b College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
c College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
This study develops a supramolecular/dynamic covalent polymerization method to construct optical glass with excellent optical properties, strong mechanical toughness, high hardness, and outstanding processability. The glass displays rapid optical self-healing performance through the combination of light-induced regeneration of poly[thioctic acid] and hydrogen bond-triggered rapid curing.
Transparent materials that are broadly applicable have played essential roles throughout the long history of human beings [1]. The evolution of transparent materials has gone through natural and artificial processes [2]. In ancient times, naturally derived minerals and resins were used as raw materials for the fabrication of transparent devices and apparatus [3]. With the development of glass industrial, the versatility of glass products has led to artificial glass becoming synonymous with transparent materials that have infiltrated every aspect of our daily lives and productive activities as a consequence [4]. The unequalled optical performance of modern inorganic glass makes it the most reliable and practical transparent material [5]. Inorganic glass is normally inherently brittle and chemically inert owing to its ultra-high viscosity, high cohesive energy density, and extremely weak chain motion [6,7]. Accordingly, the surface and internal structures of glass are vulnerable to permanent damage during long-term practical applications [8].
Over the past decade, a new design concept that involves with non-covalent interactions was conceived, providing new possibilities for constructing “soft” transparent materials, with hydrogels, organogels, and ionic gels as the most typical representatives [9–11]. Due to the existence of a large amount of solvent molecules, polymeric species in supramolecular gels show good mobility, thus facilitating the regeneration of non-covalent bonding structures in the destroyed areas via the movement of monomers. Dynamic assembly, reversible gelation, and high water content synergistically endow transparent gels with remarkable healing abilities [12–14]. However, healing frequently occurs at the cost of poor toughness and/or low hardness, and it is difficult to achieve a balance between mechanical toughness and reparirability [15]. Although the toughness of gels can be effectively enhanced by various strategies [16–18], such as double networks, solvent exchange, metal coordination, and cross-linking, the majority of tough gels are poorly hard and rigid, with their hardness values significantly lower than those of traditional polymers [19–21]. Therefore, the long-term service of gels risks their structural integrities. To overcome this shortcoming, attention has shifted to the development of “hard” materials. Non-covalently conjugated glass and plastics with high optical transmittances and good mechanical strengths have been successfully prepared and actively investigated [22–25]. Unfortunately, most of them are fragile when subjected to internal forces and hardly inherit healable capacity, and only a limited examples of supramolecular plastics are repairable [26–29].
Herein, we report an optically healable and mechanically tough supramolecular glass driven by the dynamic covalent/non-covalent polymerization of two small molecules, thioctic acid (TA) and (±)-trans-1,2-diaminocyclohexane (DC). Thermo-induced ring-opening polymerization of TA yields poly[TA] as the polymeric backbone in the bulk, which is further cross-linked by DC through multiple hydrogen bonds (Fig. 1a) [30–33]. The solvent-free state and cross-linking pattern of poly[DC/TA] endow it with excellent mechanical properties. The photopolymerization behavior of poly[TA] enables damaged areas in poly[DC/TA] to be effectively healed in-situ [34]. The repaired areas rapidly cure and mechanically toughen by regenerating hydrogen bonding between poly[TA] and DC.
|
Download:
|
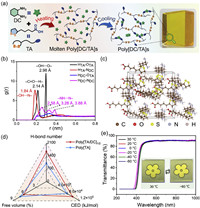
| Fig. 1. Preparation and characterization of poly[DC/TA]. (a) Preparation process of poly[DC/TA]. (b) RDFs of molecular models of poly[DC/TA]1/2. (c) Molecular models between TA and DC. (d) Hydrogen bond number, CED, and lower free volume of poly[DC/TA]1/2 and poly[TA]. (e) Transmittances of poly[DC/TA]1/2 at different temperatures. | |
The preparation of poly[DC/TA] involves two distinguishable aspects: (a) the ring-opening polymerization of TA to poly[TA], and (b) the cross-linking of poly[TA] with DC to yield poly[DC/TA] in the bulk (Fig. 1a). Because the conversion of TA into poly[TA] has been extensively studied, we mainly focused on the supramolecular chemistry between poly[TA] and DC [30–36]. New peaks and obvious chemical-shift changes were observed in the 1H NMR spectra of poly[DC/TA] in CDCl3, when comparing with those of DC and TA (Fig. S1 in Supporting information). The new peaks are corresponded to poly[TA] and the chemical shifts might be attributed to the covalently or non-covalently bonding between DC and poly[TA] [36]. Therefore, additional analysis of NMR data was carried out. The spectrum of poly[DC/TA] shows a lack of changes in the number of carbon signals, subjected to 13C NMR spectroscopy (Fig. S2 in Supporting information). Meanwhile, only one set of carbon signal at 180.56 ppm was observed, which is assigned to the carboxylic group of TA, indicating that there is not amidation reaction between TA and DC during the poly[DC/TA] formation. Those results from the NMR spectra reveal that TA and DC are not covalently bound. Poly[TA]/DC cross-linking should be driven by non-covalent interactions when poly[DC/TA] is solvent-free and in the bulk state.
