B-like toxin in biological samples from NE-afflicted chickens using capture enzyme-linked immunosorbent assay[J]. Poult Sci, 2021, 100(7): 101190. DOI:10.1016/j.psj.2021.101190 |
| [87] |
中华人民共和国农业部. 中华人民共和国农业部公告第1125号[R]. 中华人民共和国农业部公报, 2009, (1): 46-47. Ministry of Agriculture of the People's Republic of China. Announcement No. 1125 of the Ministry of agriculture of the People's Republic of China[R]. Bull Minist Agric People's Republ China, 2009, (1): 46-47. |
| [88] |
Janik E, Ceremuga M, Saluk-Bijak J, et al. Biological toxins as the Potential tools for bioterrorism[J]. Int J Mol Sci, 2019, 20(5): 1181. DOI:10.3390/ijms20051181 |
| [89] |
Recommendations of the CDC Strategic Planning Workgroup. Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup[J]. MMWR Recomm Rep, 2000, 49(RR-4): 1-14. |
| [90] |
Dewey-Mattia D, Manikonda K, Hall AJ, et al. Surveillance for foodborne disease outbreaks-United States, 2009-2015[J]. MMWR Surveill Summ, 2018, 67(10): 1-11. DOI:10.15585/mmwr.ss6710a1 |
| [91] |
Scallan E, Hoekstra RM, Mahon BE, et al. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years[J]. Epidemiol Infect, 2015, 143(13): 2795-2804. DOI:10.1017/s0950268814003185 |
| [92] |
Abdelrahim AM, Radomski N, Delannoy S, et al. Large-scale genomic analyses and toxinotyping of Clostridium perfringens implicated in foodborne outbreaks in France[J]. Front Microbiol, 2019, 10: 777. DOI:10.3389/fmicb.2019.00777 |
| [93] |
Azimirad M, Gholami F, Yadegar A, et al. Prevalence and characterization of Clostridium perfringens toxinotypes among patients with antibiotic-associated diarrhea in Iran[J]. Sci Rep, 2019, 9(1): 7792. DOI:10.1038/s41598-019-44281-5 |
| [94] |
Motamedi H, Fathollahi M, Abiri R, et al. A worldwide systematic review and meta-analysis of bacteria related to antibiotic-associated diarrhea in hospitalized patients[J]. PLoS One, 2021, 16(12): e0260667. DOI:10.1371/journal.pone.0260667 |
| [95] |
Wang BY, Dong WJ, Ma LY, et al. Prevalence and genetic diversity of Clostridium perfringens isolates in hospitalized diarrheal patients from central China[J]. Infect Drug Resist, 2021, 14: 4783-4793. DOI:10.2147/idr.s338593 |
 2022, Vol. 43
2022, Vol. 43