文章信息
- 冯录召, 彭质斌, 王大燕, 杨鹏, 杨娟, 张延炀, 陈健, 姜世强, 徐莉立, 康敏, 陈涛, 郑亚明, 郑建东, 秦颖, 赵梦娇, 谭亚运, 李中杰, 冯子健.
- Feng Luzhao, Peng Zhibin, Wang Dayan, Yang Peng, Yang Juan, Zhang Yanyang, Chen Jian, Jiang Shiqiang, Xu Lili, Kang Min, Chen Tao, Zheng Yaming, Zheng Jiandong, Qin Ying, Zhao Mengjiao, Tan Yayun, Li Zhongjie, Feng Zijian.
- 中国流感疫苗预防接种技术指南(2018-2019)
- Technical guidelines for seasonal influenza vaccination in China, 2018-2019
- 中华流行病学杂志, 2018, 39(11): 1413-1425
- Chinese Journal of Epidemiology, 2018, 39(11): 1413-1425
- http://dx.doi.org/10.3760/cma.j.issn.0254-6450.2018.11.001
-
文章历史
收稿日期: 2018-10-10
2. 102206 北京, 中国疾病预防控制中心病毒病预防控制所;
3. 100013 北京市疾病预防控制中心传染病地方病控制所;
4. 200032 上海, 复旦大学公共卫生学院;
5. 450016 郑州, 河南省疾病预防控制中心免疫预防与规划所;
6. 200336 上海市疾病预防控制中心传染病防治所;
7. 518055 深圳市南山区疾病预防控制中心免疫规划科;
8. 810007 西宁, 青海省疾病预防控制中心传染病预防控制所;
9. 511430 广州, 广东省疾病预防控制中心传染病预防控制所;
10. 250021 济南市疾病预防控制中心应急管理科;
11. 215004 苏州市疾病预防控制中心传染病防治科;
12. 102206 北京, 中国疾病预防控制中心
2. National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3. Infectious Disease & Endemic Disease Control, Beijing Center for Disease Prevention and Control, Beijing 100013, China;
4. School of Public Health, Fudan University, Shanghai 200032, China;
5. Institute for Immunization Prevention and Planning, Henan Provincial Center for Disease Control and Prevention, Zhengzhou 450016, China;
6. Institute for Communicable Disease Control and Prevention, Shanghai Municipal Center for Disease Control and Prevention, Shanghai 200336, China;
7. Department for Immunization Prevention and Planning, Nanshan District Center for Disease Control and Prevention, Shenzhen 518055;
8. Institute for Communicable Disease Control and Prevention, Qinghai Center for Disease Prevention and Control, Xining 810007, China;
9. Institute for Communicable Disease Control and Prevention, Guangdong Provincial Center for Disease Control and Prevention, Guangzhou 511430, China;
10. Department for Emergency Management, Jinan Center for Disease Control and Prevention, Jinan 250021, China;
11. Department for Communicable Disease Control and Prevention, Suzhou Center for Disease Control and Prevention, Suzhou 215004, China;
12. Chinese Center for Disease Control and Prevention, Beijing 102206, China
流感是由流感病毒引起的对人类健康危害较重的呼吸道传染病,其抗原性易变,传播迅速,每年可引起季节性流行,在学校、托幼机构和养老院等人群聚集的场所可发生暴发疫情。对孕妇、婴幼儿、老年人和慢性病患者等高危人群的危害尤为严重。接种流感疫苗是预防流感的最有效手段。2014年,中国CDC制定了《中国季节性流感疫苗应用技术指南(2014-2015)》(2014年版指南),为我国流感疫苗应用提供了技术指导。几年来,国内外陆续发表了新的研究证据,而我国也于2018年批准上市了四价流感灭活疫苗。为更好地指导我国流感预防控制和疫苗应用工作,中国CDC国家免疫规划技术工作组流感疫苗工作组综合国内外最新研究进展,在2014年版指南的基础上进行了更新和修订,形成了《中国流感疫苗预防接种技术指南(2018-2019)》。
一、病原学基础、临床特点和实验室诊断流感病毒属于正粘病毒科,是单股、负链、分节段的RNA病毒,根据病毒核蛋白和基质蛋白分为甲、乙、丙、丁(或A、B、C、D)4型[1]。甲型流感病毒根据病毒表面的血凝素(hemagglutinin,HA)和神经氨酸酶(neuraminidase,NA)的蛋白结构和基因特性,可分为多种亚型,目前已发现的18个HA(H1~18)和11个NA(N1~11)亚型[2]。甲型流感病毒除感染人外,在动物中也广泛存在,可感染禽类、猪、马、海豹以及鲸鱼和水貂等。乙型流感分为Victoria和Yamagata系,可在人群中循环传播,近期研究发现海豹也可被乙型流感病毒感染。丙型流感病毒感染人、犬和猪,但仅导致上呼吸道感染的散发病例[3]。丁型流感病毒主要感染猪、牛等动物,尚未发现人感染丁型流感病毒的病例[4-6]。目前,引起流感季节性流行的流感病毒主要是甲型流感病毒的H1N1、H3N2亚型及乙型流感病毒的Victoria和Yamagata系。
流感一般表现为急性起病、发热(部分病例可出现高热,达39~40 ℃),伴畏寒、寒战、头痛、肌肉、关节酸痛、极度乏力、食欲减退等全身症状,常有咽痛、咳嗽,可有鼻塞、流涕、胸骨后不适、颜面潮红,结膜轻度充血,也可有呕吐、腹泻等症状[7]。轻症流感常与普通感冒表现相似,但其发热和全身症状更明显。重症病例可出现病毒性肺炎、继发细菌性肺炎、急性呼吸窘迫综合征、休克、弥漫性血管内凝血、心血管和神经系统等肺外表现及多种并发症[7-8]。流感的症状是临床常规诊断和治疗的主要依据。但由于流感的症状、体征缺乏特异性,易与普通感冒和其他上呼吸道感染相混淆[9],流感确诊有赖于实验室诊断,检测方法包括病毒核酸检测、病毒分离培养、抗原检测和血清学检测[10]。
二、流行病学1.传染源、传播方式及潜伏期:流感患者和隐性感染者是季节性流感的主要传染源,主要通过其呼吸道分泌物的飞沫传播,也可以通过口腔、鼻腔、眼睛等黏膜直接或间接接触传播[11-12]。常见潜伏期为1~4 d(平均2 d),从潜伏期末到发病的急性期都有传染性。一般感染者在临床症状出现前24~48 h即可排出病毒,排毒量在感染后0.5~1 d显著增加,在发病后24 h内达到高峰[13]。成年人和较大年龄儿童一般持续排毒3~8 d(平均5 d),患者感染不同毒株的排毒时间也会有差异。住院成年人患者可在发病后持续一周或更长的时间排毒,排毒量也更大[3]。低龄儿童发病时的排毒量与成年人相同,但排毒量下降更慢,排毒时间更长[14]。与成年人相比,婴幼儿病例中长期排毒更常见(1~3周)。老年人和HIV感染者等免疫功能低下或缺陷人群的病毒清除能力更差,排毒时间更长[13, 15]。
