文章信息
- 李志丽, 李昱, 陈秋兰, 杨孝坤, 赵宏婷, 姜欣丽, 范思萌, 李丹, 秦颖, 彭质斌, 余建兴, 毛乃颖, 李中杰.
- Li Zhili, Li Yu, Chen Qiulan, Yang Xiaokun, Zhao Hongting, Jiang Xinli, Fan Simeng, Li Dan, Qin Ying, Peng Zhibin, Yu Jianxing, Mao Naiying, Li Zhongjie
- 复检核酸阳性的新型冠状病毒感染者流行分布及传染性特征分析
- Distribution and infectious characteristics of re-positive cases infected with SARS-CoV-2
- 中华流行病学杂志, 2021, 42(0): 0-0
- Chinese Journal of Epidemiology, 2021, 42(0): 0-0
- http://dx.doi.org/10.3760/cma.j.cn112338-20210506-00367
-
文章历史
收稿日期: 2021-05-06
2. 中国疾病预防控制中心传染病预防控制所, 北京 102206;
3. 中国疾病预防控制中心病毒病所, 北京 102206
2. National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3. National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China
新型冠状病毒肺炎(COVID-19)是由新型冠状病毒(新冠病毒)导致的传染性疾病。有关新冠病毒感染者的研究显示,新冠病毒排出可能持续数天至数周[1-3],部分感染者可在较长时间内检测出病毒核酸阳性[4]。其中,COVID-19患者(含有症状的病例和无症状感染者)在出院或解除隔离后的随访过程中,新冠病毒核酸复检阳性(复检阳性者)提示在样本中可以检测到新冠病毒核酸片段,但这并不表示一定存在具有复制能力或传染性的活病毒。
研究复检阳性者在新冠病毒感染者中的占比及其传播的风险,对于制定新冠病毒感染者的随访与隔离管理措施十分重要。本研究仅对单次感染的复检阳性者(复检阳性者)的特征进行综述,通过对国内外的新冠病毒感染者排毒持久性相关研究文献进行综述分析,为当前我国制定相应的防控措施提供参考依据。
一、复检阳性者分类理论上,复检阳性者可以分为两种情况,一是新冠病毒单次感染的复检阳性者,由于机体间歇性排毒[5]、不同的样本类型[5]、核酸检测结果假阴性[6]等因素影响,导致新冠病毒单次感染的同一病程中,某一时间点的样本核酸检测为阴性,出院随访过程中样本核酸检测又出现阳性。二是新冠病毒二次感染的复检阳性者(二次感染者),少数感染者由于初次感染后未能产生足够的中和抗体、免疫反应随时间减弱、病毒变异引起免疫逃逸或病毒再次感染不同的器官,使人体初次清除病毒后再次感染新冠病毒。新冠病毒基因测序可区分首次阳性和再次阳性标本中的病毒是否所属同一分支或型别,若不同时期检测出的新冠病毒分属不同的分支或型别,即为二次感染[7]。全球有文献报道的二次感染者较为罕见。由于试验所需的设备及环境条件较为严苛,开展基因测序相对困难,二次感染者数量有可能被低估。未来需要进一步开展有关深入研究和监测工作。
二、国内外新冠病毒感染者入院及出院或解除隔离标准1. 国内外新冠病毒感染者的入院标准:国内外对于新冠病毒感染者收治入院的标准存在较大差异。我国所有确诊患者全部收入定点医院接受治疗[8],无症状感染者进行隔离医学观察[9]。而WHO推荐综合评估新冠病毒感染者的临床表现、潜在风险、家庭条件等因素,允许轻度及中度感染者在家中自我隔离[10]。美国建议对中度(病毒性肺炎但无低氧血症)和严重COVID-19(呼吸困难、低氧血症或肺浸润 > 50%)病例开展评估和监测,收治入院治疗[11]。