According to the radial distribution function (RDF), peaks were observed in poly[DC/TA] within a limited scale range (<0.5 nm), reflecting the short-range ordered structures, which may be due to hydrogen-bonded molecular motifs (Fig. 1b) [37,38]. Meanwhile, compared with other hydrogen bonding formations (2.58–3.88 Å) in poly[DC/TA], the H-bonds between DC and TA have the shortest bond length (1.84–2.14 Å). Multiple hydrogen bonding patterns are simulated: multiple hydrogen bonds and various molecular recognition motifs, including TA@TA, TA@DC, DC@DC, are observed in poly[DC/TA], which demonstrates that the formation of bulk poly[DC/TA] is mainly triggered by hydrogen bonding (Fig. 1c and Fig. S3 in Supporting information). These results demonstrate that the introduction of DC can endow poly[DC/TA] with the greater diversity of intermolecular interactions (Fig. 1d). Quantitatively, poly[DC/TA] has a higher hydrogen bond (H-bond) number than poly[TA] (1631 vs. 997), which leads to a higher cohesive energy density (CED, 9.38 × 104 vs. 7.33 × 104 kJ/mol) and a lower free volume (6.04% vs. 7.02%). In additional, poly[DC/TA] was subjected to X-ray photoelectron spectroscopy (XPS), showing that poly[DC/TA] contains H-bonded amino groups from DC and H-bonded hydroxyl groups from poly[TA] (Fig. S4 in Supporting information) [5]. Taking together, these differences highlight that DC molecules can significantly increase the cross-linking density of poly[TA] through hydrogen bonds [35].
Structural analysis reveals the amorphous nature of poly[DC/TA], because only a broad peak between 10° to 30° was found in its powder X-ray diffraction (PXRD) spectrum (Fig. S5 in Supporting information). XPS etching experiments of poly[DC/TA] at different depths (10 and 20 nm) display very similar binding energy spectra, indicating that poly[DC/TA] is in a homogeneous state (Fig. S4). Meanwhile, according to the energy dispersive X-ray spectroscopy maps, poly[TA] and DC are uniformly distributed in poly[DC/TA] without any obvious signs of phase separation or aggregation (Figs. S6 and S7 in Supporting information). Simulation results further confirm that poly[DC/TA] is a homogeneous material (Fig. S8 in Supporting information).
The applications of transparent materials are directly related to their optical performances. Poly[DC/TA] is a bulk material with good optical transparency. A colorful painting was visible, when covered with a thin piece of poly[DC/TA]. Poly[DC/TA] shows high values of transmittance (>90%) in the 420–1000 nm spectral range; that is to say it is transparent in the visible and near-infrared regions (Fig. 1e). In contrast, poly[TA] is opaque. The optical behavior of poly[DC/TA] is resistant to low temperatures; it remains transparent even at −60 ℃. No transmittance decay was found, when the testing temperature was fluctuated between 30 ℃ and −60 ℃.
In general, three conditions are required to obtain optical transparency: (a) The surface is smooth to avoid light scattering; (b) It is macroscopically homogeneous to suppress light scattering; and (c) There is no strong intrinsic absorption in the visible region [3,5]. Additional experiments were performed to study the optical behavior of poly[DC/TA]. Poly[DC/TA] has a smooth and uniform surface structure with a root-mean-square roughness of 16.5 nm (Fig. S9 in Supporting information); hence, light scattering caused by a rough surface is suppressed. Meanwhile, poly[DC/TA] is homogeneous and isotropic from a macroscopic perspective; therefore, the light scattering is further attenuated. Moreover, poly[TA] and DC intrinsically absorb in the ultraviolet region because solid and liquid UV–vis spectroscopy clearly show that the absorption of poly[DC/TA] locates in the 200–400 nm wavelength range (Figs. S10 and S11 in Supporting information). All the above factors together endow poly[DC/TA] with excellent optical transparency in the visible and near-infrared regions.
