b Liver Transplant Center, Transplant Center, West China Hospital, Sichuan University, Chengdu 610041, China;
c Chengdu New Radiomedicine Technology Co., Ltd., Chengdu 610200, China;
d National Atomic Energy Agency Nuclear Technology (Nonclinical evaluation of radiopharmaceuticals) Research and Development Center, China Institute for Radiation Protection, Taiyuan 030006, China
Liver cancer currently ranks fifth most common malignant tumors globally and the third leading cause of cancer related death worldwide [1]. It is expected that the number of new confirmed cases and deaths of liver cancer worldwide will increase by 55% by 2040 [2]. Additionally, the liver is a common metastatic site for tumors originating in organs drained by the portal vein, including colorectal, pancreatic and stomach malignancies [3]. Especially, colorectal malignancies, with the current incidence rate ranking the third among people worldwide, have a notable tendency to metastasize exclusively to the liver [4]. About 50% of colorectal cancer patients will experience hepatic metastases during the disease progression [5]. While surgical resection is considered the primary treatment option for both primary and metastatic liver malignancies, only 10%–20% of patients can directly benefit from this approach [2,6]. Other treatments include ablation, liver transplantation, chemotherapy, embolization, immunotherapy, and radiation therapy [7]. Selective internal radiation therapy (SIRT), as a radioactive embolization therapy method, has been widely used in the treatment of unresectable primary and secondary liver malignancies and has shown good tolerance, potent anti-tumor effect [8].
SIRT is also known as transarterial radioembolization (TARE). In this treatment, radioactive microspheres were injected into artery feeding the tumor through catheter intervention and carried by the bloodstream into the microvascular that supplies the tumors [9]. The treatment of malignant tumor is achieved by continuously emitting lethal high energy radiation [9]. Over the past two decades, clinical practice of 90Y-resin microspheres (SIR-SpheresⓇ), 90Y-glass microspheres (TheraSphereⓇ) and 166Ho-resin microspheres (QuiremSphereⓇ) has provided valuable insight of the relationship between the characteristics of radioactive microspheres (Radionuclide properties, specific activity, quantity, particle size and density of microspheres) and the clinical effects, side effects, dose-effect, etc. [10–13]. Moreover, such experience paved the way for designing microspheres with better performance and greater clinical benefits to overcome the shortcomings of existing radioactive microspheres. The limitations include perfusion stasis, reflux, heterogeneous distribution, ectopic distribution, insufficient dosage, and inconvenient clinical administration [11,13].
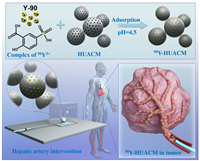
In this study, we report a new radioactive microsphere, 90Y-carbon microsphere (90Y-HUACM), which has high uniformity, high specific activity and good availability for selective internal radiation therapy for liver cancer (Fig. 1). It is prepared by planar molecular complex adsorption and chemical deposition solidification technology. Y-90, recognized as the most widely used, high-performance, and cost-effective radioactive nuclide in selective internal radiation therapy, has shown significant advantages in treating rapidly proliferating malignant tumors such as liver cancer and colorectal cancer with liver metastasis, as supported by extensive clinical data [14]. Carbon microspheres are porous, high strength and low relative density carbon materials with outstanding adsorption performance, excellent biocompatibility, and good physicochemical stability [15,16]. With a particle size of 20–45 µm and mono-dispersity, it prevents ectopic, non-targeted and non-uniform distribution, enabling precisely delivery and uniform distribution in tumors. Leveraging the exceptional properties of Y-90 and carbon microspheres, 90Y-HUACM was delivered into rabbit VX2 liver tumors through microcatheters via hepatic arteries. It resulted in outstanding local precise distribution, in vivo stability, and remarkable tumor inhibitory effects. With such high performance, 90Y-HUACM emerges as a promising radioactive microsphere for precise local internal radiation therapy. It also holds the potential to address many clinical application shortcomings associated with current radioactive microspheres.
