文章信息
- 施佳, 田勇, 雷媛, 康皓.
- Shi Jia, Tian Yong, Lei Yuan, Kang Hao.
- 母体孕期主动或被动吸烟与后代多指(趾)畸形的病例对照研究
- Active and passive maternal smoking during pregnancy and risk of having a child with polydactyly: a case-control study
- 中华流行病学杂志, 2018, 39(11): 1482-1485
- Chinese Journal of Epidemiology, 2018, 39(11): 1482-1485
- http://dx.doi.org/10.3760/cma.j.issn.0254-6450.2018.11.012
-
文章历史
收稿日期: 2018-03-27
2. 430030 武汉, 华中科技大学同济医学院附属同济医院呼吸内科
2. Department of Respiratory Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
多指(趾)畸形是最常见的先天性手部畸形之一[1-2],在新生儿中的发病率为0.03%~0.19%[3-5]。男孩的发病率高于女孩,近年来总体发病率呈增加趋势[1, 3]。虽然多指(趾)畸形可以在出生后进行手术矫正[6],但部分患儿可能需要进行多次矫正手术以达到预期的外观,对孩子的身心甚至整个家庭都有一定程度的负面影响。研究证明遗传因素与多指(趾)之间存在风险关系[7],如GLI家族锌指3 (GLI3)和基因IQCE均可致病[8-10]。然而,大多数多指(趾)畸形患者并没有家族病史,这促使我们研究环境中可能存在的危险因素。怀孕期间的不利因素会增加胎儿肢体异常的风险[11-17]。母亲孕期饮酒、接触可卡因、妊娠期糖尿病、从事具有暴露风险的职业均可能导致先天性出生缺陷[11, 17-20]。然而母亲孕期吸烟是否会增加后代肢体畸形尤其是多指(趾)畸形仍然存在争议。部分研究表明孕期主动吸烟会增加胎儿出生缺陷的发病率[18],但是母亲孕期的被动吸烟问题仍被忽视。本研究基于病例对照研究,探讨经多因素分层和控制混杂因素后母亲孕期主动或被动吸烟与后代多指(趾)畸形发病的关系,为预防胎儿先天性多指(趾)畸形提供科学依据。
对象与方法1.研究对象:选取2015年9月至2017年10月华中科技大学同济医学院附属同济医院骨科或小儿外科确诊为先天性多指(趾)畸形的住院患儿的母亲为病例组。诊断标准为临床确诊为先天性多指(趾)畸形,由≥2名具有≥5年临床工作经验的医师独立确诊患儿为多指(趾)畸形,并由影像学资料共同证实。对照组为连续性选取相同时间段因外伤就诊的患儿的母亲,以年龄相差不超过1岁进行1 : 2配对。剔除有任何慢性病史或家族病史和因记忆障碍或精神症状不能准确回答问题者。本研究通过华中科技大学伦理委员会批准,所有研究对象均签署了知情同意书。
2.研究方法:由经统一培训的2位调查员对患儿母亲进行一对一面对面调查,另1位调查员在问卷开始前对调查对象进行编号并记录其患儿是否为多指(趾)畸形,以降低询问过程中的主观偏倚。问卷调查员之间互相独立完成询问并对对方的结果不知情,最终调查结果由两份问卷对比后得出并剔除问卷填写不合格者。
问卷内容包括一般情况(孕龄、产前体重、身高、文化程度、户籍类型、职业、家庭人均月收入、患儿性别与出生体重)、母亲孕期情况(主动吸烟、被动吸烟、饮酒、孕期情绪、有效睡眠时间、阴道出血、发热、感冒、先兆流产、妊娠剧吐、贫血、化学物质、射线或噪音接触以及孕期合并症)和家族病史(母亲是否有慢性病史以及患儿父母双方是否有家族多指(趾)病史或近亲婚配史)。主动吸烟定义为吸烟行为天数≥3 d/周[21],被动吸烟定义为≥30 min/周[22];饮酒定义为≥1次/周[23]。
3.统计学分析:采用EpiData 3.1软件建立数据库并进行数据双录入,R语言和易侕软件进行统计学分析。病例组和对照组的均值和比例均为正态分布的数值变量。t检验用于分析人口特征的差异,χ2检验和OR值检验风险因素与多指(趾)畸形的关系。在对混杂变量进行调整后,通过多元logistic回归分析评价母体孕期吸烟与后代多指(趾)畸形的关系。以P<0.05为两组间差异有统计学意义。
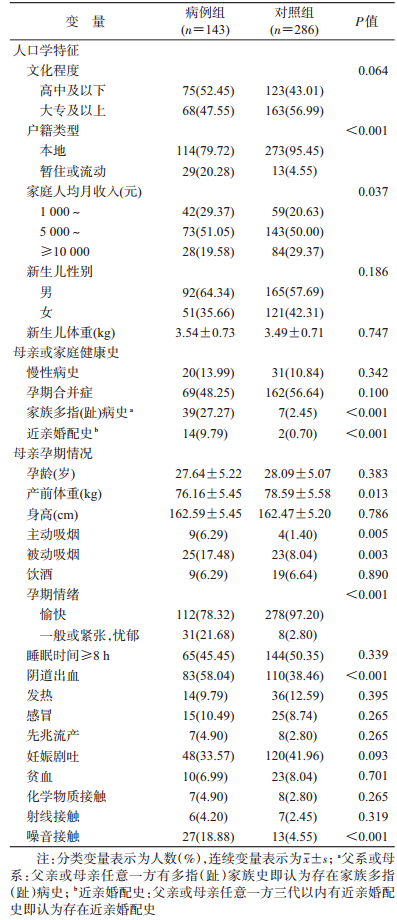
结果1.一般情况:本次研究共调查2015年9月至2017年10月于同济医院骨科或小儿外科住院拟行多指(趾)截指(趾)矫形术的160例患儿的母亲。经过剔除标准筛选,共纳入143名患儿母亲。经过患儿年龄匹配,对照组共纳入286名其他疾病患儿的母亲。两组在家庭人均月收入、家庭户籍类型、家族多指(趾)病史(父系或母系)、近亲婚配史、母亲孕期情绪状态、孕期是否吸烟(主动或被动)、阴道出血、产前体重和孕期是否接触噪音等变量间差异有统计学意义(P<0.05)(表 1)。
2.影响因素分析:
(1) 单因素分析:家庭人均月收入、家族多指(趾)病史、近亲婚配史、主动或被动吸烟、孕期阴道出血和噪音接触均与后代是否发生多指(趾)畸形显著相关(表 2)。
(2) 多元logistic回归分析:通过交互作用分析和协变量筛选进一步检测母亲孕期吸烟与后代多指(趾)畸形的关系。单因素分析时,母亲孕期吸烟(主动吸烟:OR=4.74,95%CI:1.43~15.65,P=0.011;被动吸烟:OR=2.42,95%CI:1.32~4.44,P=0.004)与后代多指(趾)畸形的关系是独立存在的(表 2);调整混杂变量[母亲孕期情绪、户籍类型、家庭人均月收入、家族多指(趾)病史、近亲婚配史、母亲孕期情绪、阴道出血]后,这种关联依旧存在(主动吸烟:aOR=7.27,95%CI:1.72~30.72,P=0.007;被动吸烟:aOR=2.41,95%CI:1.11~5.23,P=0.026)。
