b School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China
Carbazoles and their derivatives are important nitrogen-containing heterocyclic chemicals. Their special structures endow distinct functions, properties and biological activities, and therefore have wide applications in the field of photoelectrical materials, dyes, medicinal agents, and supramolecular recognition [1–4]. Among them, 2-hydroxycarbazole and 4-hydroxycarbazole are versatile chemicals which can be easily modified via functionalization of the carbazole ring and/or the hydroxyl group and thus broadly used in organic electroluminescent materials, hole transport materials, and pharmaceutical field [5–8]. Synthesis methods of 2-hydroxycarbazole consist of thermochemical route and photochemical route. The former mainly includes palladium-catalyzed oxidation of 2‑hydroxy-tetrahydrocarbazole [9], Diels-Alder reaction of 2-nitroindoles [10], reductive cyclization of 3-nitro-4-phenylphenol [11], and catalytic cyclization of biphenyl halides [4,12], which often requires harsh reaction conditions, multiple reaction steps, and the use of expensive reagents. State of the art yield of 2-hydroxycarbazole is ~30% and the reaction time is typically in hours or even days. On the contrary, the photochemical route is more environmentally friendly and can be operated at milder reaction conditions [13–15]. The photochemical synthesis of 2-hydroxycarbazole has been carried out in semi-batch reactors, resulting in a similar level of product yield as the one from thermochemical route. However, the reported reaction time was still long, e.g., several hours. In addition, the reaction volume for the semi-batch operation is typically limited to less than 1 mL. When the batch photoreactors are scaled-up, photon transfer efficiency and its even distribution within the reaction medium can hardly be maintained [16–18] due to the attenuation effect of photon transport according to Bouguer-Lambert-Beer law, causing longer reaction time, over irradiation of the reaction mixture, and lower selectivity [19–23]. On the other hand, continuous-flow photomicroreactors have advantages of larger specific surface area, faster photon transfer rates, and ease of scale-up via numbering-up [24–27]. Nevertheless, the small liquid holdup of typical photomicroreactor severely limited its throughput and hindered its potential for industrial-scale applications [28–30].
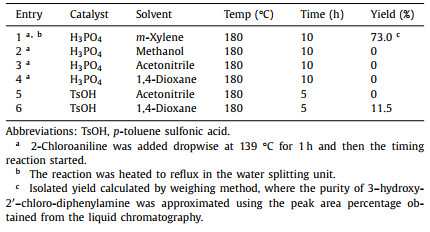
Herein, we developed a green route for the continuous and high-throughput synthesis of 2-hydroxycarbazole and its isomer, 4-hydroxycarbazole, via photochemical intramolecular cyclization of 3‑hydroxy-2′‑chloro-diphenylamine using a self-designed millimeter scale photoreactor (Scheme 1). Firstly, high-yield (73%) synthesis of 3‑hydroxy-2′‑chloro-diphenylamine, the feedstock for 2-hydroxycarbazole and 4-hydroxycarbazole, was realized using 2-chloroaniline and resorcinol as reactants and phosphoric acid as catalyst in m-xylene solvent. Then, a millimeter scale photoreactor with a decent liquid holdup (6.8 mL) was designed based on collective efforts of sizing-up and numbering-up strategies by means of contraction and expansion of the reaction channel consisting of multiple mixing units in series. Femtosecond laser engraving technique was applied to realize high-precision manufacturing of the glass photoreactor. Then, the photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole was studied in the millimeter scale photoreactor under the illumination of 365 nm UV-LED with dimethyl sulfoxide as photochemical solvent and potassium t-butoxide as base catalyst. It was found that under optimized conditions a 2-hydroxycarbazole yield of 31.6% and a 4-hydroxycarbazole yield of 11.1% could be obtained with a residence time of only 1 min. Compared to semi-batch operations, the reaction time was shortened by 1–2 orders of magnitude. As a result, a throughput of 11.3 g/day 2-hydroxycarbazole and 4.0 g/day 4-hydroxycarbazole can be achieved from the millimeter scale photoreactor with a size of 145 mm × 122 mm × 5 mm. Its characteristic size of 1.2 mm proved to contribute to the decent liquid holdup without compromising the photon transfer efficiency. To the best of our knowledge, this work demonstrates, for the first time, the use of a millimeter scale photoreactor system for rapid, high-throughput, and green production of 2-hydroxycarbazole and 4-hydroxycarbazole, which reveals a new avenue to boost the commercial development of these carbazoles.