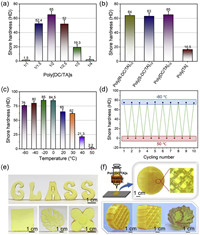
The mechanical toughness of artificial glass profoundly affects its functionalization and practical applications [39]. In this section, the mechanical properties of poly[DC/TA] were systematically studied using a combination of experimental and theoretical techniques. The hardness of poly[DC/TA] is closely related to the DC-to-TA weight ratio. Poly[DC/TA], with a 1:2 (w/w) of DC and TA ratio (i.e., poly[DC/TA]1/2) exhibits the highest hardness value of 65 HD, which is comparable to those of some traditional plastic materials (>60) (Fig. 2a). In contrast, poly[DC/TA]1/1 and poly[DC/TA]1/3 show hardness values of 1.5 and 19.3 HD, respectively, which are only 2.3% and 29.7% that of poly[DC/TA]1/2, respectively. Meanwhile, the structural configuration of DC was not the decisive factor that determines the hardness of poly[DC/TA], which is consistent with our previous study (Fig. 2b) [40]. For example, poly[R-DC/TA] has a Shore hardness of 64 HD, which is similar to that of poly[S-DC/TA] (63 HD) (herein, S-DC/R-DC are (1S,2S)-(+)−1,2-diaminocyclohexane and (1R,2R)-(-)−1,2-diaminocyclohexane, respectively). A possible explanation is that the spatial configuration-induced differences were fully mitigated by non-covalent polymerization. Compared with poly[TA], the introduction of DC can yield a higher hardness within a less cooling time. For example, the values of hardness of poly[DC/TA] and poly[TA] after cooling for 1 h are 65 and 16.5 HD, respectively.
|
Download:
|
| Fig. 2. The properties and processability of poly[DC/TA]. (a) Shore hardness of poly[DC/TA]1/2 (20 ℃, 50 RH%). (b) Shore hardness of poly[R-DC/TA]1/2, poly[S-DC/TA]1/2, poly[DC/TA]1/2 and poly[TA] (20 ℃, 50 RH%). (c) Reversible temperature-dependent viscosity of poly[DC/TA]1/2. (d) Reversible temperature-dependent Shore hardness of poly[DC/TA]1/2. (e) Macroscopic views of poly[DC/TA]1/2 models via thermal forming. (f) 3D printing process of poly[DC/TA]1/2. (g) 3D printed poly[DC/TA]1/2 models. R/S-DC are trans-(1R,2R)−1,2-diaminocyclohexane and trans-(1S,2S)−1,2-diaminocyclohexane, respectively. | |
Because the cross-linking behavior of poly[DC/TA] is driven by weak interactions, it is a thermoplastic material with a glass transition temperature at ca. 32 ℃ (Fig. S12 in Supporting information), according to the differential scanning calorimeter measurements: poly[DC/TA] has good mobility and low viscosity at high temperature, while the movements and rearrangements of polymer chain segments become frozen and it rapidly transforms into a solid during cooling. Quantitatively, the viscosities and hardness values of poly[DC/TA] were measured to be 2.54 × 107 and 1.48 × 103 Pa s, and 65 and 0, respectively, at 20 and 80 ℃ (Fig. 2c and Fig. S13 in Supporting information). These fluctuations in hardness and viscosity are reversible as evidenced by repeated heating and cooling (Fig. 2d). The thermal degradation temperature of poly[DC/TA] is around 180 ℃, which is much higher than its melting point (Fig. S14 in Supporting information). The thermo-responsiveness and thermoplastic nature of poly[DC/TA] inspired us to explore its processing potential. Macroscopic experiments intuitively demonstrate that poly[DC/TA] exhibits good thermal processability and can be cast into samples with various sizes and shapes (Fig. 2e and Fig. S15 in Supporting information). In addition to normal thermal-forming technology, poly[DC/TA] is available for fused deposition modeling (FDM) 3D printing (Figs. S16 and S17 in Supporting information) [41–43]. Irregular and complicated modes were successfully printed using a customized 3D printer with poly[DC/TA] as the consumable material (Fig. 2f). The printed models are mechanically tough (Fig. S18 in Supporting information).