(3) 分层分析:按新生儿性别、孕龄、家族多指(趾)病史、文化程度、家庭人均月收入情况进行分层,调整户籍类型、近亲婚配史、母亲孕期情绪、阴道出血后,母亲孕期吸烟与后代多指(趾)畸形的关系稳定存在(表 3)。
本研究结果表明母亲孕期主动或被动吸烟与新生儿多指(趾)畸形的风险增加显著相关。调整混杂因素后,其相关性仍然显著存在(主动吸烟:aOR=7.27,95%CI:1.72~30.72,P=0.007;被动吸烟:aOR=2.41,95%CI:1.11~5.23,P=0.026)。怀孕期间主动或被动吸烟会造成胎儿DNA的细胞遗传损害,导致染色体不稳定,这种损害与其他可能导致畸形的环境或化学诱变物质具有协同作用[24-25]。
过往研究发现母亲孕期主动吸烟会增加多种出生缺陷的发病风险[18, 26],还会显著降低胎儿出生体重并增加早产、死产风险[27-28];被动吸烟也可能会造成胎儿出现严重的出生缺陷[29]。母亲孕期吸烟不仅影响妊娠结局,在后代长期的认知能力(低学业成就和一般认知能力等)和外化行为(刑事定罪和药物滥用等)上也有显著的不良影响[30-31]。然而,这些研究未将多指(趾)畸形单独列出,而是将其纳入先天性肢端畸形中进行笼统研究,这势必增加了母亲孕期吸烟与后代多指(趾)畸形关系中的不确定性。而本研究将多指(趾)畸形作为独立结局进行研究,单独研究分析了母亲孕期主动或被动吸烟与后代多指(趾)畸形的相关性。
本研究存在局限性。首先,本研究采用问卷调查形式的病例研究,容易出现回忆偏倚;另外,本研究样本量较小,仍需要大样本量的队列研究对结果加以验证。此外,本研究采用回顾性病例对照分析方法,调查对象无法准确的回忆孕期吸烟或被动吸烟的具体数量,但我们在问卷设计中对主动吸烟和被动吸烟等变量进行了严格定义,在一定程度上可减少偏倚。
综上所述,孕妇在孕期主动或被动吸烟是胎儿多指(趾)畸形的危险因素,提示二手烟暴露也可能影响到新生儿健康。相关部门应努力提高对孕妇吸烟,尤其是更容易被忽略的二手烟危害的认识,加大关于烟草使用带来的不良后果的宣传,鼓励孕妇及其家人减少吸烟或戒烟,以减少孕期烟草暴露。
利益冲突 无
| [1] |
Comer GC, Potter M, Ladd AL. Polydactyly of the hand[J]. J Am Acad Orthop Surgeons, 2018, 26(3): 75-82. DOI:10.5435/JAAOS-D-16-00139 |
| [2] |
Malik S. Polydactyly:phenotypes, genetics and classification[J]. Clin Genet, 2014, 85(3): 203-212. DOI:10.1111/cge.12276 |
| [3] |
Xiang Y, Bian JX, Wang ZG, et al. Clinical study of 459 polydactyly cases in China, 2010 to 2014[J]. Congen Anomal, 2016, 56(5): 226-232. DOI:10.1111/cga.12163 |
| [4] |
Yeshayahu Y, Sagi A, Silberstein E. Polydactyly in the multiethnic 'Negev' population at southern Israel[J]. J Pediatr Orthoped B, 2014, 23(3): 274-276. DOI:10.1097/BPB.0000000000000039 |
| [5] |
Farrugia MC, Calleja-Agius J. Polydactyly:A review[J]. Neon Netw, 2016, 35(3): 135-142. DOI:10.1891/0730-0832.35.3.135 |
| [6] |
Singer G, Thein S, Kraus T, et al. Ulnar polydactyly-an analysis of appearance and postoperative outcome[J]. J Pediatr Surg, 2014, 49(3): 474-476. DOI:10.1016/j.jpedsurg.2013.06.029 |
| [7] |
Biesecker LG. Polydactyly:How many disorders and how many genes? 2010 update[J]. Dev Dyn, 2011, 240(5): 931-942. DOI:10.1002/dvdy.22609 |
| [8] |
Xiang Y, Jiang LM, Wang B, et al. Mutational screening of GLI3, SHH, preZRS, and ZRS in 102 Chinese children with nonsyndromic polydactyly[J]. Dev Dyn, 2017, 246(5): 392-402. DOI:10.1002/dvdy.24488 |
| [9] |
