文章信息
- 杨继春, 于树青, 高乐, 周庆欣, 詹思延, 孙凤.
- Yang Jichun, Yu Shuqing, Gao Le, Zhou Qingxin, Zhan Siyan, Sun Feng
- 全球肝癌筛查指南制订现状的系统综述
- Current global development of screening guidelines for hepatocellular carcinoma: a systematic review
- 中华流行病学杂志, 2020, 41(7): 1126-1137
- Chinese Journal of Epidemiology, 2020, 41(7): 1126-1137
- http://dx.doi.org/10.3760/cma.j.cn112338-20190814-00597
-
文章历史
收稿日期: 2019-08-14
2. 北京大学公共卫生学院流行病与卫生统计学系 100191;
3. 北京大学循证医学中心 100191
2. Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China;
3. Center of Evidence-based Medicine and Clinical Research, Peking University, Beijing 100191, China
2018年全球癌症统计报告指出:肝癌发病率位于常见癌症发病率的第6位,死亡率位于第4位;全球每年约有84.1万肝癌新发病例和78.2万人死于肝癌[1]。肝癌在蒙古国、日本、韩国等地区发病率和死亡率居高不下[2-4],欧州、美国等地区近几年也有上升趋势[5-7]。我国肝癌发病率和死亡率与日本等东亚地区相当,发病率约为26/10万,死亡率为23/10万。因人口基数大,全球50%的肝癌新发病例人数和死亡人数在我国[8]。
筛查是早发现、早诊断、早治疗癌症的关键。若能通过筛查发现癌前病变,进行早期干预,不仅会降低晚期癌症的发生,更能极大降低死亡率;从而降低并发症发生、降低医疗费用、提高患者生活质量,获益明显[9]。
作为全球疾病筛查项目评估的典范,美国预防服务工作组(US Preventive Services Task Force,USPSTF)已建立了较为完善、科学、透明的癌症筛查指南制订体系。目前,USPSTF已经完成了乳腺癌、膀胱癌、宫颈癌、结直肠癌、肺癌、口腔癌、卵巢癌、前列腺癌、皮肤癌和睾丸癌的筛查指南的制订和更新[10-19],系统总结评价癌症发生发展的每一环节的相关证据,遵循风险-获益的原则(risk-benefit),最终给出不同危险度人群筛查起始年龄、方式、间隔等推荐意见。但是,目前USPSTF尚未针对肝癌制订相应的筛查循证指南。只有美国肝病研究协会(American Association for the Study of Liver Diseases,AASLD)、欧洲肝脏研究协会(European Association for the Study of the Liver,EASL)、亚太肝脏研究协会(Asian Pacific Association for the Study of the Liver,APASL)等机构发布的肝癌临床实践指南(Clinical practice guidelines,CPG)中简要给出肝癌筛查推荐意见。我国国家卫生健康委员会发布的《原发性肝癌诊疗规范》仅在借鉴AASLD发布的肝癌CPG,提出了我国肝癌筛查相关推荐意见[20]。
本研究使用系统综述的方法对全球范围内肝癌筛查指南进行系统梳理,深入了解现有指南制订现状与推荐意见形成过程,并结合USPSTF等机构已提出的其他癌症筛查指南的制订框架,为后续制订专用的肝癌筛查循证指南形成初步建议。
资料与方法1.文献检索:系统检索英文数据库(PubMed、Embase、Web of Science、Google scholar)和中文数据库(CNKI、万方、维普、SinoMed),检索截止到2019年1月3日。此外,还检索美国肝病研究协会(American Association for the Study of Liver Diseases,AASLD)、国际指南协作网(Guideline International Network,GIN)、中国临床指南文库(China Guideline Clearinghouse,CGC)等与筛查指南可能相关的网站。中文关键词为“肝癌”+“筛查”+“指南/推荐意见”,英文关键词为“hepatocellular cancer”“screening”“guidelines/recommendations”,并考虑各个关键词的不同表达形式,见图 1。辅助文献追溯法,尽可能查到详尽的资料。

|
| 图 1 PubMed检索策略 |
2.文献筛选:文献筛选的整个过程均使用EndNote X9软件对检索到的文献进行管理,并根据系统综述的PRISMA报告规范的要求,绘制整个文献纳入排除流程图。在初步筛选文献时,制订一套筛选流程及说明,并统一培训文献筛选者。利用EndNote X9软件自动检测重复文献,排除重复文献。然后根据以下纳入排除标准,进行双人独立平行筛选文献,完成后进行不一致核查。对于意见不一致的文献,与导师交流后进行判定。
(1)纳入标准:①公开发布的原创、最新的与肝癌筛查相关的指南,如有多次更新版本,以最新一版为主;②官方制订或非官方(行业学会、协会、学术机构、专家组)发布均纳入;③语种仅限中、英文。
(2)排除标准:①关于肝癌筛查指南的介绍、评析、应用指导、应用效果评价、勘误表等;②排除肝癌筛查原始研究、不涉及肝癌筛查的指南;③重复文献;④非英文和中文语种的指南;⑤非最新版本指南。
3.信息提取:将通过初筛后的文献在EndNote X9软件中建立初筛文献库,使用Excel 2010软件进行双人独立提取数据,完成后进行不一致核查。对于意见不一致的文献,与导师讨论后进行确定。提取的信息包括:①指南基本信息(发布国家/地区、发布时间、发布机构、指南名称、指南版本等);②肝癌筛查推荐意见(筛查人群、筛查措施、筛查间隔、指南采用的证据分级系统、推荐意见分级系统、使用证据情况等);③为了更好地了解肝癌筛查指南的制订进展,对同一指南不同更新版本的肝癌筛查更新内容和理由进行对比。
4.数据分析:对所有肝癌筛查相关指南的发布情况、筛查推荐意见、证据来源等方面进行汇总描述;根据权威组织制订的癌症筛查循证指南制订框架,总结和评价肝癌筛查指南制订现状。
结果1.文献检索结果:共检索到976篇文献,去重后获得文献813篇。根据预先制订的纳入排除标准,通过阅读文题和摘要初筛获得41篇文献。进一步阅读全文复筛,最终纳入17篇文献[20-36]。筛选流程见图 2。
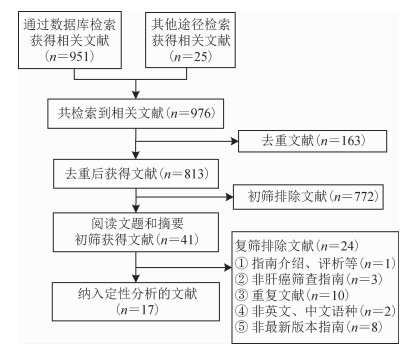
|
| 图 2 文献筛选流程图 |
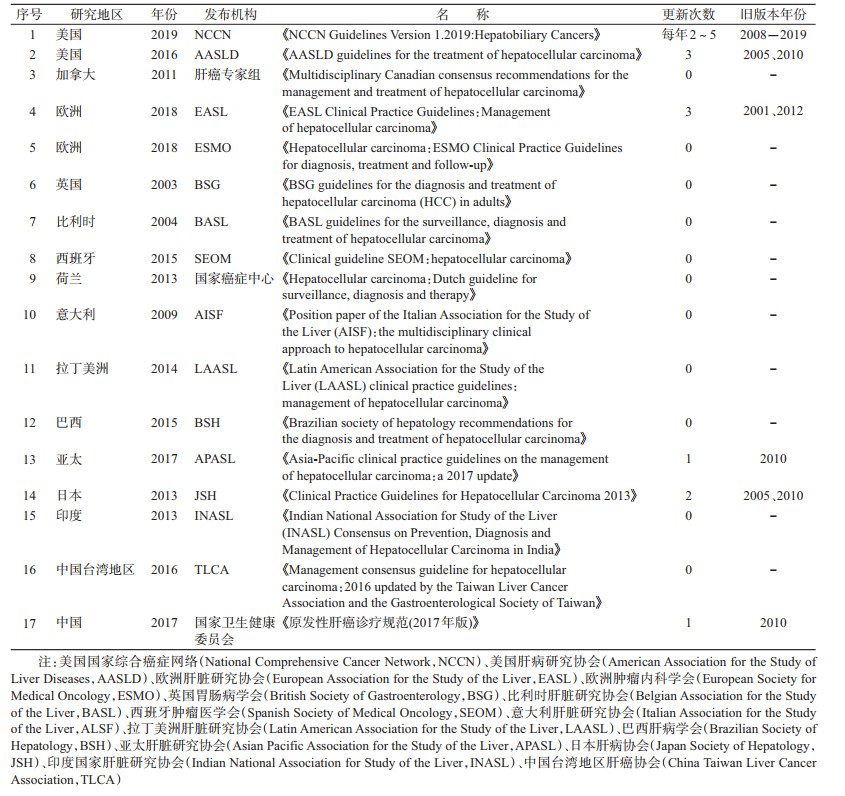
2.肝癌筛查相关指南的基本信息:目前尚无独立的肝癌筛查指南,肝癌筛查推荐意见只是肝癌CPG的一部分内容。纳入的17个指南基本信息见表 1。按发布指南的国家/地区、发布时间、指南名称、发布机构、指南更新次数、各个版本发布年份等对肝癌筛查指南的基本信息进行梳理。汇总各机构发布的最新指南,发布时间范围为2003-2019年。指南发布机构共涉及各国/各地区的肿瘤/肝病研究协会、国家癌症中心、国家卫生健康委员会等相关部门。只有美国国家综合癌症网络(National Comprehensive Cancer Network,NCCN)、美国肝病研究协会(AASLD)、欧洲肝脏研究协会(EASL)、亚太肝脏研究协会(APASL)、日本肝病协会(Japan Society of Hepatology,JSH)、中国国家卫生健康委员会6个相关部门(占35.29%)对相关指南进行更新[20-21, 23, 25, 31, 34]。
3.筛查指南推荐意见和证据来源:指南的制订主要考虑目标人群的选择以及筛查措施的评价两个环节。各肝癌指南的筛查推荐意见见表 2,从筛查人群、筛查间隔、筛查措施、证据质量和推荐意见分级系统、证据推荐来源等方面进行阐述。
(1)筛查人群:目前各国指南只推荐对肝癌高危人群进行肝癌筛查,未推荐全人群筛查策略。各国指南都将慢性乙肝、丙肝和肝硬化患者确定为肝癌筛查的高危人群;美国和欧洲地区的指南对肝硬化患者的Child-Pugh的不同分级推荐了不同的筛查策略:Child-Pugh A级和B级肝硬化患者推荐进行肝癌筛查,Child-Pugh C级肝硬化患者如果不进行肝移植不推荐筛查,指南认为肝硬化伴晚期肝功能衰竭(Child-Pugh C级),无法进行有效的肝癌治疗除非进行肝移植[21, 23, 26, 35, 37]。EASL、中国指南还把具有肝癌家族史的人群确定为肝癌高危人群,推荐对具有肝癌家族史的人群也进行肝癌筛查[20, 23]。日本指南中把既患肝炎又有肝硬化的人群列为超高危人群,对其采取更短的筛查间隔进行筛查[31]。中国指南指出具备肝癌危险因素而且年龄>40岁的男性,患肝癌风险更大。但是,中国指南未指出对上述人群是否采取不同的筛查策略[20]。
(2)筛查间隔:除了日本指南推荐对超高危人群进行每3~4个月1次的筛查[31],其他各指南均推荐对肝癌高危人群进行每6个月1次的筛查[20-30, 32, 36]。
(3)筛查措施:各国指南都推荐使用超声(ultrasound,US)对肝癌高危人群进行肝癌筛查。欧美等西方国家指南认为甲胎蛋白(alpha-fetoprotein,AFP)进行肝癌筛查的灵敏度和特异度低,对AFP是否与US联合使用进行筛查不做严格要求[21, 25, 27, 29, 33, 35];亚太地区各国指南均推荐US和AFP连用对高危人群进行肝癌筛查,证据支持亚洲地区乙肝患病率较高,肿瘤标志物对乙肝患者进行肝癌筛查的灵敏度和特异度较高[20, 31-32, 34, 36];巴西和拉丁美洲地区指南指出,US不可用的条件下可使用AFP进行肝癌筛查[26, 30]。日本指南推荐对超高危人群(既有病毒性肝炎又有肝硬化)使用计算机断层扫描(Computed Tomography,CT)、磁共振成像(Magnetic Resonance Imaging,MRI)进行筛查,以提高小肝癌检出率[31]。
(4)证据质量和推荐意见分级体系:只有7个指南(占总量的42%)使用循证医学证据分级体系对证据强度进行了分级,并给出了推荐意见,使用的主要的分级工具为指南证据体质量评价(Grading of Recommendations Assessment Development and Evaluation,GRADE)和牛津大学循证医学中心(Oxford Centre for Evidence-based Medicine,OCEBM)分级系统[21, 23, 29, 31, 34-36]。
(5)证据推荐来源:指南的证据支持来源于肝硬化、乙肝、丙肝等肝癌高危人群患病率、患肝癌风险的原始研究、筛查措施进行肝癌筛查灵敏度和特异度的研究,以及比较不同筛查间隔筛查效果的原始研究等;大部分肝癌筛查指南都参考了AASLD、EASL的指南,几乎每个指南都引用了中国上海地区乙肝患者肝癌筛查有效性评价的RCT研究[38];AASLD指南(2016版)第一次引用了2014年发表的肝硬化患者肝癌筛查有效性的Meta分析,其他指南引用的肝癌筛查有效性研究均为原始研究[39]。
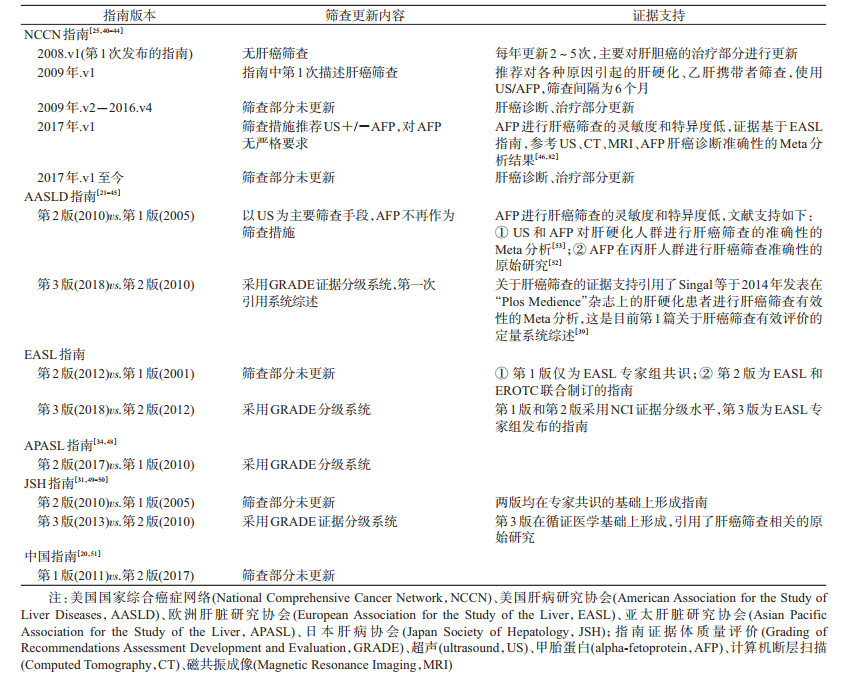
4.筛查指南更新情况:仅有6个相关部门对肝癌筛查指南进行更新,各肝癌筛查指南更新情况见表 3。NCCN肝胆临床实践指南于2008年发布第1版[40],此后每年更新2~5次,筛查部分的更新主要是2017年后的指南不再推荐使用AFP筛查[25, 40-44]。AASLD肝癌临床实践指南于2008年发布第1版[21],2010、2016年分别进行了更新,2010年版指南不再推荐使用AFP进行肝癌筛查[45],2018年版指南使用GRADE分级系统证据和推荐意见进行分级[21]、第一次引用Singal等[39]于2014年发表的评价肝硬化患者进行肝癌筛查有效性的Meta分析。EASL肝癌临床实践指南于2001年发布第1版,2012、2018年分别进行了更新,2018年版指南使用GRADE分级系统对证据和推荐意见进行分级[23, 46-47]。APASL肝癌临床实践指南于2010年发布第1版,2017年进行更新,2017年版指南使用GRADE分级系统对证据和推荐意见进行分级[34, 48]。JSH肝癌临床实践指南于2005年发布第1版,2010、2013年分别进行了更新,2013年版指南使用GRADE分级系统对证据进行分级,未给出推荐意见分级[31, 49-50]。中国国家卫生健康委员会参考国际肝癌临床实践指南,2011年发布第1版原发性肝癌诊疗规范,2017年进行更新,两版之间肝癌筛查部分信息未更新[20, 51]。
5.肝癌筛查指南制订现状总结和评价:根据图 3所示的USPSTF的乳腺癌、肺癌、结直肠癌等其他癌症筛查指南制订框架[10-19],结合肝癌筛查的各个环节和关键要素,对上述的17部肝癌筛查指南制订现状进行相应总结和评价[20-36]。
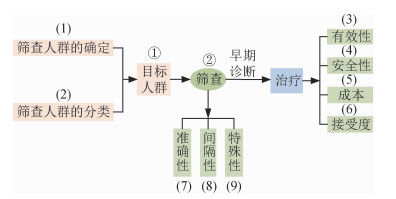
|
| 图 3 USPSTF癌症筛查指南制订框架 |
USPSTF的癌症筛查制订理论框架,共包括目标人群选择、筛查策略评价两个环节,共9个关键要素。筛查指南制定的第一个环节为目标人群选择,目标人群的确定主要包括筛查人群的确定、筛查人群的分类两个关键要素。目前的肝癌筛查指南仅推荐对肝癌高危人群进行筛查,部分指南对筛查人群进行分类,推荐对不同的筛查人群实施不同的筛查策略。筛查指南制定的第二个环节为筛查策略评价,评价一项筛查策略是否值得实施包括筛查有效性、筛查安全性、筛查成本、人群接受度、筛查措施的准确性、筛查间隔性、筛查特殊性7个关键要素。目前的肝癌筛查指南对筛查策略的评价主要考虑筛查措施准确性、筛查成本、筛查特殊性几个关键要素进行推荐,仅少数指南考虑筛查有效性、安全性、间隔性、人群接受度等关键要素。
(1)筛查人群的确定:目前的肝癌筛查指南仅推荐对肝癌高危人群进行肝癌筛查,没有指南推荐对全人群进行肝癌筛查。风险人群的确定根据HBV感染、HCV感染、肝硬化、肝癌家族史等肝癌危险因素确定肝癌高危人群。
(2)筛查人群的分类:欧美指南对肝硬化患者的Child-Pugh的不同分级推荐了不同的筛查策略:Child-Pugh A和B级肝硬化患者推荐进行肝癌筛查,Child-Pugh C级肝硬化患者如果不进行肝移植不推荐筛查,指南认为肝硬化伴晚期肝功能衰竭(Child-Pugh C级),无法进行有效的肝癌治疗对其筛查无实际效益。亚洲等地区指南可根据本地区实际情况,考虑是否对不同Child-Pugh分级的肝硬化患者采取不同的筛查推荐策略。日本指南将肝癌筛查人群分为肝癌高危人群和超高危人群,即慢性乙肝、丙肝、肝硬化人群为高危人群,既有病毒性肝炎又患有肝硬化的人群确定为超高危人群。推荐US和AFP联合使用每隔6个月对高危人群进行筛查,使用CT或MRI每隔3~4个月对超高危人群进行筛查[31]。其他地区/国家指南在指南制订过程中可根据本地区实际情况,考虑是否对筛查人群进行分类对其采取不同的筛查策略。
(3)有效性评价:筛查主要结局指标包括总死亡率、肝癌死亡率,次要结局包括生存率、寿命延长、重要的额外发现、生活质量提高等,中间结局包括早期肝癌的检出比例。目前大部分肝癌筛查指南有效性推荐意见仅来自Zhang等[38]上海的RCT研究。有的指南无筛查有效性证据,仅借鉴了其他指南或者从筛查工具准确性角度进行推荐。
(4)安全性评价:安全性结局包括各筛查工具的直接不良事件与后续诊断或治疗可能带来的安全性结局事件。各筛查工具的直接不良事件:使用CT或MRI进行筛查可能导致筛查人群的辐射暴露。筛查后续过程的安全性事件:因为筛查假阳性、筛查结果不确定性而进一步通过CT、MRI等影像学诊断过程中遭受的辐射暴露;筛查过程中因为假阳性或筛查结果不确定性而进一步通过病理学诊断过程中,筛查人群遭受的肝脏活检,活检过程中可能会导致出血、感染等安全性结局;由于筛查阳性而使患者因为自己患癌症而产生心理焦虑等负面情绪;肝癌筛查导致的过度诊断等。目前的肝癌筛查指南尚未引用筛查安全性研究证据,综合评估肝癌筛查的获益风险。目前尚未发现有指南提供筛查安全性证据。
(5)成本效益分析:主要指标有筛查花费、诊断/治疗/随访/监测花费、治疗相关生命周期成本、净花费;发现1例肝癌花费、挽救1例生命花费、挽救1个生命年花费等。目前的肝癌筛查指南引用了一些不同筛查措施的成本比较研究,进行相应推荐。
(6)人群的接受度与价值观:可纳入考虑的指标有接受度、满意度、依从性、患者偏好等。
(7)各筛查工具的准确性确定:评价指标包括检出率、灵敏度、特异度、阳性预测值、阴性预测值、符合率、阳性似然比、阴性似然比等。大部分指南在进行筛查措施推荐时引用了各筛查工具在高危人群中进行肝癌筛查的灵敏度和特异度研究。
(8)不同筛查间隔的筛查效果:缩短筛查间隔会增加筛查成本,造成资源浪费。延长筛查间隔,可能会导致筛查效果降低。目前肝癌筛查指南主要是根据肝脏肿瘤体积倍增时间来推荐筛查间隔,尚未系统梳理不同筛查间隔的筛查效果比较研究。
(9)肝癌筛查的特殊性:不同国家/地区应结合地区疾病发病率、经济水平,医疗公平性、筛查工具可及性等问题,提出适合本国/地区的肝癌筛查策略。各国家/地区的指南推荐意见均考虑当地实际情况。
综上所述,目前肝癌筛查指南主要基于筛查人群患肝癌风险、筛查工具准确性、筛查成本等要素推荐相应筛查策略,尚未综合考虑筛查有效性、安全性等关键要素。
讨论目前国内外均无独立的肝癌筛查指南。欧美国家无独立的肝癌筛查指南的原因可能与欧美国家肝癌发病率和死亡率较低,开展肝癌筛查较少有关。但是日本、韩国、中国等肝癌高发地区,应根据实际情况制订适合本国的肝癌筛查指南,以指导本国的肝癌筛查项目的开展。
我国作为肝癌大国,肝癌筛查的概念已有了大范围的传播和广泛的实践。国家卫生健康委员会发布的《原发性肝癌诊疗规范(2017年版)》中提出了相应的肝癌筛查推荐意见[20]。国家癌症中心和癌症基金会发布的农村癌症早诊早治项目(2005年)、淮河流域癌症早诊早治项目(2007年)、城市癌症早诊早治项目(2012年)等多项大规模癌症筛查项目中均包括肝癌筛查推荐方案,推荐对40~74岁的肝癌高危人群使用US和AFP联合的方式进行筛查[83-84]。但是,我国目前尚未形成肝癌筛查的循证指南。作为肝癌大国,我国应根据本国实际情况,全面收集肝癌筛查相关的证据,形成高质量的肝癌筛查循证指南,以更好指导我国肝癌筛查项目的实施。
目前肝癌筛查指南形成的肝癌筛查推荐意见主要基于筛查人群患肝癌风险、筛查工具灵敏度和特异度等角度进行考虑。大部分指南仅引用了2004年发表的中国上海乙肝人群肝癌筛查的RCT研究,作为肝癌筛查有效性证据[38]。尚未有指南综合评估筛查策略有效性和安全性而给出相应推荐意见。筛查是否有效、安全是评价筛查项目是否值得推荐的重要指标。
目前的肝癌筛查指南仅推荐对肝癌高危人群进行肝癌筛查,没有指南推荐对全人群进行肝癌筛查。各国指南都将慢性乙肝、丙肝患者、各种原因引起的肝硬化患者确定为肝癌高危人群。相关研究表明在全球范围内,54%的肝癌是因为HBV感染、31%的肝癌是因为HCV感染。HBV感染、HCV感染、过量酒精摄入、脂肪肝等都会发展成肝硬化,而85%~95%的肝硬化患者最终会发展成肝癌[85-86]。AASLD、EASL等欧美指南推荐按Child-Pugh分级对肝硬化人群进行更具体的筛查推荐。指南认为Child-Pugh C级且不进行肝移植的肝硬化患者预期生存率很低,遂不推荐对Child-Pugh C级且不进行肝移植的肝硬化患者进行肝癌筛查。
大部分肝癌筛查指南均推荐对肝癌高危人群进行每6个月1次的筛查。仅日本指南推荐对既有病毒性肝炎又患有肝硬化的人群,每3~4个月进行1次肝癌筛查。EASL、APASL指南指出肿瘤筛查间隔取决于肿瘤体积倍增时间,相关研究表明肝肿瘤体积倍增时间范围为93.5~210 d[23, 34]。所以指南推荐肝癌筛查间隔为6个月,其他指南参考EASL的指南。
欧美最新指南均推荐使用US进行筛查,对AFP筛查未作严格要求。亚洲最新指南均推荐使用US和AFP联合使用。APASL指南强调不推荐单独使用AFP进行肝癌筛查。欧美指南和亚洲指南对AFP推荐意见不同,与欧美和亚洲地区肝癌主要危险因素不同以及AFP特性有关。相关研究表明在非洲和东亚由乙肝引起的肝癌占60%,而在西方国家仅有20%的肝癌是由乙肝引起的。西方国家肝癌危险因素主要为酒精摄入等各种原因引起的肝硬化以及肥胖引起的脂肪肝等[86]。AFP不仅会在肝癌患者中有升高、在肝硬化患者或活动性肝炎中也会有所升高,导致其在肝硬化患者进行肝癌筛查的准确性较低。AFP作为血清肿瘤标志物,在乙肝患者中使用其进行肝癌筛查有一定的灵敏度和特异度[87]。
建议相关机构参考USPSTF等机构的癌症筛查制订理论框架,考虑筛查指南制订的各个环节和关键要素,系统收集肝癌筛查现有证据,遵循风险-获益的原则(risk-benefit),结合本国/地区情况,从临床医生、患者、决策者等的角度权衡利弊后形成推荐意见,制订肝癌筛查循证指南,以更好地指导本国/地区的肝癌筛查的实施,使肝癌筛查真正获益。
利益冲突 所有作者均声明不存在利益冲突
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. ACJC, 2018, 68(6): 394-424. DOI:10.3322/caac.21492 |
| [2] |
Chimed T, Sandagdorj T, Znaor A, et al. Cancer incidence and cancer control in Mongolia:results from the national cancer registry 2008-12[J]. Int J Cancer, 2017, 140(2): 302-309. DOI:10.1002/ijc.30463 |
| [3] |
Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009:a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project[J]. Jpn J Clin Oncol, 2015, 45(9): 884-891. DOI:10.1093/jjco/hyv088 |
| [4] |
Jung KW, Won YJ, Oh CM, et al. Prediction of cancer incidence and mortality in Korea, 2017[J]. Cancer Res Treat, 2017, 49(2): 306-312. DOI:10.4143/crt.2017.130 |
| [5] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. ACJC, 2019, 69(1): 7-34. DOI:10.3322/caac.21551 |
| [6] |
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe:Estimates for 40 countries and 25 major cancers in 2018[J]. Eur J Cancer, 2018, 103: 356-387. DOI:10.1016/j.ejca.2018.07.005 |
| [7] |
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe:estimates for 40 countries in 2012[J]. Eur J Cancer, 2013, 49(6): 1374-1403. DOI:10.1016/j.ejca.2012.12.027 |
| [8] |
陈万青, 孙可欣, 郑荣寿, 等. 2014年中国分地区恶性肿瘤发病和死亡分析[J]. 中国肿瘤, 2018, 27(1): 1-14. Chen WQ, Sun KX, Zheng RS, et al. Report of cancer incidence and mortality in different areas of China, 2014[J]. Chin Cancer, 2018, 27(1): 1-14. DOI:10.11735/j.issn.1004-0242.2018.01.A001 |
| [9] |
WHO. Cancer Screening 2012[EB/OL].[2019-08-01]. http://www.who.int/cancer/prevention/diagnosis-screening/screening/en/.
|
| [10] |
U.S. Preventive Services Task Force. Screening for breast cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2009, 151(10): 716-726. DOI:10.7326/0003-4819-151-10-200911170-00008 |
| [11] |
Moyer VA. Screening for bladder cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2011, 155(4): 246-251. DOI:10.7326/0003-4819-155-4-201108160-00008 |
| [12] |
Moyer VA, U. S.. Preventive Services Task Force. Screening for cervical cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2012, 156(12): 880-891. DOI:10.7326/0003-4819-156-12-201206190-00424 |
| [13] |
U.S. Preventive Services Task Force. Screening for colorectal cancer:U.S. Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2008, 149(9): 627-637. DOI:10.7326/0003-4819-149-9-200811040-00243 |
| [14] |
Moyer VA, U. S.. Preventive Services Task Force. Screening for lung cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2014, 160(5): 330-338. DOI:10.7326/M13-2771 |
| [15] |
Moyer VA, U. S.. Preventive Services Task Force. Screening for oral cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2014, 160(1): 55-60. DOI:10.7326/M13-2568 |
| [16] |
Moyer VA. Screening for ovarian cancer:U.S. preventive services task force reaffirmation recommendation statement[J]. Ann Intern Med, 2012, 157(12): 900-904. DOI:10.7326/0003-4819-157-11-201212040-00539 |
| [17] |
Moyer VA. Screening for prostate cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2012, 157(2): 120-134. DOI:10.7326/0003-4819-157-2-201207170-00459 |
| [18] |
U.S. Preventive Services Task Force. Screening for skin cancer:U.S. preventive services task force recommendation statement[J]. Ann Intern, 2009, 150(3): 188-193. DOI:10.7326/0003-4819-150-3-200902030-00008 |
| [19] |
U.S. Preventive Services Task Force. Screening for testicular cancer:U.S. preventive services task force reaffirmation recommendation statement[J]. Ann Intern Med, 2011, 154(7): 483-486. DOI:10.7326/0003-4819-154-7-201104050-00006 |
| [20] |
国家卫生健康委员会. 原发性肝癌诊疗规范(2017年版)[J]. 传染病信息, 2017, 30(3): 111-127. National Health Commission. Guidelines for diagnosis and treatment of primary liver cancer (2017 edition)[J]. Infect Dis Inf, 2017, 30(3): 111-127. DOI:10.3969/j.issn.1007-8134.2017.03.001 |
| [21] |
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma[J]. Hepatology, 2018, 67(1): 358-380. DOI:10.1002/hep.29086 |
| [22] |
BSG. BSG guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults[EB/OL].(2002)[2019-08-01]. https://www.bsg.org.uk/resource/bsg-guidelines-for-the-diagnosis-and-treatment-of-hepatocellular-carcinoma-hcc-in-adults.html.
|
| [23] |
Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines:management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI:10.1016/j.jhep.2018.03.019 |
| [24] |
Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma:ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2018, 29 Suppl 4: iv238-255. DOI:10.1093/annonc/mdy308 |
| [25] |
NCCN. NCCN Guidelines Version 3. 2019: Hepatobiliary Cancers[EB/OL]. (2019)[2019-08-01]. http://guide.medlive.cn/guideline/10532.
|
| [26] |
Carrilho FJ, de Mattos AA, Vianey AF, et al. Brazilian society of hepatology recommendations for the diagnosis and treatment of hepatocellular carcinoma[J]. Arquivos Gastroenterol, 2015, 52(52 Suppl 1): 2-14. DOI:10.1590/s0004-28032015000500001 |
| [27] |
van Vlierberghe H, Borbath I, Delwaide J, et al. BASL guidelines for the surveillance, diagnosis and treatment of hepatocellular carcinoma[J]. Acta Gastroenterol Belg, 2004, 67(1): 14-25. |
| [28] |
Eskens FALM, van Erpecum K, de Jong K, et al. Hepatocellular carcinoma:Dutch guideline for surveillance, diagnosis and therapy[J]. Neth J Med, 2014, 72(6): 299-304. |
| [29] |
Sherman M, Burak K, Maroun J, et al. Multidisciplinary Canadian consensus recommendations for the management and treatment of hepatocellular carcinoma[J]. Curr Oncol, 2011, 18(5): 228-240. DOI:10.3747/co.v18i5.952 |
| [30] |
Méndez-Sánchez N, Ridruejo E, de Mattos AA, et al. Latin American Association for the Study of the Liver (LAASL) clinical practice guidelines:management of hepatocellular carcinoma[J]. Ann Hepatol, 2014, 13 Suppl 1: S4-40. DOI:10.1016/S1665-2681(19)30919-6 |
| [31] |
JSH. Clinical Practice Guidelines for Hepatocellular Carcinoma 2013[EB/OL]. (2013)[2019-08-01]. http://www.jsh.or.jp/English/guidelines_en/Guidelines_for_hepatocellular_carcinoma_2013.