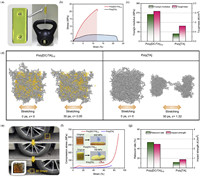
No deformations or other structural damages were observed in a long-term (>12 months) weight-load experiment in which a piece of poly[DC/TA] (5.0 × 2.0 × 0.5 cm3) was used to hang an 8-kg weight (Fig. 3a). The tensile strength of poly[DC/TA] was measured to be 21.6 MPa (Fig. 3b), which is much higher than those of poly[TA] (4.53 MPa) and our reported TA-based soft materials (<1.0 MPa) [35,36,44]. Meanwhile, the Young's modulus and toughness of poly[DC/TA] were calculated to be 256.3 MPa and 1.59 MJ/m3, respectively. The mechanical properties of poly[DC/TA] and poly[TA] are compared in Fig. 3c, which reveals that the cross-linked structure effectively leads to poly[DC/TA] having improved mechanical toughness [43]. Simulations were used to study the dynamic mechanical properties of poly[DC/TA] (Fig. 3d). The structural evolution of poly[DC/TA] shows that only slight deformation takes place, the entire 3D network is intact, and DC molecules are firmly cross-linked with poly[TA] when the simulated elongation process is set at a tensile stress of 5.5 × 103 kJ mol-1 nm-1. In contrast, poly[TA] first experiences serious deformation, after which hollow structures and structural fractures initiate under the same simulation conditions. Poly[TA] and poly[DC/TA] exhibit deformation rates of 132% and 5%, respectively, after being stretched for 30 ps [43].
|
Download:
|
| Fig. 3. Mechanical properties of poly[DC/TA]. (a) Weight loading of poly[DC/TA]1/2. (b) Tensile stress-strain curves of poly[DC/TA]1/2 and poly[TA] (100 mm/min). (c) Young's moduli and toughness of poly[DC/TA]1/2 and poly[TA]. (d) Simulated structure evolutions of poly[DC/TA]1/2 and poly[TA] during the elongation process at 5.5 × 103 kJ mol-1 nm-1 tesile stress. (e) Photos of macroscopic compression tests of poly[DC/TA]1/2 (3.0 × 3.0 × 1.2 cm, weight of the car: 1600 kg). (f) Compression stress-strain curves of poly[DC/TA]1/2 and poly[TA] (50 mm/min). (g) Rebound rates and impact strengths of poly[DC/TA]1/2 and poly[TA]. | |
Macroscopic pressing experiments revealed that a poly[DC/TA] sample (3.0 × 3.0 × 1.2 cm3) remained intact when it was repeatedly rolled over by a car (1600 kg) for >30 times (Fig. 3e). A poly[DC/TA] cube (1.7 × 1.7 × 1.5 cm3) was flattened without obvious cracks at a pressure of 600 MPa (Fig. 3f); however, poly[TA] exhibits multiple cracks when a pressure of 150 MPa was applied (Figs. S19 and S20 in Supporting information). The same trend was observed during quantitative compressive testing. While poly[DC/TA] exhibits a strain of 85% at a compressive stress of 400 MPa, poly[TA] deforms by 70% at a compressive stress of only 28 MPa. The simulation results further confirm the superior anti-compressibility of poly[DC/TA] compared to that of poly[TA] (Fig. S21 in Supporting information) [45].
Rebounding and impact experiments were designed and performed to study the energy dissipation of poly[DC/TA] (Fig. 3g and Fig. S22 in Supporting information). Poly[DC/TA] has a rebound rate of poly[DC/TA] of 53.2%, which is much higher than that of poly[TA] (11.6%) [35]. This observation indicates that a large portion of the impact energy is dissipated as elastic potential energy by sacrificing the dynamic structure of poly[DC/TA]; it also indicates that poly[DC/TA] may have impact-resistant mechanical features [36]. Poly[DC/TA] exhibits an impact energy of 2.47 kJ/m2, while that of poly[TA] is only 0.88 kJ/m2, which demonstrates that DC strongly increases the rigidity of poly[TA] via cross-linking. Additionally, poly[DC/TA] maintains good impact strength over a wide temperature range (–20~30 ℃, 1.98–2.64 kJ/m2, Fig. S23 in Supporting information).
Three essential conditions are required for poly[DC/TA] to exhibit optical healing: (a) It possesses strong adhesive bonding; (b) The photo-generated material is mobile to rapidly cover the damaged area of poly[DC/TA]; and (c) The newly healed material can cure and toughen quickly to regenerate mechanical toughness and hardness [12].