2.流感在我国的流行特点和季节性:流感在每年冬春季周循环出现的季节性特征在温带地区已有广泛研究,但在热带和亚热带地区的季节性及其驱动因素尚未解决[16-18]。越来越多研究表明,热带地区尤其在亚洲,流感的季节性呈高度多样化,既有半年或全年周期性流行,也有全年循环流行[17-20]。2013年,一项针对我国不同区域流感季节性的研究显示[21],我国甲型流感的年度周期性随纬度增加而增强,且呈多样化的空间模式和季节性特征:北纬33°以北的北方省份,呈冬季流行模式,每年1-2月出现单一年度高峰;北纬27°以南的最南方省份,在每年4-6月出现单一年度高峰;两者之间的中纬度地区,呈现每年1-2月和6-8月的双周期高峰。而乙型流感在我国大部分地区呈单一冬季高发形式。2018年一项研究对我国2005-2016年度乙型流感流行特征进行了系统分析发现[22],我国乙型流感的流行强度整体低于甲型流感;但在部分地区和年份,乙型流感的流行强度高于甲型流感,且乙型Yamagata系和Victoria系交替占优,以冬、春季流行为主,不同系的流行强度在各年间存在差异。
3.疾病负担:
(1)健康负担:
① 全人群:全人群对流感普遍易感。对全球32个流感疫苗接种随机对照队列中未接种疫苗人群的流感罹患率统计结果显示,有症状流感在成年人中的罹患率为4.4%(95%CI:3.0%~6.3%),>65岁人群为7.2%(95%CI:4.3%~12.0%);所有流感(包括无症状感染)在成年人中的罹患率为10.7%(95%CI:4.5%~23.2%)[23]。全球1970-2009年19个流行季未接种流感疫苗的成年人的年均流感罹患率为3.5%(95%CI:2.3%~4.6%)[24]。流感在全球每年可导致29万~65万人死亡[25]。在中国北方和南方城市,流感相关的呼吸和循环系统疾病超额死亡率分别为年均12.4例/10万人年和8.8例/10万人年[26]。1998- 2013年中国香港地区流感相关住院和死亡研究显示,流感相关呼吸系统疾病平均超额死亡6.27例/10万人年,占全部死亡病例的1.4%,平均超额住院184例/10万人年[27]。流感相关住院集中在0~5岁儿童和≥65岁老年人,而超额死亡集中在≥65岁老年人。
② 慢性基础性疾病患者:与同龄健康成年人相比,慢性基础性疾病患者感染流感病毒后,更易出现严重疾病或死亡,其流感相关住院率和超额死亡率更高。全球流感住院监测网络分析发现,北半球2013-2014年流感季,40%的流感相关住院病例患有慢性基础性疾病;对于大多慢性基础性疾病而言,甲型H3N2、H1N1亚型和乙型Yamagata系所致重症流感的风险无显著差异[28]。我国2011-2013年的住院严重急性呼吸道感染病例(severe acute respiratory infection,SARI)哨点监测数据显示,重症流感病例中37%患者患有慢性基础性疾病,其中心血管疾病(21.5%)、COPD(7.7%)和糖尿病(7.4%)最为常见[29]。与健康人群相比,慢性基础性疾病患者流感相关死亡率明显增高。综述研究发现,流感流行季节COPD患者甲型流感相关超额病死率超过30%,明显高于健康人群(≤0.1%)[30]。
③ 孕妇:流感对孕妇的健康危害比较严重。怀孕后,由于机体免疫和生理上的变化,孕妇感染流感病毒后易出现呼吸系统、心血管系统和其他器官的并发症[31]。流感季中,孕妇比未怀孕的育龄妇女更容易发生呼吸系统疾病相关的住院(RR=4.3,95%CI:1.96~9.41)[32]。孕妇流感死亡负担研究显示,孕晚期孕妇流感相关死亡率最高(约为3.1例/100万活产)[33];与未怀孕的健康育龄妇女相比,孕妇出现严重疾病的风险增加至3.3倍,孕中期(6.1倍)和孕晚期(7.6倍)出现严重疾病的风险进一步增加[34]。孕妇患流感还可对胎儿和新生儿产生影响,出现死产、婴儿死亡、早产和出生低体重等[35-37]。
④ 儿童:每年流感流行季节,儿童流感罹患率约为20%~30%[23, 38];在某些高流行季节,儿童流感年感染率可高达50%左右[39-40]。<5岁儿童感染流感后并发重症疾病的风险较高,流感相关SARI住院率可达2 021/10万人年至2 349/10万人年;其中,6~11月龄婴儿住院率最高(3 603/10万人年至3 805/10万人年)[41]。儿童感染流感可导致死亡,且患基础性疾病的儿童死亡风险显著高于健康儿童,但也有将近半数的死亡病例发生在健康儿童[38],每年全球约有9 243~105 690名<5岁儿童死于流感相关呼吸道疾病[25]。
⑤ 学生:学校作为封闭的人群密集场所,容易造成流感病毒的传播[42-43]。我国每年报告的流感暴发疫情中,>90%发生在学校和托幼机构。与其他人群相比,学龄儿童的流感感染率最高[44-45],且学龄儿童在学校、家庭和社区的流感传播中发挥重要的作用,流感流行还可引起大量学龄儿童缺课和父母缺勤[46-47]。
⑥ 医务人员:医务人员在日常诊疗活动中接触流感患者的机会较多,因而感染流感病毒的风险高于普通人群。未接种流感疫苗的医务人员每季节实验室确诊的流感发病率为18.7%(95%CI:15.8%~22.1%),是健康成年人的3.4倍(95%CI:1.2~5.7)[48]。中国香港地区2009年甲型H1N1流行期间,2.6%的医务人员确诊感染,其中护理人员占53.4%[49]。医务人员感染流感病毒可增加院内感染的风险。在感染流感病毒的医务人员中,35%为无症状感染者[50],>75%的医务人员在出现流感样症状后仍继续工作[51-52],从而导致流感的院内传播。
⑦ 老年人:流感感染是老年人的重要死因。我国流感超额死亡研究显示,≥65岁老年人流感相关的呼吸和循环系统疾病、全死因超额死亡率分别为64/10万~147/10万、75/10万~186/10万[53-55],与新加坡[54, 56]、葡萄牙[57]、美国[58]等发达国家接近。与其他年龄组相比,流感相关死亡风险在老年人最高,≥65岁老年人流感相关超额死亡率远高于0~64岁组,84%~95%的流感相关超额死亡发生在≥65岁老年人[24, 53-55]。流感还可导致老年人出现相当高的住院负担。2010-2012年研究发现,≥65岁老年人中确诊流感导致的SARI住院率为89/10万至141/10万[59];在不同的年龄组中,≥60岁年龄组流感确诊病例的住院率最高(16%)[60]。此外,养老院、疗养院等老年人聚集机构容易出现流感疫情暴发。
(2)经济负担和健康效用:我国流感门诊病例的直接医疗成本为156~595元/人,间接成本为198~366元/人[47, 61-63]。流感住院病例的经济负担约为门诊病例的10倍。流感感染还可明显影响患者的生命质量。>60%的流感门诊和住院病例报告具有疼痛/不适和焦虑/沮丧。患流感期间,门诊和住院病例的健康效用值(health utility)分别为0.614 2和0.585 1,损失的质量调整生命天(quality adjusted life days,QALD)为1.62和3.51 d[64];而有慢性基础性疾病的流感患者其门诊和住院费均高于无基础性疾病的流感患者(门诊:186美元比146美元;住院:1 800美元比1 189美元)[63],同时,具有基础性疾病的患者其健康相关生存质量显著低于无基础性疾病者[64]。流感同样会造成成年人群生产力的下降。未接种流感疫苗的医务人员年平均每人因流感样病例而缺勤1.75 d[65],儿童流感病例的缺课天数和家长缺勤天数分别为1.3 d和1.4 d[66]。
4.流感的治疗预防措施:每年接种流感疫苗是预防流感最有效的手段,可以显著降低接种者罹患流感和发生严重并发症的风险。奥司他韦、扎那米韦、帕拉米韦等神经氨酸酶抑制剂是甲型和乙型流感的有效治疗药物,早期尤其是发病48 h之内应用抗流感病毒药物能显著降低流感重症和死亡的发生率。抗病毒药物应在医生的指导下使用。使用奥司他韦、扎那米韦等药物预防不能代替疫苗接种,只能作为没有接种疫苗或接种疫苗后尚未获得免疫能力的重症流感高危人群的紧急临时预防措施。
保持良好的个人卫生习惯是预防流感等呼吸道传染病的重要手段。除了勤洗手以外,在流感流行季节应尽量避免去人群聚集场所,避免接触呼吸道感染患者。出现流感样症状后,要保持良好的呼吸道卫生习惯,咳嗽或打喷嚏时,用纸巾、毛巾等遮住口鼻;咳嗽或打喷嚏后洗手,尽量避免触摸眼睛、鼻或口。家庭成员出现流感患者时,要尽量避免相互接触,尤其是家中有老年人与慢性病患者时。当家长带有流感症状的患儿去医院就诊时,应同时做好患儿及自身的防护(如戴口罩),避免交叉感染。学校、托幼机构等集体单位中出现流感样病例时,患者应居家休息,减少疾病传播。