|
Download:
|
| Fig. 1. Schematic of the formulation and application in selective internal radiation therapy (SIRT) of 90Y-HUACM. | |
The carbon microspheres were produced through a highly controllable synthesis route involving high-temperature carbonization and activation of resin microsphere, which were initially prepared by emulsion polymerization. This method allows for approximately 30 g of carbon microspheres in a single batch. The particle size and morphology of carbon microspheres can be controlled by stirring speed, the ratio of aqueous and organic phases, etc. Preparation of 90Y-HUACM follows the method outlined in patent [17]. The efficient labeling of Y-90 onto carbon microspheres primarily involves the adsorption of the complexes of 5-sulfosalicylic acid with Y-90 ions on carbon microspheres, precipitation and solidification with sodium phosphate solution, cleaning with sterile injection water, and steam sterilization treatment. To investigate the morphology, structure and properties of 90Y-HUACM, nonradioactive carbon microspheres (denoted as Y-HUACM) were prepared using 89YCl3 instead of 90YCl3 by following the same synthesis procedures for 90Y-HUACM.
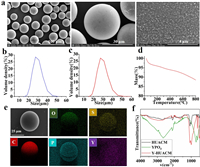
SEM and particle size distribution were employed to characterize the morphology and size of HUACM, Y-HUACM and 90Y-HUACM. HUACM (Fig. S1a in Supporting information), Y-HUACM (Figs. 2a and b) and 90Y-HUACM (Fig. 2c and Fig. S1b in Supporting information) exhibit sizes of 30 ± 15 µm, with > 90% of the microspheres falling into 20–45 µm, and have uniform morphology and monodisperse. Following Y-90 loading and steam sterilization, no significant changes were observed in the particle size and morphology. In liver tumors, the typical diameter of capillaries ranges from 5 µm to 10 µm and the diameter of arterioles ranges from 10 µm to 20 µm [18,19]. It is essential for radioactive microsphere used for selective internal radiation therapy of liver cancer to have a particle size of 20–60 µm for better delivery and distribution [9,11]. Existing marketed radioactive microsphere products such as 90Y-glass microspheres and 90Y-resin microspheres with a mean diameter of 25 ± 10 and 32 ± 10 µm, respectively [11,20]. 90Y-HUACM have an ideal particle size design, which is expected to prevent perfusion reflux and stasis, avoid ectopic distribution, and achieve precise local tumor embolization.
|
Download:
|
| Fig. 2. Characteristics of Y-HUACM and 90Y-HUACM. (a) SEM images of Y-HUACM. (b) Size distribution of Y-HUACM. (c) Size distribution of 90Y-HUACM. (d) TGA spectra of Y-HUACM. (e) Energy dispersion spectroscopy (EDS) mapping of Y-HUACM. (f) FT-IR spectrum of HUACM, YPO4 and Y-HUACM. | |
Based on the thermal gravimetric analysis (TGA) results, Y-HUACM exhibits thermal weight loss < 5% below 300 ℃ and < 10% below 600 ℃, which has a high thermal stability (Fig. 2d). Energy dispersion spectroscopy (EDS) mapping (Fig. 2e) of Y-HUACM revealed that phosphorus, oxygen and yttrium elements were clearly presented and uniformly distributed on carbon microspheres. The Fourier transform-infrared (FT-IR) spectroscopy registered for YPO4, HUACM and Y-HUACM are shown in Fig. 2f. The peaks at 638, 1009, and 1072 cm−1 in the spectrum of HUACM are shifted to 635, 994, and 1075 cm−1, respectively and shows a notably absorption increase for Y-HUACM [21]. Since the band at 994 and 1075 cm−1 was tentatively ascribed to the VS(C-O-C) and VS(O-P-O) structures of Y-HUACM [22]. Furthermore, the X-ray photoelectron spectroscopy (XPS) spectra (Fig. 3a) confirm absorption peaks of Y 3s, Y 3p, Y 3d, P 2s, P 2p, O 1s and C 1s were clearly observed and the absorption peaks of Y-HUACM and YPO4 are almost overlap. From high-resolution XPS spectra of Y-HUACM (Figs. S2a and b in Supporting information), the absorption peaks at 161.06, 159.08, 134.08 eV corresponding to Y 3d3/2, Y 3d5/2 and P 2p [23,24]. Y-HUACM displayed multiple new absorption peaks which almost completely coincide with YPO4. Additionally, the crystallographic structure of HUACM was measured by X-ray diffraction (XRD). As shown in Fig. 3b, no crystal structures were observed. These results indicated that yttrium on Y-HUACM was loaded in the form of amorphous yttrium phosphate.