Crapster JA, Hudgins L, Chen JK, et al. A novel missense variant in the GLI3 zinc finger domain in a family with digital anomalies[J]. Am J Med Genet A, 2017, 173(12): 3221-3225. DOI:10.1002/ajmg.a.38415 |
| [10] |
Umair M, Shah K, Alhaddad B, et al. Exome sequencing revealed a splice site variant in the IQCE gene underlying post-axial polydactyly type A restricted to lower limb[J]. Eur J Human Genet, 2017, 25(8): 960-965. DOI:10.1038/ejhg.2017.83 |
| [11] |
Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects:a systematic review based on 173687 malformed cases and 11.7 million controls[J]. Human Reprod Update, 2011, 17(5): 589-604. DOI:10.1093/humupd/dmr022 |
| [12] |
Parazzini F, Cipriani S, Bulfoni G, et al. The risk of birth defects after assisted reproduction[J]. J Assist Reprod Genet, 2015, 32(3): 379-385. DOI:10.1007/s10815-014-0398-6 |
| [13] |
Karaman Mİ, Gürdal M, Öztürk M, et al. Maternal exposure to diethylene glycol monomethyl ether:A possible role in the etiology of retrocaval ureter[J]. J Pediatr Surg, 2002, 37(8): 1-2. DOI:10.1053/jpsu.2002.34500 |
| [14] |
Rizzi M, Cravello B, Renò F. Textile industry manufacturing by-products induce human melanoma cell proliferation via ERK1/2 activation[J]. Cell Prolifer, 2014, 47(6): 578-586. DOI:10.1111/cpr.12132 |
| [15] |
Duong HT, Hoyt AT, Carmichael SL, et al. Is maternal parity an independent risk factor for birth defect[J]. Birth Defects Res A:Clin Mol Teratol, 2012, 94(4): 230-236. DOI:10.1002/bdra.22889 |
| [16] |
Bayrampour H, McDonald S, Tough S. Risk factors of transient and persistent anxiety during pregnancy[J]. Midwifery, 2015, 31(6): 582-589. DOI:10.1016/j.midw.2015.02.009 |
| [17] |
Engel LS, O'Meara ES, Schwartz SM. Maternal occupation in agriculture and risk of limb defects in Washington State, 1980-1993[J]. Scand J Work, Environ Health, 2000, 26(3): 193-198. DOI:10.5271/sjweh.531 |
| [18] |
Man LX, Chang B. Maternal cigarette smoking during pregnancy increases the risk of having a child with a congenital digital anomaly[J]. Plastic Reconstr Surg, 2006, 117(1): 301-308. DOI:10.1097/01.prs.0000194904.81981.71 |
| [19] |
Vinceti M, Malagoli C, Rothman KJ, et al. Risk of birth defects associated with maternal pregestational diabetes[J]. Eur J Epidemiol, 2014, 29(6): 411-418. DOI:10.1007/s10654-014-9913-4 |
| [20] |
Almberg KS, Turyk M, Jones RM, et al. A study of adverse birth outcomes and agricultural land use practices in Missouri[J]. Environ Res, 2014, 134: 420-426. DOI:10.1016/j.envres.2014.06.016 |