2. 国内外新冠病毒感染者的出院标准:我国基于核酸检测策略,明确规定无症状感染者或临床症状改善的患者连续2次(至少间隔24 h)核酸检测阴性才能解除隔离或出院[8-9]。而WHO、欧盟及美国与我国的出院标准不同,基于症状与核酸检测相结合的策略,无症状感染者核酸检测阳性10 d后或有症状的病例在症状改善3 d后,无需核酸检测就可解除隔离[12]。欧盟CDC认为COVID-19患者发病后6周内呼吸道样本可持续阳性,但在症状出现15.3(95%CI:13.4~17.2)d后,分离到传染性活病毒的概率 < 5%。推荐根据病情严重程度,在症状出现10~20 d后出院或解除隔离[13]。美国CDC认为康复患者的上呼吸道标本中核酸阳性可长达12周。但88%和95%的患者标本在出现症状10 d和15 d后不产生具有复制能力的病毒,因此,无症状感染者核酸首次阳性之日起10 d内解除隔离,对于有症状的患者,在出现症状10 d后(患者无需使用退热药物且其他症状改善及退热超过24 h)解除隔离[14]。
三、复检阳性比例复检阳性者在出院或随访人群中所占的比例存在较大差异,不同研究的阳性率可能受研究方法、随访时长、随访人群特征、样本类型、检测策略与检测方法等多种因素影响。
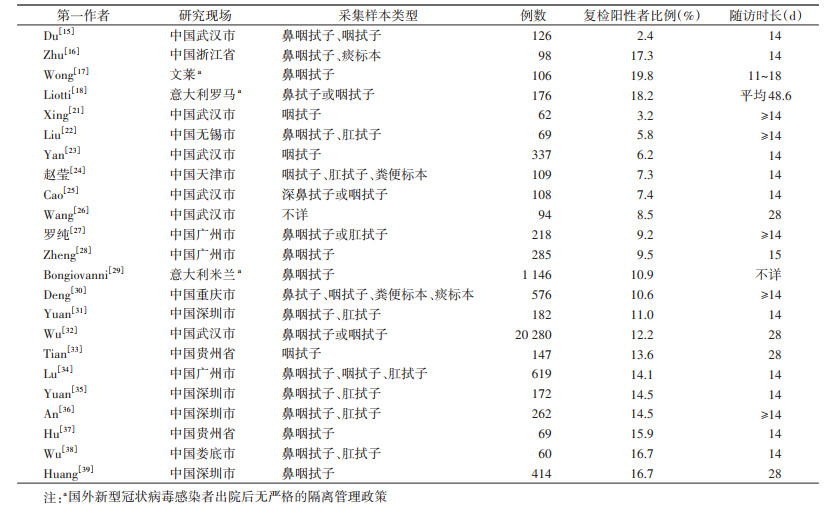
国内研究的复检阳性比例介于2.4%~17.3%[15-16]。意大利和文莱的两项研究显示,复检阳性比例约为19%[17-18],见表 1。2篇系统综述对相关文献进行荟萃分析后,估算总体复检阳性率分别为12.2%(95%CI:10.6%~13.7%)[19]和13.5%(95%CI:10.2%~16.8%)[20]。
复检阳性者首次复检阳性时间的中位数为出院后4~15 d[16, 23, 27, 32, 40-41]。复检阳性后经过治疗核酸转阴性的M(P25,P75)为10.63(7.67,15.63)d[32]。
五、复检阳性者特征1. 基本人口学特征:与未出现复检阳性的新冠病毒感染者相比,复检阳性者年龄更小[36],中位年龄范围为27~56岁[23, 32, 34, 37, 40],女性病例占12.5%~62.5%[23-25, 32, 37]。另外,有相当比例的复检阳性者报告了基础性疾病。61例复检阳性者中,慢性基础性疾病占39.3%[30],高血压和糖尿病较为常见[42],心脑血管疾病、肺部及内分泌系统等疾病也有报道[23]。
2. 临床分型特征:多篇研究认为病情越重的患者核酸持续阳性时间越长[43-44]。然而复检阳性者首次感染表现为轻症和普通型居多,占比85.26%[32],这可能与病情较轻患者的病毒载量较低[44]而导致出院时的核酸检测假阴性有关。再次入院后表现为无症状(72%)或轻症(28%)[40]。一项对20例复检阳性者的症状和影像学表现的详细报告中,无一例提示疾病发生进展[27]。绝大多数出现了临床症状的复检阳性者严重程度较第一次有所减轻[27, 36]。但也有复检阳性后病情恶化的报道,例如1例使用高剂量类固醇药物的无症状感染者和1例重症COVID-19患者出院后复检阳性病情加重[26, 45],3例具有多种基础疾病的老年感染者隔离期内复检阳性时疾病状况恶化,最终死亡[46]。
3. 抗体水平:复检阳性者复检阳性后新冠病毒特异性IgM、IgG、IgA抗体血清阳性率分别为11.11%~86.08%、52.00%~100.00%、61.54%~100.00%,总抗体和中和抗体的血清阳性率分别为98.72%和88.46%[31, 40-41, 47-49],提示绝大部分复检阳性者体内产生了新冠病毒特异性抗体。58例复检阳性者(98.3%)中和抗体滴度 > 4(范围4~1 024),与未出现复检阳性的新冠病毒感染者和住院患者类似[34]。
六、复检阳性者传染性新冠病毒核酸检测阳性,提示在样本中可以检测到新冠病毒核酸片段,但无法明确该病毒片段是否具有活性,亦无法判断该感染者是否具有传染性。因此,需进行病原学病毒分离培养实验且结合流行病学调查资料,综合研判复检阳性者的传染性。
1. 病原学证据:96%的复检阳性者的最大病毒载量 < 5 log10拷贝数/ml[40],研究显示当样本中病毒载量 < 5.4 log10拷贝数/ml时,分离出新冠病毒的概率较低[50]。复检阳性者首次住院的Ct值低于复检阳性后的Ct值,提示复检阳性后病毒载量较低[51]。