三、流感疫苗1.国内外上市的流感疫苗:目前国际上已经上市的流感疫苗有灭活流感疫苗(inactivated influenza vaccine,IIV)、流感减毒活疫苗(live attenuated influenza vaccine,LAIV)和重组疫苗(recombinant influenza vaccines,RIV)。IIV包括三价(IIV3)和四价(IIV4)两种类型,IIV3组份含有甲型H3N2、H1N1亚型与乙型毒株的一个系,IIV4组份含有甲型H3N2、H1N1亚型和乙型Victoria系、Yamagata系。近年来,国外上市了皮内接种的IIV、高剂量IIV、佐剂疫苗等。我国批准上市的为IIV3和IIV4,包括裂解疫苗和亚单位疫苗。截至2018年9月,2018-2019年度有6家厂家供应IIV,包括裂解疫苗和亚单位疫苗。
2. IIV3和IIV4接种后的免疫反应、免疫持久性:欧盟药品评价局和美国食品药品管理局的标准要求流感疫苗接种后:①血凝素抑制(hemagglutination inhibition,HI)抗体≥1 : 40;②血清阳转率,即免疫接种前HI抗体<1 : 10,免疫后HI抗体≥1 : 40,或免疫接种前HI抗体≥1 : 10,免疫接种后HI抗体几何平均滴度(geometric mean titers,GMT)增长≥4倍。人体对感染流感病毒或接种流感疫苗后获得的免疫力会随时间衰减,衰减程度与年龄和身体状况、疫苗抗原等因素有关。临床试验的证据提示,接种IIV对抗原类似毒株的保护作用可维持6~8个月[67]。接种后1年,血清抗体水平显著降低,但部分毒株的保护作用持续时间可更长。为匹配不断变异的流感病毒,WHO在多数季节推荐的流感疫苗组份会更新一个或多个毒株,疫苗毒株与前一季节完全相同的情况也存在。鉴于多数接种者抗体滴度已显著下降[68-72],为保证接种人群得到最大程度的保护,建议无论前一季节是否接种,在当年流感季节来临前仍需接种流感疫苗。
中国2018-2019年度可供应四价流感裂解疫苗,根据其说明书,接种四价流感裂解疫苗后,甲型H1N1、H3N2亚型和乙型Yamagata、Victoria系的HI抗体阳转率分别为78.5%、53.3%、78.3%和62.9%,HI抗体GMT平均增长倍数分别为12.0、4.0、7.9和5.2,血清抗体保护率分别为87.7%、98.7%、93.6%和77.2%。3项指标均达到欧盟药品评价局和美国食品药品管理局的标准,提示该疫苗具有较好的免疫原性。
3. IIV3和IIV4的免疫原性、效力和效果:免疫原性是指抗原能够刺激机体形成特异抗体或致敏淋巴细胞的能力,评价指标主要为病毒株特异性HI抗体水平和血清抗体阳转率,评价结果会受接种者年龄、免疫功能和接种前抗体水平的影响。疫苗的效力通常是指其在上市前随机对照试验(randomized controlled trial,RCT)中理想条件下的有效性;疫苗的效果则指其在人群中实际应用的有效性。评价流感疫苗效力和效果的结局指标主要包括血清抗体水平和阳转率、实验室确诊流感、急性呼吸道疾病或流感样病例就诊、流感和肺炎相关住院或死亡等。
(1)全人群:IIV在健康成年人中免疫原性良好。在健康成年人中,随机对照试验估计,IIV约可预防59%(95%CI:51%~66%)的实验室确诊流感[71-72]。队列研究结果显示,IIV对我国成年人群流感样疾病的预防效果约为42%~47%[72-73]。IIV对不同型别和亚型的流感的预防效果有明显差异,其中乙型流感为54%(95%CI:46%~61%),甲型H1N1 pdm09亚型(2009年及以后)为61%(95%CI:57%~65%),甲型H1N1亚型(2009年之前)为67%(95%CI:29%~85%),甲型H3N2亚型为33%(95%CI:26%~39%)[74]。在≥18岁成年人中,IIV4与IIV3在相同疫苗株的血清保护率和抗体阳转率方面无显著性差异,IIV4中增加的乙型流感系的抗体保护率和抗体阳转率明显高于IIV3[75-76]。
(2)孕妇:妊娠期接种IIV,既可保护孕妇,也可通过胎传抗体保护其新生儿免于罹患流感[77]。接种流感疫苗的孕妇的发热呼吸道疾病患病率降低36%(95%CI:4%~57%);其生产的婴儿出生6个月内实验室确诊流感感染减少63%(95%CI:5%~85%),发热呼吸道疾病患病率降低29%(95%CI:7%~46%)[78]。
(3)儿童:>6月龄儿童按推荐的免疫程序接种IIV3后可产生对流感病毒感染的保护作用。IIV3对6~59月龄的儿童的疫苗保护效果约为40%~80%、6~35月龄的保护效果约为49.5%[71, 79]。IIV对确诊流感住院总的保护效果为65.6%(95%CI:42.7%~79.3%),对甲型流感的保护效果为66.0%(95%CI:3.4%~88.0%),对乙型流感的保护效果为65.3%(95%CI:39.5%~80.1%)[80]。为获得最大程度的保护,≤8岁的儿童首次接种流感疫苗应接种2剂次[81]。同时,对于儿童群体,IIV4对乙型流感的免疫原性优于IIV3[82]。
(4)学生:开展基于学校的流感疫苗接种可有效减少学龄儿童流感感染的发生。在流感病毒疫苗株与流行株不匹配的情况下,学生接种IIV3的保护效果仍可达到38%(95%CI:12%~57%)[83]。幼儿园儿童中,IIV3接种效果为16%~20.6%[84-85]。在流感病毒疫苗株与流行主导株匹配的流行季,开展流感疫苗大规模集中接种可使流感集中发热疫情的发生风险大幅降低[86]。
(5)老年人:无论流感疫苗与流行株是否匹配,接种IIV均可有效保护老年人群:匹配时的保护效果约为42%~58%;不匹配时仍有18%~20%的保护效果[73, 87-91]。接种流感疫苗还可降低老年人流感相关并发症发生率,减少流感相关住院及死亡。当存在流感病毒循环的情况下,老年人接种流感疫苗还能预防28%(95%CI:26%~30%)的流感相关致命性或非致命性并发症、39%(95%CI:35%~43%)的流感样症状和49%(95%CI:33%~62%)的确诊流感[92]。
(6)慢性基础性疾病患者:我国开展的队列研究表明,接种IIV3可以减少COPD和慢性支气管炎的急性感染和住院[93-96]。IIV3接种3、6个月后COPD急性加重的住院天数分别减少3.3和7.1 d[93]。IIV对儿童和成年人哮喘患者有较好免疫原性[97],哮喘患者接种IIV能够有效减少流感感染和哮喘发作[98]。IIV在心血管疾病患者中免疫原性良好,能够保护心血管病患减少流感感染,降低心血管疾病事件的合并症的发生率[99]。对于糖尿病患者,流感疫苗的免疫原性主要与年龄和既往抗体水平滴度有关,而与是否患糖尿病无关[100]。老年糖尿病患者接种IIV后,住院和死亡的风险降低,IIV对住院的保护效果是23%,对全死因的保护效果是38%~56%[101]。
(7)医务人员:医务人员接种流感疫苗可保护自身健康,可以减少42%的临床诊断流感和29%的全病因死亡[102-103]。医务人员接种流感疫苗还可以减少缺勤、流感样疾病和呼吸系统疾病的发生和就诊[104-105],降低心脑血管疾病和糖尿病的就诊率[105]。此外,医务人员罹患流感除可能导致院内流行而直接感染患者外[105],流感导致的医务人员不足可影响医疗系统运转。因此,医务人员接种流感疫苗尤其重要。
4. IIV3和IIV4的安全性:接种流感疫苗是安全的,但也可能会出现不良反应。流感疫苗常见的副作用主要表现为局部反应(接种部位红晕、肿胀、硬结、疼痛、烧灼感等)和全身反应(发热、头痛、头晕、嗜睡、乏力、肌痛、周身不适、恶心、呕吐、腹痛、腹泻等)。通常是轻微的,并在几天内自行消失,极少出现重度反应[106-110]。我国原有的IIV3和新近上市的IIV4均为肌肉注射的灭活疫苗。众多研究表明IIV3和IIV4在安全性上没有差别[111-118],国产和进口流感疫苗相比安全性也无显著性差异[119-121]。
疑似预防接种异常反应(adverse event following immunization,AEFI)是指在预防接种后发生的怀疑与预防接种有关的不良反应或医学事件。我国于2010年发布《全国疑似预防接种异常反应监测方案》,要求责任报告单位和报告人发现属于报告范围的AEFI(包括接到受种者或其监护人的报告)后应当及时向受种者所在地的县级卫生行政部门、药品监督管理部门报告,相关信息将通过AEFI信息管理系统进行网络报告。2011-2014年AEFI信息管理系统的监测数据分析显示,我国流感疫苗相关的严重AEFI的发生率很低(1.9例/100万剂次至3.3例/100万剂次),非严重AEFI的发生率在159例/100万剂次至172例/100万剂次[122-125]。
5.疫苗成本效果、成本效益:接种流感疫苗能有效减少流感相关门急诊、住院和死亡人数,继而降低治疗费用,产生明显的经济效益[126]。从全社会的角度,对儿童、孕产妇、高危人群和医务人员开展流感疫苗接种具有成本效果[127]。近期研究结果显示:与IIV3相比,接种IIV4的增量成本效果比(incremental costs-effectiveness ratio,ICER)为每多获得一个质量调整生命年需多支出3 015美元;当支付意愿为10 000美元时,具有成本效果的概率为98%[128]。