|
Download:
|
| Fig. 3. Structure and stability characteristics of 90Y-HUACM. (a) XPS spectra of YPO4 and Y-HUACM. (b) XRD spectrum of Y-HUACM. (c) Pore size distribution of HUACM by BET. (d) Pore size distribution of Y-HUACM by BET. (e) The variation of adsorption rate with the amount of Y-90 loaded on carbon microspheres (MBq/mg). (f) Stability of different specifications of 90Y-HUACM in 37 ℃ saline. | |
The results of pore distribution and specific surface area analysis indicated that HUACM possesses rich in microporous and mesoporous structures, with the adsorption average pore diameter is about 16.8 Å, and specific surface area exceeding 1900 m2/g (Fig. 3c and Fig. S3a in Supporting information). Almost no microporous or mesoporous structures were detected on Y-HUACM (Fig. 3d and Fig. S3b in Supporting information), and the Brunauer-Emmet-Teller (BET) surface area were decreased from 1990.1 m2/g to 317.7 m2/g. Furthermore, scanning electron microscopy (SEM) analysis revealed almost no aggregation or deposition of yttrium phosphate particles on the surface of carbon microspheres. These results proved that yttrium phosphate forms sediment in the pores of carbon microspheres. Compared with the surface loading, loading in pores helped avoid or to some extent reduced the dissolving and/or falling off of Y-90 into solution, which may mitigate the safety risks associated with ectopic distribution in clinical application.
As shown in Fig. 3e, over 92 MBq of Y-90 nuclide can be loaded onto 1 mg of carbon microspheres and achieved a loading rate exceeding 95%. This adsorption process exhibited rapid kinetic. The high loading rates and rapid adsorption kinetics of 90Y-HUACM can significantly improve the production efficiency, and make the production process more environmentally friendly and economical. The exceptional Y-90 loading efficiency and capacity of HUACM enable precise regulation to achieve optimal specific activity and suitable quantity of 90Y-HUACM for liver cancer treatment. Further assessment of quantity indicated ≈6.0 × 104 particles in every milligram of HUACM. When 67 MBq of Y-90 were loaded on each milligram of HUACM, it translated to about 1110 Bq of Y-90 was loaded on each microsphere. To further evaluate the in vitro stability of 90Y-HUACM, with a specification of 1, 3, 10, 13 GBq, the microspheres were dispersed in 37 ℃ saline for 8 days. As shown in Fig. 3f, the results indicated that although the leaching rate slowly increased, the Y-90 leaching rate of 90Y-HUACM remained consistently far below 0.1% throughout the entire testing cycle. Such a low leaching rate of Y-90 is expected to have no significant impact on the safety and effectiveness of 90Y-HUACM.
In clinical application, SIR-SpheresⓇ is supplied as a 3 GBq dose at calibration, containing approximately 60 million microspheres per vial, with a specific activity of 50 Bq per sphere [25]. However, due to its low specific activity, achieving sufficient therapeutic dose requires injecting 40–80 million microspheres in tumors of each patient [11,26]. The large quantity of microspheres can lead to early stasis in about 20% of the perfusion procedures, resulting in an incomplete delivery of the prescribed activity and a suboptimal dose to tumors [27,28]. Consequently, some patients may experience refluxing or abnormal deposition, leading to radiation inflammation and other adverse reactions in the lungs, gastrointestinal tract, etc. [11,29]. TheraSphereⓇ can be customized to provide in six activity specifications 3, 5, 7, 10, 15, and 20 GBq [11,30]. Its specific activity is 2500 Bq per sphere, with a corresponding microsphere quantity ranging from 1.2 million to 8 million per dose [11]. Due to the relatively small quantity of microspheres, no evidence of flow stasis or reflux was observed [31]. However, microspheres with limited quantity induce a more heterogeneous dose distribution, especially in some large tumors, although contributes to reduced toxicity [11].