2项研究从48例复检阳性者中发现4例样本(1份鼻/咽拭子和3份肛拭子)存在亚基因组信使RNA(sgmRNA)[18, 52],而检出sgmRNA意味着感染细胞存在病毒复制,提示感染者体内可能存在活病毒[50, 52]。但是,目前大多数新冠病毒核酸阳性标本未能成功分离活病毒[34, 40, 53],仅有少数研究从3例经基因测序排除二次感染的复检阳性者标本中分离培养到活病毒。1例是免疫缺陷的新冠病毒感染者[54],另外2例复检阳性者的CT影像学结果异常[55]。
2. 流行病学证据:基于流行病学调查,结合新冠病毒核酸或抗体检测,多项研究中复检阳性者在复检阳性前后可能传染期内有近5 000例密切接触者[17, 26, 32, 36, 38, 40, 42, 45, 56-57]。仅一项研究报告了1例使用高剂量类固醇药物的无症状感染者,核酸检测阴性出院后10 d核酸结果再次阳性,其3例仅与该患者接触的家庭成员出现呼吸道症状且核酸检测阳性[45]。其他研究中均无证据显示复检阳性者在密切接触者中引起传播。
七、讨论COVID-19康复者有一定比例出现复检阳性现象,国内外复检阳性比例分别为2.4%~17.3%和10.9%~19.8%,考虑到国外新冠病毒流行水平较高,流行病学证据强度稍弱于国内,可能在一定程度上造成报告的复检阳性比例略高于国内。复检阳性后表现为无症状感染者或轻症的可能性大,后续发展为重症的风险较小,但患有多种基础疾病的老年人以及免疫缺陷或使用高剂量类固醇药物的复检阳性者病情恶化也见报道。因此,对于此类有基础疾病或免疫缺陷的特殊复检阳性人群,需要密切关注各项指标的变化,预防病情恶化。
尽管现有证据显示复检阳性者造成传播的风险较小,但有1例使用高剂量类固醇药物的复检阳性者引起密切接触者发病的研究报道[45],也有既往研究在免疫缺陷或影像学异常的复检阳性者中分离到活病毒[54-55],提示极个别的复检阳性者可能存在传染性风险,特别是免疫低下或有异常影像学表现者。基于目前的证据,应重点关注影像学异常或曾应用免疫抑制剂的个体。对于免疫功能低下的康复者,在常规防控措施之外可延长随访时间,加强对其出院后密切接触者的健康状况监测,敦促严格落实个人防护措施。
本研究探讨的“复检阳性”是一个较为广泛的概念。关于复检阳性的解释有很多假设,主要分为两个方面:
1. 出院前的新冠病毒核酸检测结果不准确,其结果受检测试剂质量、标本质量和采样部位等因素的影响[58]。新冠病毒核酸检测作为确诊COVID-19的常规手段,试剂盒的灵敏度、不适当的采样等人为因素均可能导致假阴性和漏检。我国已经批准多个企业生产的新冠病毒检测产品上市,不同产品的灵敏度不同。可考虑在医疗资源较为充足的地区,对准备出院人员和出院后隔离人员同时采用2个不同生产厂家的试剂分别检测。在此基础之上,定期对采样人员进行标准化的培训和考核,尽可能地保证核酸检测结果的准确性。此外,在不同的采样部位均可检出新冠病毒核酸,如上呼吸道、下呼吸道和粪便标本。但不同部位的病毒载量不同[59]且核酸持续时间存在差异[60],有研究显示鼻部病毒载量高于咽部[59]。粪便标本的核酸阳性持续时间更长[60],然而检出核酸片段也不等同于具有传染性,对传播的意义尚不明确。目前仍以呼吸道标本(尤其是鼻咽拭子)核酸阴性作为出院或解除隔离的参考标准。
2. 恢复期感染者体内病毒载量波动[5]或残留[61]可能是复检阳性的主要原因。即使感染者体内不存在活病毒,但其体内的核酸清除需要一定的时间(体内可检测到核酸的持续时间要远长于可检测到活病毒的时间),由于个体间存在差异,部分恢复期感染者病毒核酸检出持续时间较长,或由于应用免疫抑制剂或免疫缺陷等原因使免疫反应减弱,抑制病原体清除[62],从而可能出现病毒载量波动或病毒残留。1例死于心血管意外的感染者连续3次新冠病毒核酸检测皆为阴性,最后在其肺细胞中检出残留的新冠病毒[61]。也有研究提示新冠病毒潜伏在免疫细胞内[63],从而导致感染者康复期复检阳性。可考虑对于复检阳性的标本开展检测,若Ct值< 34.3[64]或病毒载量 > 5.4 log10拷贝数/ml[50],可进一步开展病毒分离培养实验,若提示存在活病毒则需延长住院或隔离时间。