四、2018-2019年度接种建议每年接种流感疫苗是预防流感最有效的措施。目前,流感疫苗在我国大多数地区属于第二类疫苗,公民自费、自愿接种。为促进我国流感疫苗的预防接种,降低流感对公众的健康危害和经济负担,根据2018年4月国家免疫规划咨询委员会建议,应考虑采取综合政策措施积极推动流感疫苗预防接种工作,包括采用不同筹资方式和机制,提高重点人群的接种意愿和接种率、改进免疫服务公平性的效果和效率;提高医务人员和公众对流感和疫苗预防的认识,改进临床预防实践,通过制/修订临床指南、路径、专家共识等多种渠道,推动临床医生对流感疫苗预防接种建议的推荐;加快预防接种服务体系建设,为公众提供更方便、可及和规范的预防接种等[129]。
为提高公众对流感疾病特征、危害及疫苗预防作用的认识,逐步提高高危人群的疫苗覆盖率,各级CDC要积极组织开展科学普及、健康教育、风险沟通和疫苗政策推进活动,组织指导疫苗接种时,应重点把握好剂型选择、优先接种人群、接种程序、禁忌证和接种时机等技术环节。
1.抗原组份:WHO推荐的2018-2019年度北半球IIV3组份为:甲型/Michigan/45/2015(H1N1)pdm09类似株、甲型/Singapore/INFIMH-16 -0019/2016(H3N2)类似株和乙型/Colorado/06/2017(Victoria系)类似株;IIV4组份为上述3个毒株及乙型/Phuket/3073/2013(Yamagata系)类似株。与上一年度相比,甲型H3N2亚型和乙型Victoria系病毒更换了毒株。
2.疫苗种类及适用年龄组:我国批准上市的流感疫苗包括IIV3和IIV4。IIV3为裂解疫苗和亚单位疫苗,可用于≥6月龄人群接种,包括0.25 ml(每种组份HA 7.5 μg,适用于6~35月龄婴幼儿)和0.5 ml(每种组份HA 15 μg,适用于≥36月龄人群)两种剂型;IIV4为裂解疫苗,可用于≥36月龄人群接种,为0.5 ml剂型,含每种组份HA 15 μg。对可接种不同类型、不同厂家疫苗产品的人群,可自愿接种任一种流感疫苗,无优先推荐。
3.建议优先接种人群:流感疫苗安全、有效。原则上,接种单位应为≥6月龄所有愿意接种疫苗且无禁忌证的人提供免疫服务。国内外大量流感疾病负担的科学证据表明,不同人群患流感后的临床严重程度和结局不同,借鉴WHO立场和其他国家多年的应用经验,结合我国国情,推荐以下人群为优先接种对象。
(1)6~23月龄的婴幼儿:患流感后出现重症的风险高,流感住院负担重,应优先接种流感疫苗。疫苗在该年龄组的效果高度依赖于疫苗株与循环毒株的匹配程度。
(2)2~5岁儿童:流感疾病负担也较高,但低于<2岁儿童。该年龄组儿童接种流感疫苗后,其免疫应答反应通常优于<2岁儿童。
(3)≥60岁老年人:患流感后死亡风险最高,是流感疫苗接种的重要目标人群。虽然较多证据表明,现有流感疫苗在老年人中的效果不如年轻成年人,但疫苗接种仍是目前保护老年人免于罹患流感的最有效手段。
(4)特定慢性病患者:心血管疾病(单纯高血压除外)、慢性呼吸系统疾病、肝肾功能不全、血液病、神经系统疾病、神经肌肉功能障碍、代谢性疾病(包括糖尿病)等慢性病患者、患有免疫抑制疾病或免疫功能低下者,患流感后出现重症的风险很高,应优先接种流感疫苗。
(5)医务人员:是流感疫苗接种的重要优先人群,不仅可保护医务人员自身、维持流感流行季节医疗服务的正常运转,同时可有效减少医务人员将病毒传给流感高危人群的机会。
(6)<6月龄婴儿的家庭成员和看护人员:由于现有流感疫苗不可以直接给<6月龄婴儿接种,该人群可通过母亲孕期接种和对婴儿的家庭成员和看护人员接种流感疫苗,以预防流感。
(7)孕妇或准备在流感季节怀孕的女性:国内外大量研究证实孕妇罹患流感后发生重症、死亡和不良妊娠结局的风险更高,国外对孕妇在孕期任何阶段接种流感疫苗的安全性证据充分,同时接种疫苗对预防孕妇罹患流感及通过胎传抗体保护6月龄以内婴儿的效果明确。《WHO流感疫苗立场文件(2012年版)》将孕妇列为第一优先接种人群。但由于国内缺乏孕妇接种流感疫苗的安全性评价数据,我国上市的部分流感疫苗产品说明书仍将孕妇列为禁忌证。为降低我国孕妇罹患流感及严重并发症风险,经审慎评估,本指南建议孕妇或准备在流感季节怀孕的女性接种流感疫苗,孕妇可在妊娠任何阶段接种。
4.接种剂次:
(1)6月龄至8岁儿童:首次接种流感疫苗的6月龄至8岁儿童应接种2剂次,间隔≥4周;2017-2018年或以前接种过1剂或以上流感疫苗的儿童,则建议接种1剂。
(2)9岁及以上儿童和成年人:仅需接种1剂。见图 1。
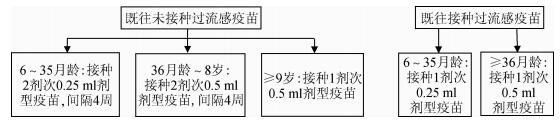
|
| 图 1 各年龄组流感疫苗接种剂次图示 |
5.接种时机:通常接种流感疫苗2~4周后,可产生具有保护水平的抗体,6~8个月后抗体滴度开始衰减。我国各地每年流感活动高峰出现的时间和持续时间不同,为保证受种者在流感高发季节前获得免疫保护,建议各地在疫苗可及后尽快安排接种工作,最好在10月底前完成免疫接种;对10月底前未接种的对象,整个流行季节都可以提供免疫服务。同一流感流行季节,已按照接种程序完成全程接种的人员,无需重复接种。孕妇在孕期的任一阶段均可接种流感疫苗,建议只要本年度的流感疫苗开始供应,可尽早接种。
6.接种部位及方法:IIV的接种采用肌肉注射(皮内注射制剂除外)[8]。成年人和>1岁儿童首选上臂三角肌接种疫苗,6月龄~1岁婴幼儿的接种部位以大腿前外侧为最佳[8]。因为血小板减少症或其他出血性疾病患者在肌肉注射时可能发生出血危险,应采用皮下注射。
7.疫苗储存:按照《疫苗储存和运输管理规范(2017年版)》的要求,应在2~8 ℃避光保存和运输,严禁冻结。
8.禁忌证:对疫苗中所含任何成分(包括辅料、甲醛、裂解剂及抗生素)过敏者。患伴或不伴发热症状的轻中度急性疾病者,建议症状消退后再接种。上次接种流感疫苗后6周内出现格林巴利综合征,不是禁忌证,但应特别注意。
《中华人民共和国药典(2015版)》未将对鸡蛋过敏者作为禁忌证。药典规定流感疫苗中卵清蛋白含量应不高于500 ng/ml。随着生产工艺的提高,疫苗中的卵蛋白含量已大大低于国家标准,以往对我国常用的流感疫苗中的卵蛋白含量测量显示含量最高不超过140 ng/ml[130]。国外学者对于鸡蛋过敏者接种IIV或LAIV的研究表明不会发生严重过敏反应[131-134]。美国免疫咨询委员会自2016年以来开始建议对鸡蛋过敏者亦可接种流感疫苗[135-136]。
9.药物相互作用:
(1)如正在或近期曾使用过任何其他疫苗或药物,包括非处方药,请接种前告知接种医生。
(2)IIV与其他灭活疫苗及减毒活疫苗可同时在不同部位接种[136];未发现影响流感疫苗和联合接种疫苗的免疫原性和安全性的证据[137]。建议≥65岁老年人同时接种IIV和肺炎球菌疫苗[137-143]。
(3)免疫抑制剂(如皮质类激素、细胞毒性药物或放射治疗)的使用可能影响接种后的免疫效果[144-145]。为避免可能的药物间相互作用,任何正在进行的治疗均应咨询医生。
(4)服用流感抗病毒药物预防和治疗期间可以接种IIV[136]。
10.接种注意事项:各接种单位要按照《预防接种工作规范(2016年版)》的要求开展IIV接种工作。接种工作中要注意以下事项:①疫苗瓶有裂纹、标签不清或失效者,疫苗出现浑浊等外观异物者均不得使用;②严格掌握疫苗剂量和适用人群的年龄范围,不能将0.5 ml剂型分为2剂次(每剂次0.25 ml)给2名婴幼儿接种;③接种完成后应告知接种对象留下观察30 min再离开;④建议注射现场备1 : 1 000肾上腺素等药品和其他抢救设施,以备偶有发生严重过敏反应时供急救使用。
志谢 感谢中国CDC尹遵栋、马超、Lawrence Everett Rodewald、殷大鹏、许文波研究员以及美国CDC中美新发和再发传染病合作项目Alexander J Millman、宋英教授的支持和帮助
利益冲突 无
| [1] |
中华人民共和国国家卫生和计划生育委员会. 流行性感冒诊疗方案(2018年版)[J]. 中华临床感染病杂志, 2018, 11(1): 1-5. National Health and Family Planning Commission of the People's Republic of China. Influenza treatment plan (2018 edition)[J]. Chin J Clin Infect Dis, 2018, 11(1): 1-5. DOI:10.3760/cma.j.issn.1674-2397.2018.01.001 |
| [2] |
USCDC. Types of Influenza Viruses[EB/OL]. (2017-09-27)[2018-09-30]. https://www.cdc.gov/flu/about/viruses/types.htm.