For enhanced effectiveness, safety, and convenient for clinical use, 90Y-HUACM can be customized for patients with specifications ranging from 1 GBq to 13 GBq per vial. And its specific activity is 1170 Bq per sphere, with corresponding 0.9–11.7 million microspheres per dose. The excellent performance and the optimized design of the quantity and specific activity of 90Y-HUACM allow for the avoidance of perfusion difficulties caused by excessive number of microspheres, as well as complete elimination of heterogeneous dose distribution. More importantly, this design ensures the accurate delivery of planned high dose to the tumor.
Furthermore, a study on simulated drug delivery was conducted using a perfusion delivery device (Fig. S6a in Supporting information). To simulate the clinical infusion process of radioactive microspheres, saline was used as the infusion solution, with a rate of 20 mL/min and volume of 60 mL. The results in Fig. S6b (Supporting information) indicated, the delivery efficiency of 90Y-HUACM with 1, 7, and 13 GBq specifications were 92.3%, 96.7%, and 97.5%, respectively. 90Y-HUACM demonstrated high delivery efficiency during the simulated delivery. The relatively low density (1.8 g/mL), good mono-dispersity and suspension characteristics of 90Y-HUACM help prevent rapid sedimentation in the delivery device or catheters and blood vessels at relatively low perfusion rate, which improves the convenience of microspheres delivery and ensures the homogeneous distribution within the lesion.
The cytotoxicity, biocompatibility, genetic toxicity, acute toxicity and chronic toxicity of Y-HUACM was evaluated by corresponding methods. The above in vitro and in vivo research results systematically demonstrated that Y-HUACM exhibits no obvious acute toxicity, chronic toxicity, or potential genotoxicity, indicated good short-term and long-term safety and biocompatibility (Supporting information).
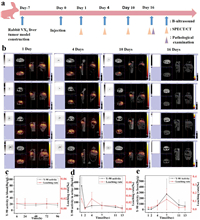
Given the excellent biocompatibility, stability, and outstanding physicochemical properties of 90Y-HUACM, a rabbit in situ VX2 liver tumor model was employed to evaluate the tumor inhibition effect and distribution in vivo. All animal experiments were approved by China Institute for Radiation Protection or West China Hospital, Sichuan University, China. New Zealand rabbit VX2 liver tumor model was successfully established and confirmed by B-ultrasound and histopathology. Y-90, half-life 64.1 h, emits pure β-particle with a maximum energy of 2.24 MeV and an average energy of 0.93 MeV [12,14]. Research has shown that Y-90 can generate continuous bremsstrahlung spectra in tissues and can be detected by Single-photon emission computed tomography (SPECT), enabling the detection and imaging of bremsstrahlung X-ray signals generated by 90Y [32,33]. For research of in vivo imaging and distribution of 90Y-HUACM, four tumor bearing rabbits from the high-dose treatment group (90Y-HUACM, 126 MBq/kg) were selected. The experiment procedures were illustrated in Fig. 4a. Single-photon emission computed tomography combined with computed tomography (SPECT/CT) imaging were performed at the 1st, 4th, 10th and 16th days after delivery of 90Y-HUACM, and the results were presented in Fig. 4b. At the 1st, 4th, and 10th days of the experiment, the 90Y signals were concentrated in the liver of the tumor bearing rabbits, especially at the liver tumor sites. No significant radioactive signals were observed in other organs. By the 16th day of the experiment, the tumor bearing rabbits no longer showed detectable X-ray signals due to the decay of 90Y. These results indicated that SPECT/CT, as a non-invasive and real-time imaging method, can track and locate 90Y-HUACM in vivo. According to Fig. 4c, the concentration of 90Y in blood samples at 6, 24, 48, 72, and 96 h after administration were all < 25 Bq/mL. Additionally, the results presented in Figs. 4d and e indicated that the activity of 90Y in urine and fecal samples were all below 350 Bq/mL or 350 Bq/g throughout the entire test cycle. In summary, the amount of 90Y leaked into blood, urine, and fecal is much lower than 0.1% of the total activity of 90Y-HUACM injected into the body. The outstanding in vivo stability of 90Y-HUACM ensures that 90Y can hardly leak into the blood and almost not excreted in urine and feces. In clinical use, the ultra-low leaching rate of 90Y in vivo not only ensures the safety and effectiveness of 90Y-HUACM but also reduce the risk of environmental contamination caused by patient excreta. The optimized particle size design and good particle size control result in 90Y-HUACM concentrated and distributed at the liver tumor site without ectopic distribution after hepatic artery interventional delivery.