既往研究显示存在新冠病毒核酸持续阳性的感染者,年龄较大、具有基础疾病的感染者病毒排出时间较长[65],这可能是由于较弱的免疫反应引起的[66]。但也有研究发现,新冠病毒核酸持续阳性的感染者其IgM抗体水平较高、持续时间较长[67],另有研究发现,长期携带者与康复者的IgM、IgG抗体以及中和抗体谱相似[55]。复检阳性者即使在血清抗体转阳后,新冠病毒核酸仍可显示阳性[66, 68]。
缺乏中和抗体的复检阳性者可能提示携带活病毒[69]。1例检出活病毒的复检阳性者,IgM、IgG抗体均为阳性,但其中和抗体滴度较低[54]。目前IgM抗体、IgG抗体、中和抗体滴度与核酸复检阳性以及长期持续阳性的关系尚不明确。
WHO认为新冠病毒感染者在症状出现9 d后分离出活病毒的情况很罕见,满足出院条件后,不再推荐进行隔离[12]。尽管复检阳性者的传播能力较低,但为避免引起疾病传播,我国多地已经实施出院后集中或居家隔离观察14 d,在医学隔离观察期内继续随访复查核酸的措施。但现有资料缺乏出院后14 d隔离期内核酸阳性样本的病毒分离证据,因此有待后续更进一步地深入评估和研究当前出院后隔离政策,科学地调整防控方案。
利益冲突 所有作者均声明不存在利益冲突
本文编辑 斗智
| [1] |
Zhou F, Yu T, Du RH, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study[J]. Lancet, 2020, 395(10229): 1054-1062. DOI:10.1016/S0140-6736(20)30566-3 |
| [2] |
Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore[J]. JAMA, 2020, 323(15): 1488-1494. DOI:10.1001/jama.2020.3204 |
| [3] |
Zhao JJ, Yuan Q, Wang HY, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019[J]. Clin Infect Dis, 2020, 71(16): 2027-2034. DOI:10.1093/cid/ciaa344 |
| [4] |
Zapor M. Persistent detection and infectious potential of SARS-CoV-2 virus in clinical specimens from COVID-19 patients[J]. Viruses, 2020, 12(12): 1384. DOI:10.3390/v12121384 |
| [5] |
Yu FT, Yan LT, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients[J]. Clin Infect Dis, 2020, 71(15): 793-798. DOI:10.1093/cid/ciaa345 |
| [6] |
Li YF, Yao L, Li JW, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19[J]. J Med Virol, 2020, 92(7): 903-908. DOI:10.1002/jmv.25786 |
| [7] |
European Center For Disease Prevention and Control (ECDC). Sequencing of SARS-CoV-2: first update[EB/OL]. (2021-01-18)[2021-03-11]. https://www.ecdc.europa.eu/en/publications-data/sequencing-sars-cov-2.