| [21] |
Bjørnholt SM, Leite M, Albieri V, et al. Maternal smoking during pregnancy and risk of stillbirth:results from a nationwide Danish register-based cohort study[J]. Acta Obstetr Gynecol Scand, 2016, 95(11): 1305-1312. DOI:10.1111/aogs.13011 |
| [22] |
Qiu J, He X, Cui H, et al. Passive smoking and preterm birth in urban China[J]. Am J Epidemiol, 2014, 180(1): 94-102. DOI:10.1093/aje/kwu092 |
| [23] |
Smith L, Savory J, Couves J, et al. Alcohol consumption during pregnancy:cross-sectional survey[J]. Midwifery, 2014, 30(12): 1173-1178. DOI:10.1016/j.midw.2014.04.002 |
| [24] |
Kuja-Halkola R, D'Onofrio BM, Larsson H, et al. Maternal smoking during pregnancy and adverse outcomes in offspring:genetic and environmental sources of covariance[J]. Behav Genet, 2014, 44(5): 456-467. DOI:10.1007/s10519-014-9668-4 |
| [25] |
Kareli D, Pouliliou S, Nikas I, et al. Effect of maternal smoking during pregnancy on fetus:a cytogenetic perspective[J]. J Maternal-Fetal Neonat Med, 2014, 27(2): 127-131. DOI:10.3109/14767058.2013.806897 |
| [26] |
Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects:validity of birth certificate data for effect estimation[J]. Public Health Rep, 2001, 116(4): 327-335. DOI:10.1093/phr/116.4.327 |
| [27] |
Andriani H, Kuo HW. Adverse effects of parental smoking during pregnancy in urban and rural areas[J]. BMC Pregnancy Childbirth, 2014, 14: 414. DOI:10.1186/s12884-014-0414-y |
| [28] |
Kitsantas P, Christopher KE. Smoking and respiratory conditions in pregnancy:associations with adverse pregnancy outcomes[J]. Southern Med J, 2013, 106(5): 310-315. DOI:10.1097/SMJ.0b013e318290c6e8 |
| [29] |
Wang M, Wang ZP, Zhang M, et al. Maternal passive smoking during pregnancy and neural tube defects in offspring:a Meta-analysis[J]. Arch Gynecol Obstetr, 2014, 289(3): 513-521. DOI:10.1007/s00404-013-2997-3 |
| [30] |
Murphy DJ, Dunney C, Mullally A, et al. Population-based study of smoking behaviour throughout pregnancy and adverse perinatal outcomes[J]. Int J Environ Res Public Health, 2013, 10(9): 3855-3867. DOI:10.3390/ijerph10093855 |
| [31] |
Bertani AL, Garcia T, Tanni SE, et al. Preventing smoking during pregnancy:the importance of maternal knowledge of the health hazards and of the treatment options available[J]. J Brasil Pneumol, 2015, 41(2): 175-181. DOI:10.1590/S1806-37132015000004482 |
 2018, Vol. 39
2018, Vol. 39