|
Download:
|
| Fig. 4. The distribution of 90Y-HUACM in rabbits after DSA-guided injection. (a) Distribution research (rabbit VX2 liver tumor model construction, injection, and monitoring regime). (b) Representative SPECT/CT images of 90Y-HUACM in rabbits at 1, 4, 10, and 16 days after DSA-guided injection (each SPECT image shows the same animal in cross-sectional, sagittal, and coronal planes from left to right). Radioactive concentration and leaching rate of Y-90 in vivo after injection of 90Y-HUACM in blood (c) at 6, 24, 48, 72 and 96 h, (d) in urine and (e) in feces on 1, 2, 4, 7, 11 and 13 days. Data were presented as a mean ± standard deviation (SD) (n = 4). | |
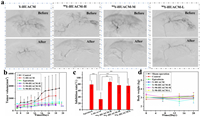
Selective internal radiation therapy, guided by digital subtraction angiography (DSA) was performed, Y-HUACM (4.61 mg/kg; 0 Gy), 90Y-HUACM (56 MBq/kg; 80 Gy), 90Y-HUACM (84 MBq/kg; 120 Gy), 90Y-HUACM (126 MBq/kg; 180 Gy), epirubicin and saline were infused through the hepatic artery. DSA angiography images before and after administration were shown in Fig. 5a. After the intervention delivery of Y-HUACM or 90Y-HUACM, most of the tiny blood supply arteries of the tumor were no longer visible, indicating that microspheres were successful entered into the microvascular bed feeding the tumor. The tumor volume, tumor weight and tumor inhibition rate were employed as the primary evaluation indicators of the treatment effectiveness. After 20 days of treatment, the average tumor volume of epirubicin, Y-HUACM and 90Y-HUACM (low, medium and high dose) groups were all lower than that of the saline group (Fig. 5b). The weight inhibition rates of epirubicin, Y-HUACM, and low (80 Gy), medium (120 Gy), and high (180 Gy) dose of 90Y-HUACM groups were 79%, 33%, 75%, 77%, and 87%, respectively (Fig. 5c). It indicated that the tumor weight inhibition rates of the epirubicin and 90Y-HUACM (low, medium, and high dose) groups were significantly different from that of the saline group (P < 0.01). 90Y-HUACM demonstrated excellent tumor inhibition effect and exhibited good dose-effect relationship. Although Y-HUACM may have a certain embolization capability, it did not exhibit significant tumor inhibitory effect. Furthermore, the results of histopathology staining (Fig. S8 in Supporting information) and analysis of tumor tissue samples (Fig. S7 in Supporting information) revealed that tumor necrosis, normal liver tissue necrosis, and liver fibrosis in 90Y-HUACM treatment groups. Therefore, this excellent therapeutic effect is attributed to the precise localization of 90Y-HUACM, enabling high-dose 90Y continuously emitting lethal high-energy β particle to the tumor. There was no significant difference in body weight between the treatment group and the control group throughout the experiment (Fig. 5d). The main toxic symptoms observed included reduced appetite, emaciation, weakness, and loose stools. These phenomena predominantly occurred in the 90Y-HUACM group, suggesting a potential correlation with the radiation effect of 90Y-HUACM on the liver and the surrounding gastrointestinal tract. No evident toxic side effects were observed during the efficacy and distribution experiments. The results indicated that 90Y-HUACM, dose ranging from 80 Gy to 180 Gy, exhibits significant therapeutic effect and good safety in selective internal radiation therapy for in situ liver tumor bearing rabbits.
|
Download:
|
| Fig. 5. Therapeutic effect of 90Y-HUACM. (a) DSA images of the rabbit VX2 liver tumor area before and after delivery of Y-HUACM and 90Y-HUACM (high, medium and low dose). (b) Changes in tumor volume determined by B-ultrasound. (c) Weight inhibition rates. (d) Body weight changes during the experimental period. Data were presented as a mean ± SD (n = 8). Statistical analysis was performed using a one-way ANOVA comparison. *P < 0.05, **P < 0.01. | |
In this study, we successfully synthesized 90Y carbon microsphere with high uniformity, high specific activity, excellent stability and superior availability using planar molecular complex adsorption and chemical deposition solidification technology. The radiolabeling efficiency of 90Y reached 99.8% and the leaching rate of 90Y was far below 0.1%. The ultra efficient and ultra stable loading of 90Y nuclide onto carbon microsphere was achieved by the strategy of loading radioactive nuclides into the pores of carbon microsphere. 90Y carbon microsphere demonstrated excellent biocompatibility, remarkable stability, precise embolization and significant anti-tumor effect in selective internal radiation therapy in a rabbit VX2 liver tumor. Additionally, the microspheres were visualized by SPECT/CT for in vivo tracing. With a density of 1.8 g/mL and good dispersion characteristics in solutions, 90Y-HUACM facilitated easy perfusion and homogeneous distribution within tumor lesion. This novel, high-performance, and customizable product emerges as a superior option for selective internal radiation treatment of advanced liver cancer. The design and synthesis strategy in this study hold promise for inspiring new developments in radioactive microsphere.
CRediT authorship contribution statementXiaosheng Zhao: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jie Gao: Validation, Supervision, Project administration, Methodology, Data curation. Kun Shi: Writing – review & editing, Supervision, Methodology, Formal analysis. Chixiang Zhang: Methodology, Investigation, Data curation. Wenliang Ma: Validation, Formal analysis, Data curation. Guo Lyu: Visualization, Project administration, Formal analysis. Jun Zhang: Visualization, Validation, Data curation. Jing Lu: Visualization, Validation, Data curation. Qiangqiang Liu: Project administration, Methodology, Investigation. Xianjin Luo: Contributed to the establishment of animal models and the conduct of animal experiments. Kunru Yu: Contributed to the development of analytical methods and the analysis and testing of samples. Jianguo Li: Project administration, Investigation. Qiang Ge: Supervision, Methodology, Investigation, Formal analysis. Jiming Cai: Resources, Project administration, Conceptualization. Chang Liu: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization. Zhiyong Qian: Writing – review & editing, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Major Scientific and Technological Special Project for "Significant New Drugs Development" (No. 2018ZX09201018–028), the nuclear energy development projects of China during the 13th Five Year Plan period, and the key research and development project of the Sichuan Provincial Department of Science and Technology (No. 18ZDYF1466).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110662.
| [1] |
D.Q. Huang, P. Mathurin, H. Cortez-Pinto, et al., Nat. Rev. Gastroenterol. Hepatol. 20 (2023) 37-49. DOI:10.1038/s41575-022-00688-6 |