|
Download:
|
| Scheme 1. Photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole by intramolecular arylation of 3‑hydroxy-2′‑chloro-diphenylamine under the illumination of 365 nm UV-LED. DMSO, dimethyl sulfoxide; t-BuOK, potassium t-butoxide. | |
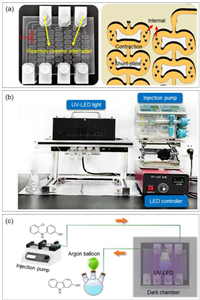
A millimeter scale butterfly-shaped photoreactor was designed based on sizing-up and numbering-up strategies. Glass with high chemical stability and high light transmittance was used as the material for the reactor. During the machining, a glass plate with a size of 145 mm × 122 mm × 5 mm was used as the substrate, which underwent femtosecond laser engraving to construct photoreactors with a characteristic length of 1.2 mm (Fig. 1a). The reaction channel consists of 51 mixing units in series, and the size of each unit was 15 mm × 8.8 mm × 1.2 mm with a liquid holdup of 112 µL. As a result, a total liquid holdup of 6.8 mL was achieved through connection of these mixing units. A butterfly-shaped obstacle was placed in the middle of each mixing unit, and rhombus internals were arranged on both sides of each mixing unit [31,32]. Photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole involves electron transfer among radical intermediates, the efficient mixing of which can promote the formation of target products [33]. High mixing performance was reported for the butterfly-shaped photoreactor as a > 99% mixing index was realized for ethanol/water (1:1, v/v) system after passing the 3rd mixing unit at a total flow of ~10 mL/min, attributed to the contraction and expansion design of the reaction channel [31]. Moreover, its characteristic size of 1.2 mm would be beneficial to the photon transfer efficiency. Therefore, the use of millimeter scale butterfly-shaped photoreactor may facilitate both the mixing efficiency among radical intermediates and the photon transfer rates, thereby enhancing the reaction rates.
|
Download:
|
| Fig. 1. Experimental equipment: (a) Photo and illustration of the butterfly-shaped photoreactor; (b) Experimental setup for the photochemical intra-molecular cyclization of 3‑hydroxy-2′‑chloro-diphenylamine to produce 2-hydroxycarbazole and 4-hydroxycarbazole; (c) Schematic diagram of the continuous synthesis of 2-hydroxycarbazole via photochemical route. | |
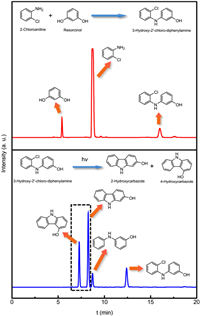
In the current work, reactants and products analysis was performed by high performance liquid chromatography (HPLC; 1260; Agilent Technologies). For analysis of 3‑hydroxy-2′‑chloro-diphenylamine synthesis, the HPLC conditions were as follow: flowing phase methanol: water (7:3), Eclipse Plus C18 column (4.6 mm × 250 mm × 5 µm; Agilent Technologies) and 250 ± 4 nm. For the analysis of 2-hydroxycarbazole and 4-hydroxycarbazole synthesis, the HPLC conditions were as follow: flowing phase methanol and acetonitrile mixture: water (7:3), Eclipse Plus C18 column (4.6 mm × 250 mm × 5 µm; Agilent Technologies) and 250 ± 4 nm. The characterization of reactants and products is shown in Fig. 2.