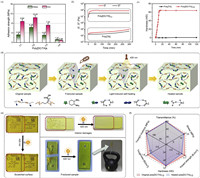
The adhesive bonding ability of poly[DC/TA] was studied by evaluating its adhesion and cohesion capabilities (Fig. 4a). Poly[DC/TA] adheres well to substrates over a wide temperature (−40~70 ℃; 1.36–12.89 MPa) or humidity (0–99 RH%; 8.64–10.42 MPa) range (Figs. S24 and S25 in Supporting information). Poly[DC/TA]1/2 exhibits the best adhesion capacity (>10 MPa) and highest cohesion energy (9.38 × 104 kJ/mol). These results demonstrate that poly[DC/TA] has strong adhesive bonding, which is the basic structural requirement for optical healing. In contrast, poly[TA] adheres poorly to substrates (<1.2 MPa).
|
Download:
|
| Fig. 4. Optically repairable capability of poly[DC/TA]. (a) Adhesion strengths of poly[DC/TA]. (b) Time-dependent rheological behaviors of poly[DC/TA]1/2 and poly[TA] after irradiation at 420 nm. (c) Time-dependent hardness of poly[DC/TA]1/2 after irradiation at 420 nm. (d) The healing process of poly[DC/TA]. (e) Optically healing of poly[DC/TA]1/2. (f) The comparison of original and healed poly [DC/TA]1/2. | |
The photopolymerization of poly[TA] was later carefully investigated. A yellow transparent gel-like soft material was obtained when poly[TA] powder was irradiated at 420 nm for 3 min, as confirmed by macroscopic observations and rheological testing (Fig. 4b, Figs. S26 and S27 in Supporting information). However, the newly formed poly[TA] did not solidify rapidly (>2 h), according to its time-dependent moduli, viscosity, and hardness (Figs. 4b and c). These observations reveal that individual poly[TA] does not meet the requirements of optical healablility. Fortunately, the introduction of DC not only greatly accelerates the solidification process of photosynthetic poly[TA], but also improves the mechanical strength of the healed areas (Fig. 4d). After irradiation with light, the newly yielded poly[DC/TA] had relatively low modulus, viscosity, and hardness values, which means that it can quickly cover damaged areas (Figs. 4b and c). The healed poly[DC/TA] then rapidly solidified to deliver excellent mechanical properties within a short time (<10 min). Not only were scratches (caused by a knife) on the surface of poly[DC/TA] effectively repaired, but interior damages (caused by freezing poly[DC/TA] in liquid nitrogen or completely fracturing it) were also perfectly healed upon irradiation at 420 nm (Fig. 4e and Figs. S28–S31 in Supporting information). The tensile strength, impact strength, and hardness of poly[DC/TA]/healed poly[DC/TA] were determined to be 21.6/20.6 MPa, 2.47/2.09 kJ/m2 and 65/65 HD, respectively (Fig. 4f). The refractive index of poly[DC/TA] does not change before or after healing, remaining at 1.54 (Fig. S28 in Supporting information). Additionally, the opaque damaged area became transparent and exhibited a transmittance above 90% after irradiation, which is consistent with the macroscopic-healing observations (Fig. 4f and Fig. S29 in Supporting information). This change in the optical properties of poly[DC/TA] is attributable to the reconstruction of a smooth surface and a homogeneous interior. Compared with some reported glasses, poly[DC/TA] exhibits a rapid and efficient healing capability (Fig. S32 in Supporting information) [46–49].
Two control experiments were used to exclude the thermally healing behavior of poly[TA]. Heating a poly[DC/TA] sample at 55 ℃ for 60 min did not repair the damaged areas (Fig. S33 in Supporting information). Meanwhile, temperature monitoring shows that the irradiated area exhibits a maximum temperature of 35 ℃ during the entire healing process (Fig. S34 in Supporting information). Those healing and control experiments support that the healing capacity of poly[DC/TA] is optically triggered.
A supramolecular/dynamic-covalent polymerization approach was developed and used to construct optical glass. Poly[DC/TA] exhibits good optical behavior, strong mechanical toughness, high hardness, and excellent processability facilitated by the synergistic assembly of non-covalently linked motifs and dynamic polymeric units. The optically healable properties of poly[DC/TA] were obtained via a combination of the light-induced regeneration of poly[TA] and DC-triggered rapid solidification. Damages to the surface and interior can be effectively repaired. The newly healed poly[DC/TA] shows good transparency and a high mechanical strength. The innovative strategy used in this study presents a promising solution to the challenges faced by supramolecular transparent materials and opens up new possibilities for the development of reliable and practical artificial transparent materials.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementQiao Zhang: Writing – original draft, Methodology, Conceptualization. Xin Tan: Writing – original draft, Software. Zihang Liu: Writing – original draft, Investigation. Jingyu Ma: Writing – original draft, Investigation. Dongqi Cao: Writing – original draft, Investigation. Fenfang Li: Writing – original draft, Software. Shengyi Dong: Writing – review & editing, Writing – original draft, Conceptualization.
AcknowledgmentsThe authors gratefully acknowledge the Natural Science Foundation of Hunan Province (No. 2024JJ6202), the National Natural Science Foundation of China (No. 22271087), the Outstanding Youth Scientist Foundation of Hunan Province (No. 2021JJ10010), the Huxiang Young Talent Program from Hunan Province (No. 2018RS3036).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110660.
| [1] |
M.W. David, Glass: A Short History. The British Museum Press, 2012.
|
| [2] |
S.C. Rasmussen, Modern materials in antiquity: an early history of the art and technology of glass, in: S.C. Rasmusse (Ed.), Chemical Technology in Antiquity, ACS Symposium Series 1211, 2015, pp. 267–313.
|
| [3] |
H. Scholze, Glass: Nature, Structure, and Properties. Springer Press, 1991: pp. 156-364. DOI:10.1007/978-1-4613-9069-5_3
|
| [4] |
N. Ma, S. Horike, Chem. Rev. 122 (2022) 4163-4203. DOI:10.1021/acs.chemrev.1c00826 |
| [5] |
S. Wu, C. Cai, X. Wang, et al., Mater. Horiz. 10 (2023) 5152-5160. DOI:10.1039/d3mh01220d |
| [6] |
F. Nie, D. Yan, Angew. Chem. Int. Ed. 62 (2023) e202302751. |
| [7] |
T. Magrini, F. Bouville, A. Lauria, et al., Nat. Commun. 10 (2019) 2794. DOI:10.1038/s41467-019-10829-2 |
| [8] |
C. Cai, S. Wu, Y. Zhang, et al., Nat. Commun. 15 (2024) 3929. |
| [9] |
X. Lin, X. Zhao, C. Xu, et al., J. Polym. Sci. 60 (2022) 2525-2542. DOI:10.1002/pol.20220154 |
| [10] |
Z. Huang, X. Chen, S.J.K. O'Neill, et al., Nat. Mater. 21 (2022) 103-109. DOI:10.1038/s41563-021-01124-x |
| [11] |
Y. Sun, L. Jiang, Y. Chen, et al., Chin. Chem. Lett. 35 (2024) 108644. |
| [12] |
B. Li, P.F. Cao, T. Saito, et al., Chem. Rev. 123 (2023) 701-735. DOI:10.1021/acs.chemrev.2c00575 |
| [13] |
H.Q. Peng, W. Zhu, W.J. Guo, et al., Prog. Polym. Sci. 137 (2023) 101635. |
| [14] |
M. Burnworth, L. Tang, J.R. Kumpfer, et al., Nature 472 (2011) 334-338. DOI:10.1038/nature09963 |