|
| [32] |
Surveillance Group. Management consensus guideline for hepatocellular carcinoma:2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan[J]. J Formos Med Assoc, 2018, 117(5): 381-403. DOI:10.1016/j.jfma.2017.09.007 |
| [33] |
Sastre J, Díaz-Beveridge R, García-Foncillas J, et al. Clinical guideline SEOM:hepatocellular carcinoma[J]. Clin Transl Oncol, 2015, 17(12): 988-995. DOI:10.1007/s12094-015-1451-3 |
| [34] |
Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma:a 2017 update[J]. Hepatol Int, 2017, 11(4): 317-370. DOI:10.1007/s12072-017-9799-9 |
| [35] |
Bolondi L, Cillo U, Colombo M, et al. Position paper of the Italian Association for the study of the Liver (AISF):the multidisciplinary clinical approach to hepatocellular carcinoma[J]. Dig Liver Dis, 2013, 45(9): 712-723. DOI:10.1016/j.dld.2013.01.012 |
| [36] |
Kumar A, Acharya SK, Singh SP, et al. The Indian National Association for Study of the Liver (INASL) consensus on prevention, diagnosis and management of hepatocellular carcinoma in india:the puri recommendations[J]. J Clin Exp Hepatol, 2014, 4 Suppl 3: S3-26. DOI:10.1016/j.jceh.2014.04.003 |
| [37] |
Sánchez-Tapias JM, Costa J, Mas A, et al. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients[J]. Gastroenterology, 2002, 123(6): 1848-1856. DOI:10.1053/gast.2002.37041 |
| [38] |
Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma[J]. J Cancer Res Clin Oncol, 2004, 130(7): 417-422. DOI:10.1007/s00432-004-0552-0 |
| [39] |
Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis:a Meta-analysis[J]. PLoS Med, 2014, 11(4): e1001624. DOI:10.1371/journal.pmed.1001624 |
| [40] |
NCCN. NCCN Guidelines Version 1. 2008 Guidelines: Hepatobiliary Cancers[J]. 2008.
|
| [41] |
NCCN. NCCN Guidelines Version 1. 2009 Guidelines: Hepatobiliary Cancers[EB/OL]. (2009)[2019-08-01]. http://guide.medlive.cn/guideline/10532.
|
| [42] |
NCCN. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers (Version 1. 2017)[EB/OL]. (2017)[2019-08-01]. http://guide.medlive.cn/guideline/12951.
|
| [43] |
NCCN. NCCN Guidelines Version 2. 2009 Guidelines: Hepatobiliary Cancers[EB/OL]. (2009)[2019-08-01]. http://guide.medlive.cn/guideline/10532.
|
| [44] |
NCCN. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers-Evidence Blocks (Version 1. 2016)[EB/OL]. (2016)[2019-08-01]. http://guide.medlive.cn/guideline/11127.
|
| [45] |
Bruix J, Sherman M. Management of hepatocellular carcinoma:an update[J]. Hepatology, 2011, 53(3): 1020-1022. DOI:10.1002/hep.24199 |
| [46] |
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver[J]. J Hepatol, 2001, 35(3): 421-430. DOI:10.1016/s0168-8278(01)00130-1 |
| [47] |
EASL-EORTC. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma[EB/OL]. (2012)[2019-08-01]. https://www.eortc.org/guidelines/.
|
| [48] |
Omata M, Lesmana LA, Tateishi R, et al. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma[J]. Hepatol Int, 2010, 4(2): 439-474. DOI:10.1007/s12072-010-9165-7 |
| [49] |
Kudo M, Okanoue T, Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan:consensus-based clinical practice manual proposed by the Japan Society of Hepatology[J]. Oncology, 2007, 72 Suppl 1: 2-15. DOI:10.1159/000111702 |
| [50] |
Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan:consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version[J]. Dig Dis, 2011, 29(3): 339-364. DOI:10.1159/000327577 |
| [51] |
卫生部. 原发性肝癌诊疗规范(2011年版)[J]. 临床肿瘤学杂志, 2011, 16(10): 929-946. Ministry of Health. Guidelines for diagnosis and treatment of primary liver cancer (2011 edition)[J]. Chin Clin Oncol, 2011, 16(10): 929-946. DOI:10.3969/j.issn.1009-0460.2011.10.017 |
| [52] |
Lok AS, Sterling RK, Everhart JE, et al. Des-γ-carboxy prothrombin and α-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma[J]. Gastroenterology, 2010, 138(2): 493-502. DOI:10.1053/j.gastro.2009.10.031 |
| [53] |
Singal A, Volk ML, Waljee A, et al. Meta-analysis:surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis[J]. Aliment Pharmacol Ther, 2009, 30(1): 37-47. DOI:10.1111/j.1365-2036.2009.04014.x |
| [54] |
Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis:a randomized trial comparing 3-and 6-month periodicities[J]. Hepatology, 2011, 54(6): 1987-1997. DOI:10.1002/hep.24545 |
| [55] |
Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival[J]. J Hepatol, 2010, 53(2): 291-297. DOI:10.1016/j.jhep.2010.03.010 |
| [56] |
Trevisani F, Santi V, Gramenzi A, et al. Surveillance for early diagnosis of hepatocellular carcinoma:is it effective in intermediate/advanced cirrhosis?[J]. Am J Gastroenterol, 2007, 102(11): 2448-2457. DOI:10.1111/j.1572-0241.2007.01395.x |
| [57] |
El-Serag HB, Kramer JR, Chen GJ, et al. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA[J]. Gut, 2011, 60(7): 992-997. DOI:10.1136/gut.2010.230508 |
| [58] |
Sato T, Tateishi R, Yoshida H, et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C[J]. Hepatol Int, 2009, 3(4): 544-550. DOI:10.1007/s12072-009-9145-y |
| [59] |
Unoura M, Kaneko S, Matsushita E, et al. High-risk groups and screening strategies for early detection of hepatocellular carcinoma in patients with chronic liver disease[J]. Hepatogastroenterology, 1993, 40(4): 305-310. |
| [60] |
Wong LL, Limm WM, Severino R, et al. Improved survival with screening for hepatocellular carcinoma[J]. Liver Transpl, 2000, 6(3): 320-325. DOI:10.1053/lv.2000.4875 |