|
Download:
|
| Fig. 2. Characterization of products using HPLC. | |
In this work, the feedstock for photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole is 3‑hydroxy-2′‑chloro-diphenylamine, which was prepared via thermochemical route using 2-chloroaniline and resorcinol as reactants and phosphoric acid as catalyst. In a typical experiment, resorcinol, m-xylene and phosphoric acid were added to a three-necked flask equipped with a water separator and heated up to 139 ℃ while stirring in an oil bath. Then, a mixture of 2-chloroaniline and m-xylene solution was fed into the flask using a constant pressure dropping funnel within 60 min, followed by heating to 180 ℃ and reacting for 10 h at 180 ℃. Afterwards, the reaction medium cooled to room temperature. The oil layer was separated by a separatory funnel and washed three times with ultrapure water. The obtained oil solution was then concentrated using a rotary evaporator to remove m-xylene and 2-chloroaniline, after which 3‑hydroxy-2′‑chloro-diphenylamine solid was obtained. The solid was qualitatively analyzed by liquid chromatography (LC, G6230B, Agilent Technologies) equipped with time-of-flight mass spectrometer (MS), the result of which was provided in the Supporting Information (Fig. S1 in Supporting information).
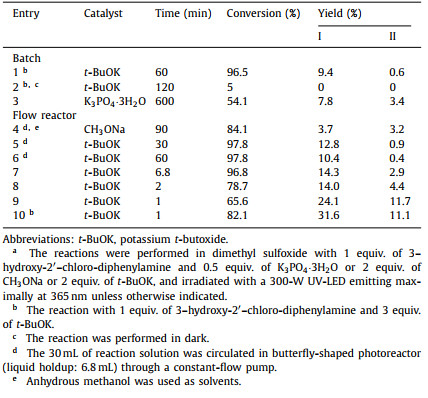
For the preparation of 3‑hydroxy-2′‑chloro-diphenylamine, a dramatic solvent effect was observed as m-xylene led to a 3‑hydroxy-2′‑chloro-diphenylamine yield of 73.0% while the use of methanol, acetonitrile, or 1,4-dioxane did not generate the target product (Table 1). The undesired solvents may facilitate the reaction between phosphoric acid and 2-chloroaniline, and thereby inhibit the target reaction (entries 2–4, Table 1). Solid formation was observed when m-xylene was used as the solvent, which is resulted from the low solubility of resorcinol in m-xylene and undesired reaction between phosphoric acid and 2-chloroaniline to generate insoluble solids. To prevent the solid formation, p-toluene sulfonic acid was chosen as the catalyst instead of phosphoric acid which did not react with 2-chloroaniline at room temperature. However, a much lower yield (11.5%) was obtained (entry 6, Table 1) during the homogeneous preparation of 3‑hydroxy-2′‑chloro-diphenylamine.
|
|
Table 1 Thermochemical synthesis of 3‑hydroxy-2′‑chloro-diphenylamine. |
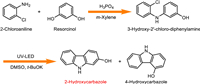
For photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole in a batch reactor, 2-hydroxycarbazole yield of 7.8% and a 4-hydroxycarbazole yield of 3.4% (Table 2, entry 3) were obtained at 3‑hydroxy-2′‑chloro-diphenylamine conversion of 54.1% after 600 min reaction under argon protection and 365 nm UV-LED irradiation with K3PO4·3H2O as catalyst and anhydrous dimethyl sulfoxide as photochemical solvent. When using potassium t-butoxide as catalyst, a 2-hydroxycarbazole yield of 9.4% and a 4-hydroxycarbazole yield of 0.6% were obtained at a conversion of 96.5% after 60 min reaction (Table 2, entry 1). In a control experiment, the reaction hardly occurred in a dark environment after 120 min under the same conditions (Table 2, entry 2). Therefore, the semi-batch operation requires reaction time of hours without achieving high yields for target products.
|
|
Table 2 Photochemical synthesis of 2-hydroxycarbazole (Ⅰ) and 4-hydroxycarbazole (Ⅱ) via intramolecular arylation of 3‑hydroxy-2′‑chloro-diphenylamine a. |
For the continuous photochemical synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole, the experimental setup is shown in Figs. 1b and c. Firstly, the butterfly-shaped photoreactor and three flasks were vacuumed and filled with argon by a circulating water multipurpose vacuum pump. Then, the anhydrous solvent was purged and deoxygenated with argon in a dark environment, and the alkali catalyst was added into the solvent. After 5 min, 3‑hydroxy-2′-chlorodiphenylamine was added into the alkali solution, and then transferred to a disposable syringe wrapped with aluminum foil. The reactant solution was then fed into the butterfly-shaped photoreactor by a syringe pump. The tubes that outside of the reactor were wrapped with aluminum foil to prevent the influence of environmental light, which were not counted as reaction volume. The reaction medium in the butterfly-shaped photoreactor was irradiated by a 300 W flat plate UV-LED with a maximum emitting wavelength of 365 nm at room temperature. Once reaching the steady state, the product was collected in brown vials.