|
| [3] |
Bischoff WE, Swett K, Leng I, et al. Exposure to influenza virus aerosols during routine patient care[J]. J Infect Dis, 2013, 207(7): 1037-1046. DOI:10.1093/infdis/jis773 |
| [4] |
WHO. Fact sheets on influenza (seasonal)[R]. (2018-01-31)[2018-09-30]. http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
|
| [5] |
李茜, 李霆, 吴绍强, 等. D型流感病毒研究概述[J]. 检验检疫学刊, 2017, 27(4): 73-75. Li X, Li T, Wu SQ, et al. An overview of research progress on influenza D viruses[J]. J Inspect Quarant, 2017, 27(4): 73-75. |
| [6] |
Hause BM, Collin EA, Liu RX, et al. Characterization of a novel influenza virus in cattle and Swine:proposal for a new genus in the Orthomyxoviridae family[J]. mBio, 2014, 5(2): e00031-14. DOI:10.1128/mBio.00031-14 |
| [7] |
医政医管局.流行性感冒诊断与治疗指南(2011年版)[R].北京: 中华人民共和国卫生健康委员会, 2011. Medical Administration and Hospital Authority. Influenza Diagnosis and Treatment Guidelines[R]. Beijing: People's Republic of China health and Health Committee, 2011. |
| [8] |
W HO. Vaccines against influenza WHO position paper-November 2012[J]. Wkly Epidemiol Rec, 2012, 87(47): 461-476. |
| [9] |
Nicholson KG, Wood JM, Zambon M. Influenza[J]. Lancet, 2003, 362(9397): 1733-1745. DOI:10.1016/S0140-6736(03)14854-4 |
| [10] |
Kim DK, Poudel B. Tools to detect influenza virus[J]. Yonsei Med J, 2013, 54(3): 560-566. DOI:10.3349/ymj.2013.54.3.560 |
| [11] |
Kelso JM. Safety of influenza vaccines[J]. Curr Opin Allergy Clin Immunol, 2012, 12(4): 383-388. DOI:10.1097/ACI.0b013e328354395d |
| [12] |
国家卫生和计划生育委员会, 国家中医药管理局. 流行性感冒诊疗方案(2018年版)[J]. 中国感染控制杂志, 2018, 17(2): 181-184. National Health and Family Planning Commission, State Administration of Traditional Chinese Medicine. Influenza treatment program (2018 edition)[J]. Chin J Infect Control, 2018, 17(2): 181-184. DOI:10.3969/j.issn.1671-9638.2018.02.020 |
| [13] |
WHO Writing Group. Nonpharmaceutical interventions for pandemic influenza, international measures[J]. Emerg Infect Dis, 2006, 12(1): 81-87. DOI:10.3201/eid1201.051370 |
| [14] |
Lau LLH, Ip DKM, Nishiura H, et al. Heterogeneity in viral shedding among individuals with medically attended influenza a virus infection[J]. J Infect Dis, 2013, 207(8): 1281-1285. DOI:10.1093/infdis/jit034 |
| [15] |
Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza:a review of volunteer challenge studies[J]. Am J Epidemiol, 2008, 167(7): 775-785. DOI:10.1093/aje/kwm375 |
| [16] |
Lipsitch M, Viboud C. Influenza seasonality:lifting the fog[J]. Proc Natl Acad Sci USA, 2009, 106(10): 3645-3646. DOI:10.1073/pnas.0900933106 |
| [17] |
Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions[J]. PLoS Med, 2006, 3(4): e89. DOI:10.1371/journal.pmed.0030089 |
| [18] |
Azziz Baumgartner E, Dao CN, Nasreen S, et al. Seasonality, timing, and climate drivers of influenza activity worldwide[J]. J Infect Dis, 2012, 206(6): 838-846. DOI:10.1093/infdis/jis467 |
| [19] |
Bloom-Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV):a global comparative review[J]. PLoS One, 2013, 8(2): e54445. DOI:10.1371/journal.pone.0054445 |
| [20] |
Zou JY, Yang H, Cui HJ, et al. Geographic divisions and modeling of virological data on seasonal influenza in the Chinese mainland during the 2006-2009 monitoring years[J]. PLoS One, 2013, 8(3): e58434. DOI:10.1371/journal.pone.0058434 |
| [21] |
Yu HJ, Alonso WJ, Feng LZ, et al. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies:spatio-temporal modeling of surveillance data[J]. PLoS Med, 2013, 10(11): e1001552. DOI:10.1371/journal.pmed.1001552 |
| [22] |
Yang J, Lau YC, Wu P, et al. Variation in influenza B virus epidemiology by lineage, China[J]. Emerg Infect Dis, 2018, 24(8): 1536-1540. DOI:10.3201/eid2408.180063 |
| [23] |
Somes MP, Turner RM, Dwyer LJ, et al. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals:a systematic review and meta-analysis[J]. Vaccine, 2018, 36(23): 3199-3207. DOI:10.1016/j.vaccine.2018.04.063 |
| [24] |
Jayasundara K, Soobiah C, Thommes E, et al. Natural attack rate of influenza in unvaccinated children and adults:a Meta-regression analysis[J]. BMC Infect Dis, 2014, 14: 670. DOI:10.1186/s12879-014-0670-5 |
| [25] |
Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality:a modelling study[J]. Lancet, 2018, 391(10127): 1285-1300. DOI:10.1016/S0140-6736(17)33293-2 |
| [26] |
Feng LZ, Shay DK, Jiang Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003-2008[J]. Bull World Health Organ, 2012, 90(4): 279-288B. DOI:10.2471/BLT.11.096958 |
| [27] |
Wu P, Presanis AM, Bond HS, et al. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998-2013[J]. Sci Rep, 2017, 7(1): 929. DOI:10.1038/s41598-017-01021-x |
| [28] |
Puig-Barberà J, Natividad-Sancho A, Trushakova S, et al. Epidemiology of hospital admissions with influenza during the 2013/2014 northern hemisphere influenza season:results from the global influenza hospital surveillance network[J]. PLoS One, 2016, 11(5): e0154970. DOI:10.1371/journal.pone.0154970 |
| [29] |
Peng ZB, Feng LZ, Carolyn GM, et al. Characterizing the epidemiology, virology, and clinical features of influenza in China's first severe acute respiratory infection sentinel surveillance system, February 2011-October 2013[J]. BMC Infect Dis, 2015, 15: 143. DOI:10.1186/s12879-015-0884-1 |
| [30] |
Plans-Rubió P. Prevention and control of influenza in persons with chronic obstructive pulmonary disease[J]. Int J Chron Obstruct Pulmon Dis, 2007, 2(1): 41-53. |
| [31] |
Goodnight WH, Soper DE. Pneumonia in pregnancy[J]. Crit Care Med, 2005, 33(10): S390-397. DOI:10.1097/01.CCM.0000182483.24836.66 |
| [32] |
Ohfuji S, Deguchi M, Tachibana D, et al. Estimating influenza disease burden among pregnant women:application of self-control method[J]. Vaccine, 2017, 35(36): 4811-4816. DOI:10.1016/j.vaccine.2017.07.006 |
| [33] |
Callaghan WM, Chu SY, Jamieson DJ. Deaths from seasonal influenza among pregnant women in the United States, 1998-2005[J]. Obstet Gynecol, 2010, 115(5): 919-923. DOI:10.1097/AOG.0b013e3181d99d85 |
| [34] |
Yu HJ, Feng ZJ, Uyeki TM, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China[J]. Clin Infect Dis, 2011, 52(4): 457-465. DOI:10.1093/cid/ciq144 |
| [35] |
Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies:a systematic review and Meta-analysis[J]. Hum Reprod, 2014, 29(4): 809-823. DOI:10.1093/humrep/det455 |
| [36] |
Steinhoff MC, MacDonald N, Pfeifer D, et al. Influenza vaccine in pregnancy:policy and research strategies[J]. Lancet, 2014, 383(9929): 1611-1613. DOI:10.1016/S0140-6736(14)60583-3 |
| [37] |
He J, Liu ZW, Lu YP, et al. A systematic review and meta-analysis of influenza a virus infection during pregnancy associated with an increased risk for stillbirth and low birth weight[J]. Kidney Blood Press Res, 2017, 42(2): 232-243. DOI:10.1159/000477221 |
| [38] |
Fraaij PLA, Heikkinen T. Seasonal influenza:the burden of disease in children[J]. Vaccine, 2011, 29(43): 7524-7528. DOI:10.1016/j.vaccine.2011.08.010 |
| [39] |
Monto AS, Koopman JS, Longini Jr IM. Tecumseh study of illness.. Influenza infection and disease, 1976-1981[J]. Am J Epidemiol, 1985, 121(6): 811-822. DOI:10.1093/oxfordjournals.aje.a114052 |
| [40] |
Cowling BJ, Perera RAPM, Fang VJ, et al. Incidence of influenza virus infections in children in Hong Kong in a 3-year randomized placebo-controlled vaccine study, 2009-2012[J]. Clin Infect Dis, 2014, 59(4): 517-524. DOI:10.1093/cid/ciu356 |
| [41] |
Yu HJ, Huang JG, Huai Y, et al. The substantial hospitalization burden of influenza in central China:surveillance for severe, acute respiratory infection, and influenza viruses, 2010-2012[J]. Influenza Other Respir Viruses, 2014, 8(1): 53-65. DOI:10.1111/irv.12205 |
| [42] |
Finnie TJR, Copley VR, Hall IM, et al. An analysis of influenza outbreaks in institutions and enclosed societies[J]. Epidemiol Infect, 2014, 142(1): 107-113. DOI:10.1017/S0950268813000733 |
| [43] |
Gaglani MJ. Editorial commentary:school-located influenza vaccination:why worth the effort?[J]. Clin Infect Dis, 2014, 59(3): 333-335. DOI:10.1093/cid/ciu344 |
| [44] |
Fiore AE, Epperson S, Perrotta D, et al. Expanding the recommendations for annual influenza vaccination to school-age children in the United States[J]. Pediatrics, 2012, 129 Suppl 2: S54-62. DOI:10.1542/peds.2011-0737C |
| [45] |
Wu S, van Asten L, Wang L, et al. Estimated incidence and number of outpatient visits for seasonal influenza in 2015-2016 in Beijing, China[J]. Epidemiol Infect, 2017, 145(16): 3334-3344. DOI:10.1017/S0950268817002369 |
| [46] |
Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season:effect on school absenteeism, parental absenteeism from work, and secondary illness in families[J]. Arch Pediatr Adolesc Med, 2002, 156(10): 986-991. DOI:10.1001/archpedi.156.10.986 |
| [47] |
Chiu SS, Chan KH, So LY, et al. The population based socioeconomic burden of pediatric influenza-associated hospitalization in Hong Kong[J]. Vaccine, 2012, 30(10): 1895-1900. DOI:10.1016/j.vaccine.2011.12.027 |
| [48] |
Kuster SP, Shah PS, Coleman BL, et al. Incidence of influenza in healthy adults and healthcare workers:a systematic review and Meta-analysis[J]. PLoS One, 2011, 6(10): e26239. DOI:10.1371/journal.pone.0026239 |
| [49] |
Seto WH, Cowling BJ, Lam HS, et al. Clinical and nonclinical health care workers faced a similar risk of acquiring 2009 pandemic H1N1 infection[J]. Clin Infect Dis, 2011, 53(3): 280-283. DOI:10.1093/cid/cir375 |
| [50] |
Kumar S, Fan J, Melzer-Lange M, et al. H1N1 hemagglutinin-inhibition seroprevalence in emergency department health care workers after the first wave of the 2009 influenza pandemic[J]. Pediatr Emerg Care, 2011, 27(9): 804-807. DOI:10.1097/PEC.0b013e31822c125e |
| [51] |
Salgado CD, Farr BM, Hall KK, et al. Influenza in the acute hospital setting[J]. Lancet Infect Dis, 2002, 2(3): 145-155. DOI:10.1016/S1473-3099(02)00221-9 |
| [52] |
Elder AG, O'Donnell B, McCruden EAB, et al. Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993-4 epidemic:results of serum testing and questionnaire[J]. BMJ, 1996, 313(7067): 1241-1242. DOI:10.1136/bmj.313.7067.1241 |
| [53] |
Wang H, Fu CX, Li KB, et al. Influenza associated mortality in southern China, 2010-2012[J]. Vaccine, 2014, 32(8): 973-978. DOI:10.1016/j.vaccine.2013.12.013 |
| [54] |
Yang L, Ma S, Chen PY, et al. Influenza associated mortality in the subtropics and tropics:results from three Asian cities[J]. Vaccine, 2011, 29(48): 8909-8914. DOI:10.1016/j.vaccine.2011.09.071 |
| [55] |
Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998-2009[J]. J Infect Dis, 2012, 206(12): 1862-1871. DOI:10.1093/infdis/jis628 |
| [56] |
Chow A, Ma S, Ling AE, et al. Influenza-associated deaths in tropical Singapore[J]. Emerg Infect Dis, 2006, 12(1): 114-121. DOI:10.3201/eid1201.050826 |
| [57] |
Nunes B, Viboud C, Machado A, et al. Excess mortality associated with influenza epidemics in portugal, 1980 to 2004[J]. PLoS One, 2011, 6(6): e20661. DOI:10.1371/journal.pone.0020661 |
| [58] |
Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States[J]. JAMA, 2003, 289(2): 179-186. DOI:10.1001/jama.289.2.179 |
| [59] |
Yu HJ, Huang JG, Huai Y, et al. The substantial hospitalization burden of influenza in central China:surveillance for severe, acute respiratory infection, and influenza viruses, 2010-2012[J]. Influenza other Respir Viruses, 2014, 8(1): 53-65. DOI:10.1111/irv.12205 |
| [60] |
李琳, 张颖, 董晓春, 等. 2015年天津市流感确诊病例流行病学特征分析及疾病负担评估[J]. 职业与健康, 2017, 33(2): 216-218, 222. Li L, Zhang Y, Dong XC, et al. Analysis on epidemiological characteristics and evaluation on disease burden of confirmed influenza cases in Tianjin in 2015[J]. Occup Health, 2017, 33(2): 216-218, 222. DOI:10.13329/j.cnki.zyyjk.2017.0064 |
| [61] |
Guo RN, Zheng HZ, Li JS, et al. A population-based study on incidence and economic burden of influenza-like illness in south China, 2007[J]. Public Health, 2011, 125(6): 389-395. DOI:10.1016/j.puhe.2011.03.004 |
| [62] |
Yang J, Jit M, Leung KS, et al. The economic burden of influenza-associated outpatient visits and hospitalizations in China:a retrospective survey[J]. Infect Dis Poverty, 2015, 4: 44. DOI:10.1186/s40249-015-0077-6 |
| [63] |
Chen J, Li YT, Gu BK, et al. Estimation of the direct cost of treating people aged more than 60 years infected by influenza virus in Shanghai[J]. Asia Pac J Public Health, 2015, 27(2): NP936-NP946. DOI:10.1177/1010539512460269 |
| [64] |
Yang J, Jit M, Zheng YM, et al. The impact of influenza on the health related quality of life in China:an EQ-5D survey[J]. BMC Infect Dis, 2017, 17(1): 686. DOI:10.1186/s12879-017-2801-2 |
| [65] |
Chan SSW. Does vaccinating ED health care workers against influenza reduce sickness absenteeism[J]. Am J Emerg Med, 2007, 25(7): 808-811. DOI:10.1016/j.ajem.2007.02.002 |
| [66] |
于佳, 张涛, 王胤, 等. 苏州市2011-2017年5岁以下儿童流感门诊病例临床特征及疾病负担[J]. 中华流行病学杂志, 2018, 39(6): 847-851. Yu J, Zhang T, Wang Y, et al. Clinical characteristics and economic burden of influenza among children under 5 years old, in Suzhou, 2011-2017[J]. Chin J Epidemiol, 2018, 39(6): 847-851. DOI:10.3760/cma.j.issn.0254-6450.2018.06.029 |
| [67] |
Cate TR, Couch RB, Parker D, et al. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines-1978[J]. Rev Infect Dis, 1983, 5(4): 737-747. DOI:10.1093/clinids/5.4.737 |
| [68] |
Ochiai H, Shibata M, Kamimura K, et al. Evaluation of the efficacy of split-product trivalent A(H1N1), A(H3N2), and B influenza vaccines:reactogenicity, immunogenicity and persistence of antibodies following two doses of vaccines[J]. Microbiol Immunol, 1986, 30(11): 1141-1149. DOI:10.1111/j.1348-0421.1986.tb03043.x |
| [69] |
Künzel W, Glathe H, Engelmann H, et al. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time[J]. Vaccine, 1996, 14(12): 1108-1110. DOI:10.1016/0264-410X(96)00061-8 |
| [70] |
Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly:Risk factors for poor immune response and persistence[J]. Vaccine, 2010, 28(23): 3929-3935. DOI:10.1016/j.vaccine.2010.03.067 |
| [71] |
Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines:a systematic review and Meta-analysis[J]. Lancet Infect Dis, 2012, 12(1): 36-44. DOI:10.1016/S1473-3099(11)70295-X |
| [72] |
Jefferson T, Di Pietrantonj C, Rivetti A, et al. Vaccines for preventing influenza in healthy adults[J]. Cochrane Database Syst Rev, 2010(7): CD001269. DOI:10.1002/14651858.CD001269.pub4 |
| [73] |
星一, 刘民. 流感灭活疫苗在中国应用效果的Meta分析[J]. 中华流行病学杂志, 2009, 30(4): 368-370. Xing Y, Liu M. Meta-analysis on the effectiveness of inactivated influenza vaccine[J]. Chin J Epidemiol, 2009, 30(4): 368-370. DOI:10.3760/cma.j.issn.0254-6450.2009.04.015 |
| [74] |
Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype:a systematic review and meta-analysis of test-negative design studies[J]. Lancet Infect Dis, 2016, 16(8): 942-951. DOI:10.1016/S1473-3099(16)00129-8 |
| [75] |
Moa AM, Chughtai AA, Muscatello DJ, et al. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults:a systematic review and meta-analysis of randomised controlled trials[J]. Vaccine, 2016, 34(35): 4092-4102. DOI:10.1016/j.vaccine.2016.06.064 |
| [76] |
Bekkat-Berkani R, Ray R, Jain VK, et al. Evidence update:GlaxoSmithKline's inactivated quadrivalent influenza vaccines[J]. Expert Rev Vaccines, 2016, 15(2): 201-214. DOI:10.1586/14760584.2016.1113878 |
| [77] |
Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy-antibody responses in mothers and infants[J]. N Engl J Med, 2010, 362(17): 1644-1646. DOI:10.1056/NEJMc0912599 |
| [78] |
Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants[J]. N Engl J Med, 2008, 359(15): 1555-1564. DOI:10.1056/NEJMoa0708630 |
| [79] |
Fu CX, He Q, Li ZT, et al. Seasonal influenza vaccine effectiveness among children, 2010-2012[J]. Influenza Other Respir Viruses, 2013, 7(6): 1168-1174. DOI:10.1111/irv.12157 |
| [80] |
Chiu SS, Kwan MYW, Feng S, et al. Interim estimate of influenza vaccine effectiveness in hospitalised children, Hong Kong, 2017/18[J]. Euro Surveill, 2018, 23(8): pii=18-00062. DOI:10.2807/1560-7917.ES.2018.23.8.18-00062 |
| [81] |
Neuzil KM, Jackson LA, Nelson J, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children[J]. J Infect Dis, 2006, 194(8): 1032-1039. DOI:10.1086/507309 |
| [82] |
Pepin S, Szymanski H, Rochin Kobashi IA, et al. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age:a phase Ⅲ randomized controlled study[J]. Hum Vaccin Immunother, 2016, 12(12): 3072-3078. DOI:10.1080/21645515.2016.1212143 |
| [83] |
Zhang L, Yang P, Thompson MG, et al. Influenza vaccine effectiveness in preventing influenza illness among children during school-based outbreaks in the 2014-2015 season in Beijing, China[J]. Pediatr Infect Dis J, 2017, 36(3): e69-75. DOI:10.1097/INF.0000000000001434 |
| [84] |
Wang Y, Chen LL, Cheng YJ, et al. Potential impact of B lineage mismatch on trivalent influenza vaccine effectiveness during the 2015-2016 influenza season among nursery school children in Suzhou, China[J]. Hum Vaccin Immunother, 2018, 14(3): 630-636. DOI:10.1080/21645515.2017.1397868 |
| [85] |
Wang Y, Chen LL, Yu J, et al. The effectiveness of influenza vaccination among nursery school children in China during the 2016/17 influenza season[J]. Vaccine, 2018, 36(18): 2456-2461. DOI:10.1016/j.vaccine.2018.03.039 |
| [86] |
Pan Y, Wang QY, Yang P, et al. Influenza vaccination in preventing outbreaks in schools:a long-term ecological overview[J]. Vaccine, 2017, 35(51): 7133-7138. DOI:10.1016/j.vaccine.2017.10.096 |
| [87] |
Jefferson T, Di Pietrantonj C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly[J]. Cochrane Database Syst Rev, 2010(2): CD004876. DOI:10.1002/14651858.CD004876.pub3 |
| [88] |
Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015-2016 season[J]. N Engl J Med, 2017, 377(6): 534-543. DOI:10.1056/NEJMoa1700153 |
| [89] |
Flannery B, Chung JR, Thaker SN, et al. Interim estimates of 2016-17 seasonal influenza vaccine effectiveness-United States, February 2017[J]. MMWR Morb Mortal Wkly Rep, 2017, 66(6): 167-171. DOI:10.15585/mmwr.mm6606a3 |
| [90] |
Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness-United States, February 2018[J]. MMWR Morb Mortal Wkly Rep, 2018, 67(6): 180-185. DOI:10.15585/mmwr.mm6706a2 |
| [91] |
Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people:an individual participant data meta-analysis of test-negative design case-control studies[J]. Lancet Respir Med, 2017, 5(3): 200-211. DOI:10.1016/S2213-2600(17)30043-7 |
| [92] |
Beyer WEP, McElhaney J, Smith DJ, et al. Cochrane re-arranged:support for policies to vaccinate elderly people against influenza[J]. Vaccine, 2013, 31(50): 6030-6033. DOI:10.1016/j.vaccine.2013.09.063 |
| [93] |
黄远东, 赵晓平, 万涛, 等. 慢性阻塞性肺病人群流感疫苗接种的效果观察[J]. 海南医学, 2011, 22(4): 29-31. Huang YD, Zhao XP, Wan T, et al. Effects of influenza vaccination in chronic obstructive pulmonary disease[J]. Hainan Med J, 2011, 22(4): 29-31. DOI:10.3969/j.issn.1003-6350.2011.04.011 |
| [94] |
王学英. 西宁地区慢性阻塞性肺病患者稳定期接种2种疫苗的效果观察[J]. 青海医药杂志, 2010, 40(7): 75-76. Wang XY. Observation on the effect of inoculation of two kinds of vaccines in patients with chronic obstructive pulmonary disease in Xining area[J]. Qinghai Med Mag, 2010, 40(7): 75-76. |
| [95] |
章琴莺. 流感疫苗和肺炎球菌疫苗预防COPD急性发作疗效观察[J]. 浙江中西医结合杂志, 2014, 24(7): 597-599. Zhang QY. Observation on the efficacy of influenza vaccine and pneumococcal vaccine in preventing acute exacerbation of COPD[J]. Zhejiang J Integr Tradit Chin Western Med, 2014, 24(7): 597-599. |
| [96] |
高忠翠, 李江涛, 展胜. 卡舒宁联合流感疫苗对老年性慢性支气管炎合并急性感染的防治效果[J]. 中国生物制品学杂志, 2011, 24(10): 1214-1216. Gao ZC, Li JT, Zhan S. Preventive and curative effects of Card Shu Ning combined with influenza vaccine on senile chronic bronchitis complicated with acute infection[J]. Chin J Biol, 2011, 24(10): 1214-1216. DOI:10.13200/j.cjb.2011.10.99.gaozhc.030 |
| [97] |
Schwarze J, Openshaw P, Jha A, et al. Influenza burden, prevention, and treatment in asthma-A scoping review by the EAACI Influenza in asthma task force[J]. Allergy, 2018, 73(6): 1151-1181. DOI:10.1111/all.13333 |
| [98] |
Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma:a systematic review and meta-analysis[J]. Clin Infect Dis, 2017, 65(8): 1388-1395. DOI:10.1093/cid/cix524 |
| [99] |
Clar C, Oseni Z, Flowers N, et al. Influenza vaccines for preventing cardiovascular disease[J]. Cochrane Database Syst Rev, 2015(5): Cd005050. DOI:10.1002/14651858.CD005050.pub3 |
| [100] |
Seo YB, Baek JH, Lee J, et al. Long-term immunogenicity and safety of a conventional influenza vaccine in patients with type 2 diabetes[J]. Clin Vaccine Immunol, 2015, 22(11): 1160-1165. DOI:10.1128/CVI.00288-15 |
| [101] |
Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus:a review[J]. Vaccine, 2017, 35(38): 5095-5101. DOI:10.1016/j.vaccine.2017.07.095 |
| [102] |
Kliner M, Keenan A, Sinclair D, et al. Influenza vaccination for healthcare workers in the UK:appraisal of systematic reviews and policy options[J]. BMJ Open, 2016, 6(9): e012149. DOI:10.1136/bmjopen-2016-012149 |
| [103] |
Vanhems P, Baghdadi Y, Roche S, et al. Influenza vaccine effectiveness among healthcare workers in comparison to hospitalized patients:a 2004-2009 case-test, negative-control, prospective study[J]. Hum Vaccin Immunother, 2016, 12(2): 485-490. DOI:10.1080/21645515.2015.1079677 |
| [104] |
吴承菊, 郑修霞, 孙菲, 等. 医务人员接种流感疫苗的效果分析[J]. 中国实用护理杂志, 2008, 24(17): 57-59. Wu CJ, Zheng XX, Sun F, et al. Effect analysis of influenza vaccination among medical staff[J]. Chin J Pract Nurs, 2008, 24(17): 57-59. DOI:10.3760/cma.j.issn.1672-7088.2008.17.029 |
| [105] |
刘民, 刘改芬, 赵伟, 等. 医务人员接种流感疫苗的效果及效益研究[J]. 中国全科医学, 2006, 9(9): 708-711. Liu M, Liu GF, Zhao W, et al. An effect and cost-benefit analysis of influenza vaccine among the healthcare worker[J]. Chin J Gen Pract, 2006, 9(9): 708-711. DOI:10.3969/j.issn.1007-9572.2006.09.006 |
| [106] |
黄少萍, 朱振颖. 儿童接种流感疫苗预防效果追踪随访对照研究[J]. 白求恩医学杂志, 2014, 12(6): 601-602. Huang SP, Zhu ZY. A follow-up study of the prevention and treatment of influenza vaccine in children[J]. Bethune Med J, 2014, 12(6): 601-602. DOI:10.3969/j.issn.1672-2876.2014.06.055 |
| [107] |
朱向军, 刘志田, 高志刚, 等. 儿童型流感疫苗(防感灵)的安全性及免疫原性研究[J]. 中国公共卫生, 2002, 18(2): 211-212. Zhu XJ, Liu ZT, Gao ZG, et al. Study on safety and immunity of influenza vaccine (Fangganling) for children[J]. China Public Health, 2002, 18(2): 211-212. DOI:10.11847/zgggws2002-18-02-56 |
| [108] |
王瑞琴, 唐雅清, 刘重程. 国产流行性感冒裂解疫苗安全性和免疫原性评价分析[J]. 中国卫生检验杂志, 2008, 18(2): 340-342. Wang RQ, Tang YQ, Liu CC. Evaluation of safety and immunogenicity of domestic influenza split vaccine[J]. Chin J Health Lab Technol, 2008, 18(2): 340-342. DOI:10.3969/j.issn.1004-8685.2008.02.063 |
| [109] |
段玮, 杨鹏, 石伟先, 等. 流感疫苗的安全性和效果效益研究[J]. 国际病毒学杂志, 2014, 21(6): 241-244. Duan W, Yang P, Shi WX, et al. Research on safety and cost-benefit of influenza vaccine[J]. Int J Virol, 2014, 21(6): 241-244. DOI:10.3760/cma.j.issn.1673-4092.2014.06.001 |
| [110] |
王萍, 张昕伟, 宋宇飞, 等. 流行性感冒病毒裂解疫苗在18岁以上健康人群中应用的安全性及免疫原性研究[J]. 中华流行病学杂志, 2011, 32(2): 120-124. Wang P, Zhang XW, Song YF, et al. Safety and immunogenicity on the formulation of trivalent split influenza vaccine among healthy people aged over 18 years[J]. Chin J Epidemiol, 2011, 32(2): 120-124. DOI:10.3760/cma.j.issn.0254-6450.2011.02.004 |
| [111] |
de Lusignan S, dos Santos G, Byford R, et al. Enhanced safety surveillance of seasonal quadrivalent influenza vaccines in English primary care:interim analysis[J]. Adv Ther, 2018, 35(8): 1199-1214. DOI:10.1007/s12325-018-0747-4 |
| [112] |
Greenberg DP, Robertson CA, Landolfi VA, et al. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age[J]. Pediatr Infect Dis J, 2014, 33(6): 630-636. DOI:10.1097/INF.0000000000000254 |
| [113] |
Tsurudome Y, Kimachi K, Okada Y, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine in healthy adults:a phase Ⅱ, open-label, uncontrolled trial in Japan[J]. Microbiol Immunol, 2015, 59(10): 597-604. DOI:10.1111/1348-0421.12316 |
| [114] |
Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, et al. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥ 18 years:a phase Ⅲ, randomized trial[J]. Vaccine, 2014, 32(13): 1480-1487. DOI:10.1016/j.vaccine.2014.01.022 |
| [115] |
Statler VA, Albano FR, Airey J, et al. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in children 6-59 months of age:a phase 3, randomized, noninferiority study[J]. Vaccine, 2018. DOI:10.1016/j.vaccine.2018.07.036 |
| [116] |
van de Witte S, Nauta J, Montomoli E, et al. A phase Ⅲ randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses[J]. Vaccine, 2018, 36(40): 6030-6038. DOI:10.1016/j.vaccine.2018.04.043 |
| [117] |
Haber P, Moro PL, Lewis P, et al. Post-licensure surveillance of quadrivalent inactivated influenza (ⅡV4) vaccine in the United States, Vaccine Adverse Event Reporting System (VAERS), July 1, 2013-May 31, 2015[J]. Vaccine, 2016, 34(22): 2507-2512. DOI:10.1016/j.vaccine.2016.03.048 |
| [118] |
胡昱, 李倩, 陈雅萍, 等. 18岁以上人群接种四价流感病毒灭活疫苗免疫原性和安全性的Meta分析[J]. 国际流行病学传染病学杂志, 2017, 44(1): 47-52. Hu Y, Li Q, Chen YP, et al. Meta-analysis on immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults aged above 18 years[J]. Int J Epidemiol Infect Dis, 2017, 44(1): 47-52. DOI:10.3760/cma.j.issn.1673-4149.2017.01.010 |
| [119] |
张佩如, 祝小平, 周良君, 等. 国产流行性感冒病毒裂解疫苗安全性及免疫效果观察[J]. 中华预防医学杂志, 2009, 43(7): 615-618. Zhang PR, Zhu XP, Zhou LJ, et al. Safety and immunological effect of domestic split influenza virus vaccine[J]. Chin J Prev Med, 2009, 43(7): 615-618. DOI:10.3760/cma.j.issn.0253-9624.2009.07.019 |
| [120] |
孙立娜, 李丽芳, 刘静芹, 等. 国产与进口流感疫苗接种的临床观察[J]. 中国免疫学杂志, 2014, 30(1): 77-79. Sun LN, Li LF, Liu JQ, et al. Clinical observation of domestic and imported influenza vaccination[J]. Chin J Immunol, 2014, 30(1): 77-79. DOI:10.3969/j.issn.1000-484X.2014.01.015 |
| [121] |
胡锦流, 王仪, 范刚, 等. 流行性感冒裂解疫苗临床安全性及免疫原性研究[J]. 现代预防医学, 2006, 33(5): 828-829. Hu JL, Wang Y, Fan G, et al. Clinical safety and immunogenicity of influenza split vaccine[J]. Mod Prev Med, 2006, 33(5): 828-829. DOI:10.3969/j.issn.1003-8507.2006.05.077 |
| [122] |
武文娣, 李克莉, 郑景山, 等. 中国2011年疑似预防接种异常反应监测数据分析[J]. 中国疫苗和免疫, 2013, 19(2): 97-109. Wu WD, Li KL, Zheng JS, et al. Analysis on surveillance data of adverse events following immunization in China, 2011[J]. Chin J Vaccines Immun, 2013, 19(2): 97-109. |
| [123] |
武文娣, 刘大卫, 李克莉, 等. 中国2012年疑似预防接种异常反应监测数据分析[J]. 中国疫苗和免疫, 2014, 20(1): 1-12, 66. Wu WD, Liu DW, Li KL, et al. Analysis on surveillance data of adverse events following immunization in China, 2012[J]. Chin J Vaccines Immun, 2014, 20(1): 1-12, 66. |
| [124] |
叶家楷, 李克莉, 许涤沙, 等. 中国2013年疑似预防接种异常反应信息管理系统数据分析[J]. 中国疫苗和免疫, 2015, 21(2): 121-131, 200. Ye JK, Li KL, Xu DS, et al. Evaluation of the adverse events following immunization information management system in China, 2013[J]. Chin J Vaccines Immun, 2015, 21(2): 121-131, 200. |
| [125] |
叶家楷, 李克莉, 许涤沙, 等. 中国2014年疑似预防接种异常反应信息管理系统监测数据分析[J]. 中国疫苗和免疫, 2016, 22(2): 125-137. Ye JK, Li KL, Xu DS, et al. Analysis of surveillance for adverse events following immunization in China, 2014[J]. Chin J Vaccines Immun, 2016, 22(2): 125-137. |
| [126] |
Peasah SK, Azziz-Baumgartner E, Breese J, et al. Influenza cost and cost-effectiveness studies globally-a review[J]. Vaccine, 2013, 31(46): 5339-5348. DOI:10.1016/j.vaccine.2013.09.013 |
| [127] |
Ting EEK, Sander B, Ungar WJ. Systematic review of the cost-effectiveness of influenza immunization programs[J]. Vaccine, 2017, 35(15): 1828-1843. DOI:10.1016/j.vaccine.2017.02.044 |
| [128] |
Yang MC, Tan ECH, Su JJ. Cost-effectiveness analysis of quadrivalent versus trivalent influenza vaccine in Taiwan:a lifetime multi-cohort model[J]. Hum Vaccin Immunother, 2017, 13(1): 81-89. DOI:10.1080/21645515.2016.1225636 |
| [129] |
彭质斌, 王大燕, 杨娟, 等. 中国流感疫苗应用现状及促进预防接种的政策探讨[J]. 中华流行病学杂志, 2018, 39(8): 1045-1050. Peng ZB, Wang DY, Yang J, et al. Current situation and related policies on the implementation and promotion of influenza vaccination, in China[J]. Chin J Epidemiol, 2018, 39(8): 1045-1050. DOI:10.3760/cma.j.issn.0254-6450.2018.08.007 |
| [130] |
庄文佳, 丛莉, 蔡兴雁, 等. 五种国内常用流感疫苗的分析比较[J]. 中外健康文摘, 2012, 9(27): 71-74. Zhuang WJ, Cong L, Cai XY, et al. Analysis and comparison of five commonly used influenza vaccines in China[J]. World Health Dig Med Period, 2012, 9(27): 71-74. DOI:10.3969/j.issn.1672-5085.2012.27.055 |
| [131] |
des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza[J]. J Allergy Clin Immunol, 2012, 130(5): 1213-1216. DOI:10.1016/j.jaci.2012.07.046 |
| [132] |
des Roches A, Samaan K, Graham F, et al. Safe vaccination of patients with egg allergy by using live attenuated influenza vaccine[J]. J Allergy Clin Immunol Pract, 2015, 3(1): 138-139. DOI:10.1016/j.jaip.2014.08.008 |
| [133] |
Turner PJ, Southern J, Andrews NJ, et al. Safety of live attenuated influenza vaccine in atopic children with egg allergy[J]. J Allergy Clin Immunol, 2015, 136(2): 376-381. DOI:10.1016/j.jaci.2014.12.1925 |
| [134] |
Turner PJ, Southern J, Andrews NJ, et al. Safety of live attenuated influenza vaccine in young people with egg allergy:multicentre prospective cohort study[J]. BMJ, 2015, 351. DOI:10.1136/bmj.h6291 |
| [135] |
USCDC. Background Document for "Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2017-18 influenza season"[EB/OL]. (2018-09-04)[2018-09-30]. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/downloads/ACIP-recs-2017-18-bkgd.pdf.
|
| [136] |
Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines:recommendations of the advisory committee on immunization practices-United States, 2018-19 Influenza Season[J]. MMWR Recomm Rep, 2018, 67(3): 1-20. DOI:10.15585/mmwr.rr6703a1 |
| [137] |
Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older[J]. J Am Geriatr Soc, 2007, 55(10): 1499-1507. DOI:10.1111/j.1532-5415.2007.01397.x |
| [138] |
Ofori-Anyinam O, Leroux-Roels G, Drame M, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine co-administered with a 23-valent pneumococcal polysaccharide vaccine versus separate administration, in adults ≥50 years of age:results from a phase Ⅲ, randomized, non-inferiority trial[J]. Vaccine, 2017, 35(46): 6321-6328. DOI:10.1016/j.vaccine.2017.09.012 |
| [139] |
Chang YC, Chou YJ, Liu JY, et al. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan-a representative population-based comparative study[J]. J Infect, 2012, 65(3): 231-238. DOI:10.1016/j.jinf.2012.04.014 |
| [140] |
Christenson B, Pauksen K, Sylvan SP. Effect of influenza and pneumococcal vaccines in elderly persons in years of low influenza activity[J]. Virol J, 2008, 5: 52. DOI:10.1186/1743-422X-5-52 |
| [141] |
Yin MJ, Huang LF, Zhang Y, et al. Effectiveness and safety of dual influenza and pneumococcal vaccination versus separate administration or no vaccination in older adults:a Meta-analysis[J]. Expert Rev Vaccines, 2018, 17(7): 653-663. DOI:10.1080/14760584.2018.1495077 |
| [142] |
Poscia A, Collamati A, Carfì A, et al. Influenza and pneumococcal vaccination in older adults living in nursing home:a survival analysis on the shelter study[J]. Eur J Public Health, 2017, 27(6): 1016-1020. DOI:10.1093/eurpub/ckx150 |
| [143] |
Li CH, Gubbins PO, Chen GJ. Prior pneumococcal and influenza vaccinations and in-hospital outcomes for community- acquired pneumonia in elderly veterans[J]. J Hosp Med, 2015, 10(5): 287-293. DOI:10.1002/jhm.2328 |
| [144] |
Farkas K, Terhes G, Deak J, et al. The efficiency of influenza vaccines in patients with inflammatory bowel disease on immunosuppressive therapy[J]. Orv Hetil, 2012, 153(47): 1870-1874. DOI:10.1556/OH.2012.29484 |
| [145] |
Huemer HP. Possible immunosuppressive effects of drug exposure and environmental and nutritional effects on infection and vaccination[J]. Mediators Inflamm, 2015, 2015: 349176. DOI:10.1155/2015/349176 |
 2018, Vol. 39
2018, Vol. 39