| [15] |
X.W. Xu, V.V. Jerca, R. Hoogenboom, Mater. Horiz. 8 (2021) 1173-1188. DOI:10.1039/d0mh01514h |
| [16] |
H. Fan, J. Wang, J. Gong, Adv. Funct. Mater. 31 (2021) 2009334. |
| [17] |
L. Zhou, C. Yang, W. Dou, et al., Chin. Chem. Lett. 35 (2024) 108669. |
| [18] |
L. Li, W. Li, X. Wang, et al., Angew. Chem. Int. Ed. 61 (2022) e202212512. |
| [19] |
W. Li, L. Li, Z. Liu, et al., Adv. Mater. 35 (2023) 2301383. |
| [20] |
R. Zhu, D. Zhu, Z. Zheng, et al., Nat. Commun. 15 (2024) 1344. |
| [21] |
M.A. Gonzalez, J.R. Simon, A. Ghoorchian, et al., Adv. Mater. 29 (2017) 1604743. |
| [22] |
J. Chen, Y. Ma, T. Chen, et al., Adv. Funct. Mater. 33 (2023) 2212564. |
| [23] |
J. Zhang, T. Bai, W. Liu, et al., Nat. Commun. 14 (2023) 3524. |
| [24] |
J. Yu, D. Qi, E. Mäkilä, et al., Angew. Chem. Int. Ed. 61 (2022) e202204611. |
| [25] |
M. Liu, P. Liu, G. Lu, et al., Angew. Chem. Int. Ed. 57 (2018) 11242-11246. DOI:10.1002/anie.201805206 |
| [26] |
K. Kato, A. Ohara, K. Michishio, et al., Macromolecules 53 (2020) 8910-8917. DOI:10.1021/acs.macromol.0c02009 |
| [27] |
J. Zhu, G.Y. Chen, L. Yu, et al., CCS Chem. 2 (2020) 280-292. DOI:10.31635/ccschem.020.201900118 |
| [28] |
X. Lu, P. Xie, X. Xiang, et al., Macromolecules 55 (2022) 2557-2565. DOI:10.1021/acs.macromol.2c00272 |
| [29] |
Z. Zhang, L. Cheng, J. Zhao, et al., Angew. Chem. Int. Ed. 59 (2020) 12139-12146. DOI:10.1002/anie.202004152 |
| [30] |
Q. Zhang, Y. Deng, C.Y. Shi, et al., Matter 4 (2021) 1-13. |
| [31] |
Q. Zhang, D.H. Qu, B.L. Feringa, et al., J. Am. Chem. Soc. 144 (2022) 2022-2033. DOI:10.1021/jacs.1c10359 |
| [32] |
Q. Zhang, C.Y. Shi, D.H. Qu, et al., Sci. Adv. 4 (2018) eaat8192. |
| [33] |
Y. Deng, Q. Zhang, C. Shi, et al., Adv. Sci. 8 (2022) eabk3286. |
| [34] |
C.Y. Shi, Q. Zhang, B.S. Wang, et al., ACS Appl. Mater. Interfaces 13 (2021) 44860-44867. DOI:10.1021/acsami.1c11679 |
| [35] |
Y. Zhang, C. Cai, F. Li, et al., Small 19 (2023) 2300857. |
| [36] |
Y. Zhang, C. Cai, K. Xu, et al., Mater. Horiz. 11 (2024) 1315-1324. |
| [37] |
M.I. Ojovan, D.V. Louzguine-Luzgin, J. Phys. Chem. B 124 (2020) 3186-3194. DOI:10.1021/acs.jpcb.0c00214 |
| [38] |
G. Camisasca, H. Pathak, K.T. Wikfeldt, et al., J. Chem. Phys. 151 (2019) 044502. |
| [39] |
N. Fei, K.Z. Wang, D. Yan, Nat. Commun. 14 (2023) 1654. |
| [40] |
Q. Zhang, Y. Yin, J. Song, et al., Chin. Chem. Lett. 34 (2023) 108126. |
| [41] |
D. Gtilve-Martinez, W. Neri, D. Horaud, et al., Adv. Funct. Mater. 33 (2023) 2214954. |
| [42] |
S.C. Ligon, R. Liska, J. Stampfl, et al., Chem. Rev. 117 (2017) 10212-10290. DOI:10.1021/acs.chemrev.7b00074 |
| [43] |
C. Cai, S. Wu, Y. Zhang, et al., Adv. Sci. 9 (2022) 2203630. |
| [44] |
S. Luo, N. Wang, Y. Pan, et al., Small 20 (2024) 2310839. |
| [45] |
W. Fang, Z. Mu, Y. He, et al., Nature 619 (2023) 293-299. DOI:10.1038/s41586-023-06117-1 |
| [46] |
J. Chen, Z. Wang, B. Yao, et al., Adv. Mater. 36 (2024) 2401178. |
| [47] |
J. Jing, B. Yao, W. Sun, et al., Angew. Chem. Int. Ed. 63 (2024) e202410693. |
| [48] |
W. Li, H. Liu, H. Wang, et al., Chem. Mater. 35 (2023) 682-691. |
| [49] |
W. Fan, Y. Jin, L. Shi, et al., J. Mater. Chem. A 8 (2020) 6757-6767. DOI:10.1039/c9ta13928a |
 2025, Vol. 36
2025, Vol. 36 