The results of the continuous photochemical synthesis show that potassium t-butoxide (Table 2, entry 5) is a better base catalyst compared to sodium methoxide (Table 2, entry 4) and dimethyl sulfoxide is a better photochemical solvent than methanol. The lower oxygen content of dimethyl sulfoxide than that of methanol may reduce the oxygen derived quenching of photochemical radical reaction and thus enhance the conversion of 3‑hydroxy-2′‑chloro-diphenylamine [33,34]. The major byproduct is 3-hydroxydiphenylamine (Table S1 in Supporting information), which was formed by fragmentation of the C—Cl bond of 3‑hydroxy-2′‑chloro-diphenylamine. The mole balance of the photochemical synthesis (Table 2 and Table S1) suggests the formation of other byproducts that were not detected by the current HPLC method. These byproducts may be formed by chlorination on the ortho and/or para hydrogens on the benzene ring which become active due to the presence of phenolic hydroxyl groups.
The following experiment used dimethyl sulfoxide as the photochemical solvent, and under optimized conditions a 2-hydroxycarbazole yield of 31.6% and a 4-hydroxycarbazole yield of 11.1% were obtained (Table 2, entry 10). Increasing the residence time did not increase the yield of target products at the expense of the reactant due to formation of byproducts (entries 7–9, Table 2 and Table S1). It was also found that higher dosage of potassium t-butoxide is beneficial to the formation of 2-hydroxycarbazole (Table 2, entries 9 and 10), which may be due to the alkali promoted deprotonation of 3‑hydroxy-2′‑chloro-diphenylamine that leading to its higher conversion rates [33]. Therefore, the higher conversion of entry 10 as compared to entry 8 is due to a higher dosage of t-BuOK for the former despite its shorter residence time. Under the same conversion or equivalent conditions, the continuous synthesis of 2-hydroxycarbazoleand and 4-hydroxycarbazole using the butterfly-shaped photoreactor (Table 2, entries 7 and 10) resulted in much shorter reaction time compared to the semi-batch operation (Table 2, entry 1) and higher selectivity to target products. The promotion effects are resulted from the high photon transfer rates and uniformity given the larger specific surface area and smaller characteristic length of the millimeter scale butterfly-shaped photoreactor compared to the batch reactor. Further improvement of the yield of target products may involve optimizing LED light sources (e.g., wavelength, photon flux, illumination area) and/or screening for other alkaline catalysts.
In summary, we explored an efficient route for the continuous synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole through photochemical intramolecular cyclization of 3‑hydroxy-2′‑chloro-diphenylamine. A millimeter scale butterfly-shaped photoreactor with a decent liquid holdup and high specific surface area was designed and fabricated via femtosecond laser engraving technique. 3‑Hydroxy-2′‑chloro-diphenylamine was used as the feedstock for the photochemical synthesis, the high-yield (73.0%) production of which was realized using 2-chloroaniline and resorcinol as reactants and phosphoric acid as catalyst. Then, the millimeter scale photoreactor was used for the synthesis of 2-hydroxycarbazole and 4-hydroxycarbazole under the illumination of 365 nm UV-LED with dimethyl sulfoxide as photochemical solvent and potassium t-butoxide as base catalyst. Under optimized conditions, a 2-hydroxycarbazole yield of 31.6% and a 4-hydroxycarbazole yield of 11.1% were obtained via the continuous photosynthesis with a residence time of only 1 min, while semi-batch operations under similar conditions required hours of reaction. Compared to the semi-batch operations, the higher production efficiency and yield of target products from the continuous photochemical synthesis are resulted from the higher photon transfer rates and more uniform photon distribution provided by the specific surface area and characteristic size of the millimeter scale photoreactor, which enhances the reaction rates and mitigates overreaction. The current photoreactor (145 mm × 122 mm × 5 mm) can provide a throughput of 11.3 g/day 2-hydroxycarbazole and 4.0 g/day 4-hydroxycarbazole. This work reveals a new avenue for green and efficient synthesis of carbazole derivatives.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was funded by the National Natural Science Foundation of China (Nos. 21991103, 21991104, 22008074, 22008072); Natural Science Foundation of Shanghai (No. 20ZR1415700), China Postdoctoral Science Foundation (Nos. 2020M671025, 2019TQ0093). The authors thank Prof. Ya Cheng and Dr. Miao Wu from East China Normal University for the fabrication of reactor via femtosecond laser engraving.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108893.