| [2] |
A.G. Singal, F. Kanwal, J.M. Llovet, Nat. Rev. Clin. Oncol. 20 (2023) 864-884. DOI:10.1038/s41571-023-00825-3 |
| [3] |
S. Li, Y. Qu, L. Liu, et al., Cell Prolif. 56 (2023) e13452. |
| [4] |
M. Housini, B. Dariya, N. Ahmed, et al., Gene 892 (2024) 147857. |
| [5] |
J. Martin, A. Petrillo, E.C. Smyth, et al., World J. Clin. Oncol. 11 (2020) 761. |
| [6] |
H. Chen, H. Cheng, W. Wu, et al., Chin. Chem. Lett. 31 (2020) 1375-1381. |
| [7] |
H. Petrowsky, R. Fritsch, M. Guckenberger, et al., Nat. Rev. Gastroenterol. Hepatol. 17 (2020) 755-772. DOI:10.1038/s41575-020-0314-8 |
| [8] |
J. Roosen, N.J.M. Klaassen, L.E.L.W. Gotby, et al., Eur. J. Nucl. Med. Mol. I. 48 (2021) 3776-3790. DOI:10.1007/s00259-021-05340-0 |
| [9] |
Y. Zhou, Y. Gao, G.X. Duan, et al., Adv. Nano. Biomed. Res. 3 (2023) 2200166. |
| [10] |
C. Mosconi, A. Cappelli, C. Pettinato, R. Golfieri, World J. Hepatol. 7 (2015) 738-752. |
| [11] |
P. d'Abadie, M. Hesse, A. Louppe, et al., Molecules 26 (2021) 3966-3979. DOI:10.3390/molecules26133966 |
| [12] |
E. Birgin, E. Rasbach, S. Seyfried, et al., Cancers 12 (2020) 294-317. DOI:10.3390/cancers12020294 |
| [13] |
A.S. Kennedy, D. Ball, S.J. Cohen, et al., J. Gastrointest Oncol. 6 (2015) 134-142. |
| [14] |
S. Asadian, H. Mirzaei, B.A. Kalantari, et al., Pharmacol. Res. 160 (2020) 105070. |
| [15] |
W. Yang, G. Xu, J. Shu, M. Wang, X. Ge, Chin. Chem. Lett. 32 (2021) 866-869. DOI:10.3390/agriculture11090866 |
| [16] |
C. Yuan, X. Wang, X. Yang, et al., Chin. Chem. Lett. 32 (2021) 2079-2085. |
| [17] |
X.S. Zhao, C.X. Zhang, J. Lu, et al., Patent (2023) CN111939276B. |
| [18] |
R. Salem, R.J. Lewandowski, Clin. Gastroenterol. Hepatol. 11 (2013) 604-611. |
| [19] |
M.J. Powerski, C. Erxleben, C. Scheurig-Münkleret, et al., Eur. J. Radiol. 84 (2015) 201-207. |
| [20] |
E. Garin, Y. Rolland, S. Laffont, J. Edeline, Eur. J. Nucl. Med. Mol. I. 43 (2016) 559-575. DOI:10.1007/s00259-015-3157-8 |
| [21] |
A. Barroso-Bogeat, M. Alexandre-Franco, C. Fernández-González, et al., Ind. Eng. Chem. Res. 55 (2016) 5200-5206. DOI:10.1021/acs.iecr.5b04563 |
| [22] |
K.K. Bamzai, N. Kachroo, V. Singh, S. Verma, J. Mater. 2013 (2013) 359514. |
| [23] |
F. Wang, C. Li, Y. Li, C.Y. Jimmy, Appl. Catal. B: Environ. 119 (2012) 267-272. |
| [24] |
J. Ryu, Y.W. Suh, D.J. Suh, D.J. Ahn, Carbon 48 (2010) 1990-1998. |
| [25] |
M.A. Westcott, D.M. Coldwell, D.M. Liu, J.F. Zikria, Adv. Radiat. Oncol. 1 (2016) 351-364. |
| [26] |
S.M. Srinivas, E.C. Nasr, V.K. Kunam, J.A. Bullen, A.S. Purysko, J. Gastrointest. Oncol. 7 (2016) 530. DOI:10.21037/jgo.2016.03.09 |
| [27] |
C.C. Pieper, W.A. Willinek, D. Thomas, et al., Eur. Radiol. 26 (2016) 2779-2789. DOI:10.1007/s00330-015-4076-6 |
| [28] |
P.M. Piana, V. Bar, L. Doyle, et al., HPB 16 (2014) 336-341. DOI:10.1111/hpb.12135 |
| [29] |
M. Feely, R. Tondon, M. Gubbiotti, et al., Am. J. Surg. Pathol. 46 (2022) 1234-1240. DOI:10.1097/pas.0000000000001901 |
| [30] |
R. Salem, R.D. Hunter, Int. J. Radiat. Oncol. 66 (2006) S83-S88. |
| [31] |
R.J. Lewandowski, J. Minocha, K. Memon, et al., Eur. J. Nucl. Med. Mol. Imaging 41 (2014) 486-493. DOI:10.1007/s00259-013-2575-8 |
| [32] |
S.A. Debebe, M. Adjouadi, S.A. Gulec, et al., J. Appl. Clin. Med. Phys. 20 (2019) 30-42. DOI:10.1002/acm2.12512 |
| [33] |
H. Ahmadzadehfar, H. Duan, A.R. Haug, et al., Eur. J. Nucl. Med. Mol. Imaging 41 (2014) 115-124. DOI:10.1007/s00259-013-2675-5 |
 2025, Vol. 36
2025, Vol. 36 