|
| [8] |
国家卫生健康委员会办公厅, 国家中医药管理局办公室. 关于印发新型冠状病毒肺炎诊疗方案(试行第八版)的通知[EB/OL]. (2020-08-18)[2021-04-01]. http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml. General Office of the National Health Commission, Office of the State Administration of Traditional Chinese Medicine. Protocol of diagnosis and treatment for COVID-19(trial version 8)[EB/OL]. (2020-08-18)[2021-04-01]. http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml. |
| [9] |
国家卫生健康委员会办公厅. 关于印发新型冠状病毒肺炎防控方案(第七版)的通知[EB/OL]. (2020-09-15)[2020-05-01]. http://www.nhc.gov.cn/jkj/s3577/202009/318683cbfaee4191aee29cd774b19d8d.shtml. General Office of National Health Commission. Prevention and control protocol for COVID-19(version 7)[EB/OL]. (2020-09-15)[2020-05-01]. http://www.nhc.gov.cn/jkj/s3577/202009/318683cbfaee4191aee29cd774b19d8d.shtml. |
| [10] |
World Health Organization (WHO). Clinical management of COVID-19[EB/OL]. (2020-05-27)[2020-01-10]. https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf
|
| [11] |
National Institutes of Health (NIH). General management of nonhospitalized patients with acute COVID-19[EB/OL]. (2021-07-08)[2020-01-10]. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-patients-general-management/
|
| [12] |
世界卫生组织. COVID-19患者解除隔离的标准[EB/OL]. (2020-06-17)[2020-12-27]. https://apps.who.int/iris/bitstream/handle/10665/332451/WHO-2019-nCoV-Sci_Brief-Discharge_From_Isolation-2020.1-chi.pdf World Health Organization (WHO). Criteria for the removal of COVID-19 patients from isolation[EB/OL]. (2020-06-17)[2020-12-27]. https://apps.who.int/iris/bitstream/handle/10665/332451/WHO-2019-nCoV-Sci_Brief-Discharge_From_Isolation-2020.1-chi.pdf |
| [13] |
European Center for Disease Prevention and Control (ECDC). Guidance for discharge and ending of isolation of people with COVID-19[EB/OL]. (2020-10-16)[2021-02-24]. https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-for-discharge-and-ending-of-isolation-of-people-with-COVID-19.pdf
|
| [14] |
Centers for Disease Control and Prevention. Interim guidance on ending isolation and precautions for adults with COVID-19[EB/OL]. (2021-03-16)[2021-06-18]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
|
| [15] |
Du HW, Chen JN, Pan XB, et al. Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients[J]. Eur J Clin Microbiol Infect Dis, 2021, 40(2): 413-417. DOI:10.1007/s10096-020-04024-1 |
| [16] |
Zhu H, Fu LY, Jin YH, et al. Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection[J]. J Clin Lab Anal, 2020, 34(7): e23392. DOI:10.1002/jcla.23392 |
| [17] |
Wong J, Koh WC, Momin RN, et al. Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: Evidence from Brunei Darussalam[J]. J Med Virol, 2020, 92(11): 2847-2851. DOI:10.1002/jmv.26199 |
| [18] |
Liotti FM, Menchinelli G, Marchetti S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results[J]. JAMA Intern Med, 2021, 181(5): 702-704. DOI:10.1001/jamainternmed.2020.7570 |
| [19] |
Ren XY, Ren XG, Lou JA, et al. A systematic review and meta-analysis of discharged COVID-19 patients retesting positive for RT-PCR[J]. EClinicalMedicine, 2021, 34: 100839. DOI:10.1016/j.eclinm.2021.100839 |
| [20] |
张奕, 孙瑛, 段玮, 等. 新型冠状病毒肺炎患者出院后核酸复阳发生率的Meta分析[J]. 国际病毒学杂志, 2021, 28(1): 6-10. Zhang Y, Sun Y, Duan W, et al. Recurrence of SARS-CoV-2 viral RNA positive in discharged COVID-19 patients: a meta-analysis[J]. Int J Virol, 2021, 28(1): 6-10. DOI:10.3760/cma.j.issn.1673-4092.2021.01.002 |
| [21] |
Xing YY, Mo PZ, Xiao Y, et al. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019(COVID-19), China, January to February 2020[J]. Euro Surveill, 2020, 25(10): 2000191. DOI:10.2807/1560-7917.ES.2020.25.10.2000191 |
| [22] |
Liu J, Xiao Y, Shen Y, et al. Detection of SARS-CoV-2 by RT-PCR in anal from patients who have recovered from coronavirus disease 2019[J]. J Med Virol, 2020, 92(10): 1769-1771. DOI:10.1002/jmv.25875 |
| [23] |
Yan N, Wang W, Gao YZ, et al. Medium term follow-up of 337 patients with coronavirus disease 2019(COVID-19) in a Fangcang shelter hospital in Wuhan, China[J]. Front Med, 2020, 7: 373. DOI:10.3389/fmed.2020.00373 |
| [24] |
赵莹, 吴伟慎, 何海艳, 等. 天津市新型冠状病毒肺炎确诊病例治愈出院后核酸阳转情况分析[J]. 第三军医大学学报, 2020, 42(9): 879-882. Zhao Y, Wu WS, He HY, et al. Analysis of re-positive nucleic acid conversion in patients recovered from COVID-19 in Tianjin[J]. J Third Milit Med Univ, 2020, 42(9): 879-882. DOI:10.16016/j.1000-5404.202003103 |
| [25] |
Cao H, Ruan L, Liu J, et al. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge[J]. J Med Virol, 2020, 92(10): 2159-2164. DOI:10.1002/jmv.26017 |
| [26] |
Wang X, Xu H, Jiang H, et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study[J]. QJM, 2020, 113(9): 657-665. DOI:10.1093/qjmed/hcaa178 |
| [27] |
罗纯, 温学良, 谭颖, 等. 广州地区新型冠状病毒肺炎出院病例核酸再次阳性的临床特征[J]. 广东医学, 2020, 41(13): 1297-1301. Luo C, Wen XL, Tan Y, et al. The clinical characteristics of recovered patients with coronavirus disease 2019 who retested positive for the virus in Guangzhou[J]. Guangdong Med J, 2020, 41(13): 1297-1301. DOI:10.13820/j.cnki.gdyx.20201082 |
| [28] |
Zheng JZ, Zhou R, Chen FJ, et al. Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: A prospective cohort study[J]. PLoS Neglect Trop Dis, 2020, 14(8): e0008648. DOI:10.1371/journal.pntd.0008648 |
| [29] |
Bongiovanni M, Vignati M, Giuliani G, et al. The dilemma of COVID-19 recurrence after clinical recovery[J]. J Infect, 2020, 81(6): 979-997. DOI:10.1016/j.jinf.2020.08.019 |
| [30] |
Deng W, Guang TW, Yang M, et al. Positive results for patients with COVID-19 discharged form hospital in Chongqing, China[J]. BMC Infect Dis, 2020, 20(1): 429. DOI:10.1186/s12879-020-05151-y |
| [31] |
Yuan B, Liu HQ, Yang ZR, et al. Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation[J]. Sci Rep, 2020, 10(1): 11887. DOI:10.1038/s41598-020-68782-w |
| [32] |
Wu XM, Wang ZM, He ZY, et al. A follow-up study shows no new infections caused by patients with repeat positive of COVID-19 in Wuhan[J]. medRxiv, 2020. DOI:10.1101/2020.11.18.20232892 |
| [33] |
Tian ML, Long YJ, Hong Y, et al. The treatment and follow-up of 'recurrence' with discharged COVID-19 patients: data from Guizhou, China[J]. Environ Microbiol, 2020, 22(8): 3588-3592. DOI:10.1111/1462-2920.15156 |
| [34] |
Lu J, Peng JJ, Xiong QL, et al. Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR[J]. Ebiomedicine, 2020, 59: 102960. DOI:10.1016/j.ebiom.2020.102960 |
| [35] |
Yuan J, Kou SL, Liang YH, et al. Polymerase chain reaction assays reverted to positive in 25 discharged patients with COVID-19[J]. Clin Infect Dis, 2020, 71(16): 2230-2232. DOI:10.1093/cid/ciaa398 |
| [36] |
An JH, Liao XJ, Xiao TY, et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test[J]. Ann Transl Med, 2020, 8(17): 1084. DOI:10.21037/atm-20-5602 |
| [37] |
Hu RJ, Jiang ZX, Gao HM, et al. Recurrent positive reverse transcriptase-polymerase chain reaction results for coronavirus disease 2019 in patients discharged from a Hospital in China[J]. JAMA Network Open, 2020, 3(5): e2010475. DOI:10.1001/jamanetworkopen.2020.10475 |
| [38] |
Wu JR, Liu XY, Liu JJ, et al. Coronavirus disease 2019 test results after clinical recovery and hospital discharge among patients in China[J]. JAMA Netw Open, 2020, 3(5): e209759. DOI:10.1001/jamanetworkopen.2020.9759 |
| [39] |
Huang J, Zheng L, Li Z, et al. Kinetics of SARS-CoV-2 positivity of infected and recovered patients from a single center[J]. Sci Rep, 2020, 10(1): 18629. DOI:10.1038/s41598-020-75629-x |
| [40] |
Yang C, Jiang M, Wang XH, et al. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study[J]. Emerg Microbes Infect, 2020, 9(1): 2368-2378. DOI:10.1080/22221751.2020.1837018 |
| [41] |
Mei Q, Li J, Du RH, et al. Assessment of patients who tested positive for COVID-19 after recovery[J]. Lancet Infect Dis, 2020, 20(9): 1004-1005. DOI:10.1016/S1473-3099(20)30433-3 |
| [42] |
Hong K, Cao W, Liu ZY, et al. Prolonged presence of viral nucleic acid in clinically recovered COVID-19 patients was not associated with effective infectiousness[J]. Emerg Microbes Infect, 2020, 9(1): 2315-2321. DOI:10.1080/22221751.2020.1827983 |
| [43] |
Fang ZX, Zhang Y, Hang CF, et al. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients[J]. J Infect, 2020, 81(1): 147-178. DOI:10.1016/j.jinf.2020.03.013 |
| [44] |
Zheng SF, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020:retrospective cohort study[J]. BMJ, 2020, 369: m144. DOI:10.1136/bmj.m1443 |
| [45] |
de Jesus RP, Silva R, Aliyeva E, et al. Reactivation of SARS-CoV-2 after asymptomatic infection while on high-dose corticosteroids. Case report[J]. SN Comprehens Clin Med, 2020, 2(11): 2402-2405. DOI:10.1007/s42399-020-00548-x |
| [46] |
Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID-19 in older patients: a description of three cases[J]. J Am Geriatr Soc, 2020, 68(10): 2179-2183. DOI:10.1111/jgs.16728 |
| [47] |
Zou Y, Wang BR, Sun L, et al. The issue of recurrently positive patients who recovered from COVID-19 according to the current discharge criteria: investigation of patients from multiple medical institutions in Wuhan, China[J]. J Infect Dis, 2020, 222(11): 1784-1788. DOI:10.1093/infdis/jiaa301 |
| [48] |
Shui TJ, Li C, Liu HB, et al. Characteristics of recovered COVID-19 patients with recurrent positive RT-PCR findings in Wuhan, China: a retrospective study[J]. BMC Infect Dis, 2020, 20(1): 749. DOI:10.1186/s12879-020-05463-z |
| [49] |
Chen J, Xu XP, Hu J, et al. Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China[J]. Aging, 2020, 12(17): 16675-16689. DOI:10.18632/aging.103795 |
| [50] |
Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019[J]. Nature, 2020, 581(7809): 465-469. DOI:10.1038/s41586-020-2196-x |
| [51] |
秦维超, 孙贵银, 张运洪, 等. 3例COVID-19出院后核酸复检阳性患者的检测分析[J]. 病毒学报, 2020, 36(4): 554-559. Qin WC, Sun GY, Zhang YH, et al. Patients with COVID-19 testing positive for nucleic acids of SARS-CoV-2 in Re-examination after discharge from hospital: an analysis of three cases[J]. Chin J Virol, 2020, 36(4): 554-559. DOI:10.13242/j.cnki.bingduxuebao.003722 |
| [52] |
Hu FY, Chen FJ, Ou ZH, et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract[J]. Cell Mol Immunol, 2020, 17(11): 1119-1125. DOI:10.1038/s41423-020-00550-2 |
| [53] |
Kang YJ. South Korea's COVID-19 infection status: from the perspective of re-positive test results after viral clearance evidenced by negative test results[J]. Disaster Med Public, 2020, 14(6): 762-764. DOI:10.1017/dmp.2020.168 |
| [54] |
Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host[J]. N Engl J Med, 2020, 383(23): 2291-2293. DOI:10.1056/NEJMc2031364 |
| [55] |
Li Q, Zheng XS, Shen XR, et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19[J]. Emerg Microbes Infect, 2020, 9(1): 2571-2577. DOI:10.1080/22221751.2020.1852058 |
| [56] |
Yang ZQ, Chen XF, Huang RB, et al. Atypical presentations of coronavirus disease 2019(COVID-19) from onset to readmission[J]. BMC Infect Dis, 2021, 21(1): 127. DOI:10.1186/s12879-020-05751-8 |
| [57] |
Wang P. Recurrent presence of SARS-CoV-2 RNA in a 33-year-old man[J]. J Med Virol, 2021, 93(2): 592-594. DOI:10.1002/jmv.26334 |
| [58] |
Zhang B, Liu SY, Dong YH, et al. Positive rectal swabs in young patients recovered from coronavirus disease 2019(COVID-19)[J]. J Infect, 2020, 81(2): e49-52. DOI:10.1016/j.jinf.2020.04.023 |
| [59] |
Yu J, Yen H, Huang H, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients[J]. N England J Med, 2020, 382(12): 1177-1179. DOI:10.1056/NEJMc2001737 |
| [60] |
Weiss A, Jellingsø M, Sommer MOA. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: A systematic review and meta-analysis[J]. Ebiomedicine, 2020, 58: 102916. DOI:10.1016/j.ebiom.2020.102916 |
| [61] |
Yao XH, He ZC, Li TY, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient[J]. Cell Res, 2020, 30(6): 541-543. DOI:10.1038/s41422-020-0318-5 |
| [62] |
Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury[J]. The Lancet, 2020, 395(10223): 473-475. DOI:10.1016/S0140-6736(20)30317-2 |
| [63] |
Elberry MH, Ahmed H. Occult SARS-CoV-2 infection; a possible hypothesis for viral relapse[J]. Med Hypotheses, 2020, 144: 109980. DOI:10.1016/j.mehy.2020.109980 |
| [64] |
Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection[J]. J Infect, 2020, 81(3): 357-371. DOI:10.1016/j.jinf.2020.06.067 |
| [65] |
Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2:a preliminary study from 56 COVID-19 patients[J]. Clin Infect Dis, 2020, 71(16): 2249-2251. DOI:10.1093/cid/ciaa460 |
| [66] |
Carmo A, Pereira-Vaz J, Mota V, et al. Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19[J]. J Med Virol, 2020, 92(10): 2227-2231. DOI:10.1002/jmv.26103 |
| [67] |
Li N, Wang X, Lv TF. Prolonged SARS-CoV-2 RNA shedding: Not a rare phenomenon[J]. J Med Virol, 2020, 92(11): 2286-2287. DOI:10.1002/jmv.25952 |
| [68] |
Liu WD, Chang SY, Wang JT, et al. Prolonged virus shedding even after seroconversion in a patient with COVID-19[J]. J Infect, 2020, 81(2): 318-356. DOI:10.1016/j.jinf.2020.03.063 |
| [69] |
van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019(COVID-19)[J]. Nat Commun, 2021, 12(1): 267. DOI:10.1038/s41467-020-20568-4 |
 2021, Vol. 42
2021, Vol. 42