| [61] |
Trevisani F, Cantarini MC, Labate AMM, et al. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis:effects on cancer staging and patient survival[J]. Am J Gastroenterol, 2004, 99(8): 1470-1476. DOI:10.1111/j.1572-0241.2004.30137.x |
| [62] |
Malek NP, Schmidt S, Huber P, et al. The diagnosis and treatment of hepatocellular carcinoma[J]. Dtsch Arztebl Int, 2014, 111(7): 101-106. DOI:10.3238/arztebl.2014.0101 |
| [63] |
van Meer S, de Man RA, Siersema PD, et al. Surveillance for hepatocellular carcinoma in chronic liver disease:evidence and controversies[J]. World J Gastroenterol, 2013, 19(40): 6744-6756. DOI:10.3748/wjg.v19.i40.6744 |
| [64] |
Trevisani F, de Notariis S, Rapaccini G, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma:effects on cancer stage and patient survival (Italian experience)[J]. Am J Gastroenterol, 2002, 97(3): 734-744. DOI:10.1016/S0002-9270(01)04119-3 |
| [65] |
Yuen MF, Lai CL. Screening for hepatocellular carcinoma:survival benefit and cost-effectiveness[J]. Ann Oncol, 2003, 14(10): 1463-1467. DOI:10.1093/annonc/mdg400 |
| [66] |
Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography-A randomised study[J]. Aliment Pharmacol Ther, 2013, 38(3): 303-312. DOI:10.1111/apt.12370 |
| [67] |
Beasley RP, Lin CC, Hwang LY, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan[J]. Lancet, 1981, 318(8256): 1129-1133. DOI:10.1016/s0140-6736(81)90585-7 |
| [68] |
Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma:a cost effectiveness analysis[J]. Gut, 2001, 48(2): 251-259. DOI:10.1136/gut.48.2.251 |
| [69] |
Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B[J]. Hepatology, 1995, 21(1): 77-82. DOI:10.1016/0270-9139(95)90411-5 |
| [70] |
Ikai I, Kudo M, Arii S, et al. Report of the 18th follow-up survey of primary liver cancer in Japan[J]. Hepatol Res, 2010, 40(11): 1043-1059. DOI:10.1111/j.1872-034X.2010.00731.x |
| [71] |
Tanaka H, Nouso K, Kobashi H, et al. Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival[J]. Liver Int, 2006, 26(5): 543-551. DOI:10.1111/j.1478-3231.2006.01270.x |
| [72] |
Tong MJ, Sun HE, Hsien C, et al. Surveillance for hepatocellular carcinoma improves survival in Asian-American patients with hepatitis B:Results from a community-based clinic[J]. Digest Dis Sci, 2010, 55(3): 826-835. DOI:10.1007/s10620-009-1059-y |
| [73] |
Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease[J]. N Engl J Med, 1993, 328(25): 1797-1801. DOI:10.1056/NEJM199306243282501 |
| [74] |
Wong GLH, Wong VWS, Tan GM, et al. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis[J]. Liver Int, 2008, 28(1): 79-87. DOI:10.1111/j.1478-3231.2007.01576.x |
| [75] |
Yu EWR, Chie WC, Chen THH. Does screening or surveillance for primary hepatocellular carcinoma with ultrasonography improve the prognosis of patients?[J]. Cancer J, 2004, 10(5): 317-325. DOI:10.1097/00130404-200409000-00009 |
| [76] |
Chen JG, Parkin DM, Chen QG. Screening for liver cancer:results of a randomised controlled trial in Qidong, China[J]. J Med Screen, 2003, 10(4): 204-209. DOI:10.1258/096914103771773320 |
| [77] |
Chen THH, Chen CJ, Yen MF, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan[J]. Int J Cancer, 2002, 98(2): 257-261. DOI:10.1002/ijc.10122 |
| [78] |
Kuo YH, Lu SN, Chen CL, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis[J]. Eur J Cancer, 2010, 46(4): 744-751. DOI:10.1016/j.ejca.2009.12.018 |
| [79] |
Sarkar M, Stewart S, Yu A, et al. Hepatocellular carcinoma screening practices and impact on survival among hepatitis B-infected Asian Americans[J]. J Viral Hepat, 2012, 19(8): 594-600. DOI:10.1111/j.1365-2893.2011.01577.x |
| [80] |
Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C:results from the HALT-C Trial[J]. J Hepatol, 2005, 43(3): 434-441. DOI:10.1016/j.jhep.2005.03.019 |
| [81] |
Paul SB, Gulati MS, Sreenivas V, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma:value of clinical symptomatology, imaging and alpha-fetoprotein[J]. Oncology, 2007, 72 Suppl. 1: 117-123. DOI:10.1159/000111717 |
| [82] |
Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma:a systematic review[J]. Am J Gastroenterol, 2006, 101(3): 513-523. DOI:10.1111/j.1572-0241.2006.00467.x |
| [83] |
陈万青, 李霓, 石菊芳, 等. 中国城市癌症早诊早治项目进展[J]. 中国肿瘤, 2019, 28(1): 23-25. Chen WQ, Li N, Shi JF, et al. Progress of cancer screening program in urban China[J]. China Cancer, 2019, 28(1): 23-25. DOI:10.11735/j.issn.1004-0242.2019.01.A003 |
| [84] |
石菊芳, 代敏. 中国癌症筛查的卫生经济学评价[J]. 中华预防医学杂志, 2017, 51(2): 107-111. Shi JF, Dai M. Health economic evaluation of cancer screening in China[J]. Chin J Prev Med, 2017, 51(2): 107-111. DOI:10.3760/cma.j.issn.0253-9624.2017.02.002 |
| [85] |
Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis:incidence and risk factors[J]. Gastroenterology, 2004, 127 Suppl 5: 35-50. DOI:10.1053/j.gastro.2004.09.014 |
| [86] |
Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level:Results from the Global Burden of Disease Study 2015[J]. JAMA Oncol, 2017, 3(12): 1683-1691. DOI:10.1001/jamaoncol.2017.3055 |
| [87] |
Yao MJ, Zhao JM, Lu FM. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma[J]. Oncotarget, 2016, 7(4): 3702-3708. DOI:10.18632/oncotarget.6913 |
 2020, Vol. 41
2020, Vol. 41