| [1] |
M.S. Joshi, A.I. Lansakara, F.C. Pigge, Tetrahedron Lett. 56 (2015) 3204-3207. DOI:10.1016/j.tetlet.2014.12.074 |
| [2] |
H.M. Kim, J.M. Choi, J.Y. Lee, Org. Electron. 43 (2017) 130-135. DOI:10.1016/j.orgel.2017.01.021 |
| [3] |
S.M. Kim, J.H. Yun, S.H. Han, J.Y. Lee, J. Ind. Eng. Chem. 84 (2020) 217-225. DOI:10.1016/j.jiec.2019.12.036 |
| [4] |
Y. Ou, N. Jiao, Chem. Commun. 49 (2013) 3473-3475. DOI:10.1039/c3cc41443d |
| [5] |
J.M. Thomas, G.C. Churchill, S. Patel, A. Galione, J. Pharmacol. Exp. Ther. 298 (2001) 644-650. |
| [6] |
S.C. Tovey, C.L. Longland, M. Mezna, F. Michelangeli, Eur. J. Pharmacol. 354 (1998) 245-251. DOI:10.1016/S0014-2999(98)00446-4 |
| [7] |
G. Smolinsky, J. Am. Chem. Soc. 83 (1961) 2489-2493. DOI:10.1021/ja01472a016 |
| [8] |
K. Lauer, E. Kiegel, DE2928483B1, 1980.
|
| [9] |
A. Kawada, M. Komori, T. Nakagawa, K. Ishikawa, Patent: JP2003160516A, 2003.
|
| [10] |
T.L.S. Kishbaugh, G.W. Gribble, Tetrahedron Lett. 42 (2001) 4783-4785. DOI:10.1016/S0040-4039(01)00854-1 |
| [11] |
R. Sanz, J. Escribano, M.R. Pedrosa, R. Aguado, F.J. Arnaiz, Adv. Synth. Catal. 349 (2007) 713-718. DOI:10.1002/adsc.200600384 |
| [12] |
O. Sonoda, T. Miyai, Patent: JP2002241365A, 2002.
|
| [13] |
T. Noel, J. Flow. Chem. 7 (2017) 87-93. DOI:10.1556/1846.2017.00022 |
| [14] |
F. Politano, G. Oksdath-Mansilla, Org. Process Res. Dev. 22 (2018) 1045-1062. DOI:10.1021/acs.oprd.8b00213 |
| [15] |
N. Hoffmann, Chem. Rev. 108 (2008) 1052-1103. DOI:10.1021/cr0680336 |
| [16] |
X. Shi, S. Liu, C. Duanmu, et al., Chem. Eng. J. 420 (2021) 129976. DOI:10.1016/j.cej.2021.129976 |
| [17] |
C. Shen, M. Shang, H. Zhang, Y. Su, AlChE J. 66 (2020) e16841. DOI:10.1002/aic.16841 |
| [18] |
Y. Wang, C. Shen, M. Qiu, M. Shang, Y. Su, AlChE J. 69 (2023) e18102. DOI:10.1002/aic.18102 |
| [19] |
T. Aillet, K. Loubiere, O. Dechy-Cabaret, L. Prat, Int. J. Chem. React. Eng. 12 (2014) 257-269. DOI:10.1515/ijcre-2013-0121 |
| [20] |
S. Bachollet, K. Terao, S. Aida, et al., Beilstein J. Org. Chem. 9 (2013) 2015-2021. DOI:10.3762/bjoc.9.237 |
| [21] |
M. