文章信息
- 邬惟为, 冯永亮, 史晓红, 张萍, 王科科, 杨飞飞, 韩天碧, 王素萍.
- Wu Weiwei, Feng Yongliang, Shi Xiaohong, Zhang Ping, Wang Keke, Yang Feifei, Han Tianbi, Wang Suping.
- 脂多糖干预对军团菌肺炎作用的实验研究
- An animal experiment regarding the effect of lipopolysaccharide intervention program on Legionella pneumonia
- 中华流行病学杂志, 2019, 40(6): 682-685
- Chinese Journal of Epidemiology, 2019, 40(6): 682-685
- http://dx.doi.org/10.3760/cma.j.issn.0254-6450.2019.06.015
-
文章历史
收稿日期: 2019-01-25
军团菌病(legionnaires’ disease)是由嗜肺军团杆菌引起的以肺炎为主的全身性疾病,常伴多系统损害,呈世界范围分布[1]。军团菌是典型的革兰阴性细菌,细胞壁上的脂多糖是其主要致病成分。军团菌进入机体后,被天然免疫受体TLR4(Toll-like receptors 4)识别,引发机体炎性反应。如果宿主炎性细胞因子失控性表达,会引发严重的感染和多脏器损伤[2],这可能是许多军团菌肺炎病例预后较差的原因。脓毒血症病例预后较差的主要临床表现也是炎性细胞因子的异常表达,已有研究发现,脂多糖耐受会在脓毒血症病例体内发挥“保护效应”[3]。脂多糖耐受是机体在前期小剂量暴露脂多糖后,会对后续暴露致死性剂量脂多糖产生保护效应。本研究拟通过建立军团菌感染动物模型,观察大肠埃希菌O111:B4脂多糖早期干预对军团菌肺炎的影响,以期为防制军团菌肺炎及改善预后提供依据。
对象与方法1.研究对象:实验动物:C3H/HeN小鼠72只,雌雄各半,SPF级,6~8周龄,购自中国北京维通利华实验动物技术有限公司[许可证号:SCXK(京)2006-2009]。菌种:嗜肺军团菌血清1型(Legionella pneumophila subsp,ATCC 33152)购自美国标准菌种保藏所(American Type Culture Collection,ATCC),以1.2×107 cfu/ml浓度配制染菌菌悬液。脂多糖E.coli O111:B4,购自美国Sigma公司。
2.分组:将实验动物随机分组,设立脂多糖干预染菌组、脂多糖未干预染菌组和对照组,每组各设12、24和48 h 3个时间点组,每组8只,雌雄各半。
3.脂多糖干预及军团菌感染模型建立:脂多糖干预组小鼠,按每只100 ng的剂量[4-5],腹腔注射进行脂多糖干预,其余组注射生理盐水。干预24 h后,对脂多糖干预组及脂多糖未干预组小鼠进行染菌。腹腔注射50 μl的10%水合氯醛进行深麻醉后,采用气管注射50 μl军团菌悬液(1.2×107 cfu/ml)造模,对照组注射50 μl的无菌生理盐水[6]。
4.指标检测:实验小鼠分别于染菌后12、24、48 h,水合氯醛腹腔注射麻醉后,采集眼眶血于EDTA采血管中,分离外周血单个核细胞(PBMC),流式细胞仪检测TLR4。解剖小鼠,分离肺脏称重,计算脏器系数(小鼠肺脏重量/体重)。取左肺中叶0.02 g组织匀浆,采用ELISA法检测肿瘤坏死因子-α(TNF-α)和白细胞介素-1β(IL-1β)。
5.统计学分析:实验数据用(x ± s)来表示,采用χ2检验、析因方差分析进行组间比较,多组间两两比较采用LSD法,以P<0.05为差异有统计学意义。
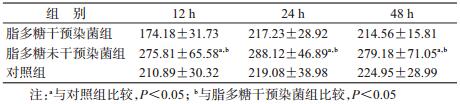
结果1.肺脏脏器系数比较:经析因分析,脂多糖干预染菌组、脂多糖未干预染菌组和对照组之间肺脏脏器系数差异有统计学意义(F=5.699,P=0.006),各时间点组间差异有统计学意义(F=10.648,P<0.001)。经LSD法两两比较,脂多糖未干预染菌组的肺脏脏器系数高于脂多糖干预染菌组和对照组(P<0.05),脂多糖干预染菌组与对照组间差异无统计学意义。见表 1。
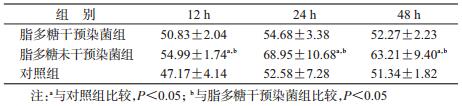
2. PBMC中TLR4蛋白表达:经析因分析,脂多糖干预染菌组、脂多糖未干预染菌组和对照组PBMC中TLR4蛋白水平表达差异有统计学意义(F=47.144,P<0.001),各个时间点差异有统计学意义(F=141.543,P<0.001),分组和时间点之间交互作用也有统计学意义(F=29.902,P<0.001)。经LSD法两两比较,脂多糖干预染菌组TLR4表达水平在12、24 h均高于对照组(P<0.05);脂多糖未干预染菌组TLR4表达水平在12 h高于对照组(P<0.05)。脂多糖干预染菌组与脂多糖未干预染菌组TLR4蛋白均在12 h达到高峰,且脂多糖干预染菌组TLR4水平高于脂多糖未干预染菌组(P<0.05);两染菌组TLR4水平随着染菌时间的延长而下降,在24、48 h两染菌组TLR4表达水平差异无统计学意义。见表 2。
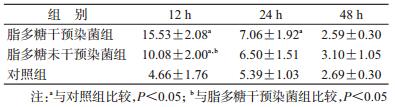
3.细胞因子TNF-α、IL-1β表达:脂多糖干预染菌组、脂多糖未干预染菌组、对照组之间TNF-α浓度差异有统计学意义(F=25.817,P<0.001),时间的主效应也有统计学意义(F=8.393,P<0.001),分组和时间点之间未发现交互作用(F=1.460,P<0.05)。经两两比较,脂多糖干预染菌组与对照组间差异无统计学意义,但脂多糖未干预染菌组各个时间点TNF-α的分泌水平均高于脂多糖干预染菌组和对照组(P<0.05)。见表 3。
脂多糖干预染菌组、脂多糖未干预染菌组、对照组之间IL-1β浓度差异有统计学意义(F=18.990,P<0.001),时间的主效应无统计学意义(F=1.323,P<0.05),分组和时间点之间也未发现交互作用(F=0.382,P>0.05)。经两两比较,脂多糖干预染菌组与对照组间差异无统计学意义,但脂多糖未干预染菌组各个时间点IL-1β的分泌水平均高于脂多糖干预染菌组和对照组(P<0.05)。见表 4。
军团菌病主要由军团菌引起,军团菌多存在于空调、冷却塔、喷泉和建筑粉尘中。随着城市化进程加快,军团菌病已成为全球范围内重要的公共卫生问题[1]。在非典型性肺炎中,军团菌肺炎是病情最重的一种,主要由军团菌脂多糖引发的失控性炎症反应和多器官功能衰竭影响预后。脂多糖耐受为改善军团菌病的预后提供了契机,目前已在脓毒血症病例体内发现,脂多糖耐受可对病例产生“保护效应”[3]。
为探讨脂多糖耐受对军团菌肺炎的影响,本研究通过建立军团菌感染动物模型,观察大肠埃希菌O111:B4脂多糖早期干预对军团菌肺炎的影响。结果显示,脂多糖早期小剂量干预后,感染军团菌,肺脏的脏器系数与对照组无显著差别,提示脂多糖耐受可能会减轻炎性反应。肺组织炎性细胞因子TNF-α、IL-1β结果也表明,脂多糖早期干预后,感染军团菌,炎性细胞因子TNF-α、IL-1β表达下降,与脏器系数结果趋势一致,提示脂多糖耐受可能会减轻军团菌感染后的炎症反应。脂多糖进入人体内,首先被TLR4识别,通过两条信号通路:MyD88依赖通路和MyD88非依赖通路,使机体产生大量细胞因子如TNF-α、IL-1、IL-6等,抵御感染并引起炎症反应[7]。但是,机体如前期接触少量脂多糖,再接触大量脂多糖或致死性剂量的脂多糖,会诱发机体脂多糖耐受,以保护机体免受过激炎症反应损害,这一现象最早在脓毒血症病例体内发现,并在不同动物模型中得以验证[8-10]。Natarajan等[11]利用小鼠构建脂多糖耐受模型时也发现,小鼠肺组织炎性细胞因子TNF-α表达显著下降。本研究结果提示,感染军团菌之前经小剂量脂多糖干预,可能会降低炎性细胞因子表达,减轻炎性反应。
军团菌是一种革兰阴性菌,主要被机体天然免疫受体TLR4识别,发挥免疫效应。本研究进一步对各组肺组织TLR4表达进行检测,结果显示,脂多糖干预组在12 h高于脂多糖未干预组,提示脂多糖干预可上调TLR4表达。目前,许多体外实验均证实,脂多糖可上调TLR4表达水平。Faure等[12]发现,人肠上皮细胞在大肠埃希菌脂多糖的刺激下,肠上皮细胞表面的TLR4表达水平提高,但TLR2无明显变化。An等[13]利用大肠埃希菌脂多糖刺激小鼠树突状细胞后,从基因水平发现TLR4 mRNA明显上调。同样,Visintin等[14]在使用大肠埃希菌脂多糖刺激诱导未成熟的树突状细胞(iDC)分化为成熟的树突状细胞(mDC)过程中,TLR4的表达水平上升。
本研究发现随着时间变化,TLR4表达水平下降,在24 h时脂多糖干预染菌组仍高于对照组,在48 h时3组间TLR4无明显差异。这一趋势与体外研究结果一致,An等[13]利用大肠埃希菌脂多糖刺激小鼠树突状细胞,TLR4 mRNA在1 h达到高峰后开始下降,在5 h时与对照组水平无显著差别。这可能是由于随着军团菌的清除,其模式识别受体TLR4表达开始下降。在48 h时,对照组也表现出TLR4表达水平下降,这可能由于在军团菌病造模时,气管有创注射引起小鼠轻微炎症,固有免疫被激活。但是,对照组无脂多糖干预或染菌,故TLR4表达一直处于较低水平。
另外,脂多糖干预染菌组的TLR4水平在12和24 h均高于脂多糖未干预染菌组,但下游细胞因子TNF-α与IL-1β表达水平却低于脂多糖未干预染菌组,这可能是脂多糖耐受的作用。现有研究表明,在发生脂多糖耐受后,TLR4- MyD88-NF-κB信号通路中的TLR4和MyD88因受到脂多糖刺激,其mRNA和蛋白表达水平均上升,但下游磷脂酰肌醇激酶通过下调蛋白激酶B表达,抑制IL-1受体相关激酶后续通路,故表现出下游炎性细胞因子如TNF-α与IL-1β表达水平的下降[15-16]。脂多糖早期干预,减少军团菌炎性细胞因子的释放,可能会改善军团菌肺炎的炎性症状。另外,虽然目前尚无脂多糖耐受对军团菌清除影响的相关研究,但在系统性细菌感染的动物模型研究中发现,脂多糖耐受可强化小鼠的细菌清除[17],机制尚需进一步研究。
综上所述,本研究通过建立军团菌感染动物模型,观察到早期小剂量脂多糖干预可能会减轻军团菌肺炎炎性症状,并上调天然免疫受体TLR4水平,同时防止机体出现过激炎症反应,改善预后。本研究存在局限性,仅对TLR4信号通路关键蛋白和候选炎性细胞因子TNF-α与IL-1β进行观察,未来尚需深入研究脂多糖干预对军团菌肺炎保护作用的内在机制,即TLR4-MyD88-NF-κB信号通路及其下游所有细胞因子的详细变化,以期为防治军团菌肺炎及改善预后提供依据。
利益冲突 所有作者均声明不存在利益冲突
| [1] |
Hofmann A, Beaulieu Y, Bernard F, et al. Fulminant legionellosis in two patients treated with infliximab for Crohn's disease:case series and literature review[J]. Can J Gastroenterol, 2016, 23(12): 829-833. DOI:10.1155/2009/836938 |
| [2] |
Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response[J]. Clin Immunol, 2009, 130(1): 7-15. DOI:10.1016/j.clim.2008.08.015 |
| [3] |
Cavaillon JM, Adib-Conquy M. Bench-to-bedside review:endotoxin tolerance as a model of leukocyte reprogramming in sepsis[J]. Crit Care, 2006, 10(5): 233. DOI:10.1186/cc5055 |
| [4] |
Eisenbarth SC, Piggott DA, Huleatt JW, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen[J]. J Exp Med, 2002, 196(12): 1645-1651. DOI:10.1084/jem.20021340 |
| [5] |
Kim YK, Oh SY, Jeon SG, et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma[J]. J Immunol, 2007, 178(8): 5375-5382. DOI:10.4049/jimmunol.178.8.5375 |
| [6] |
邬惟为, 王素萍, 张帆, 等. Toll样受体4在C3H/HeJ小鼠军团菌感染模型中作用[J]. 中国公共卫生, 2014, 30(3): 311-313. Wu WW, Wang SP, Zhang F, et al. Role of Toll-like receptor 4 in Legionella pneumophila infection in C3H/HeJ mouse model[J]. Chin J Public Health, 2014, 30(3): 311-313. DOI:10.11847/zgggws2014-30-03-18 |
| [7] |
Schmausser B, Andrulis M, Endrich S, et al. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells:an implication for interaction with Helicobacter pylori[J]. Int J Med Microbiol, 2005, 295(3): 179-185. DOI:10.1016/j.ijmm.2005.02.009 |
| [8] |
Del Fresno C, Garcia-Rio F, Gómez-Piña V, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes:demonstration in isolated monocytes from cystic fibrosis patients[J]. J Immunol, 2009, 182(10): 6494-6507. DOI:10.4049/jimmunol.0803350 |
| [9] |
Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS[J]. Micr Infect, 2002, 4(9): 903-914. DOI:10.1016/s1286-4579(02)01613-1 |
| [10] |
Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications[J]. Nature, 2007, 447(7147): 972-978. DOI:10.1038/nature05836 |
| [11] |
Natarajan S, Kim J, Remick DG. Acute pulmonary lipopolysaccharide tolerance decreases TNF-α without reducing neutrophil recruitment[J]. J Immunol, 2008, 181(12): 8402-8408. DOI:10.4049/jimmunol.181.12.8402 |
| [12] |
Faure E, Thomas L, Xu HL, et al. Bacterial lipopolysaccharide and IFN-γ induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells:role of NF-κB activation[J]. J Immunol, 2001, 166(3): 2018-2024. DOI:10.4049/jimmunol.166.3.2018 |
| [13] |
An HZ, Yu ZY, Zhang MH, et al. Involvement of ERK, p38 and NF-κB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells[J]. Immunology, 2010, 106(1): 38-45. DOI:10.1046/j.1365-2567.2002.01401.x |
| [14] |
Visintin A, Mazzoni A, Spitzer JH, et al. Regulation of Toll-like receptors in human monocytes and dendritic cells[J]. J Immunol, 2001, 166(1): 249-255. DOI:10.4049/jimmunol.166.1.249 |
| [15] |
Morris M, Li LW. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming[J]. Arch Immunol Ther Exp, 2012, 60(1): 13-18. DOI:10.1007/s00005-011-0155-9 |
| [16] |
Yan JY, Bai JN, Gao C, et al. Chronic unpredictable stress abrogates the endotoxin tolerance induced by repeated peripheral LPS challenge via the tlr4 signaling pathway[J]. Neurosci Lett, 2017, 645: 7-13. DOI:10.1016/j.neulet.2017.02.070 |
| [17] |
Wheeler DS, Lahni PM, Denenberg AG, et al. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis[J]. Shock, 2008, 30(3): 267-273. DOI:10.1097/shk.0b013e318162c190 |
 2019, Vol. 40
2019, Vol. 40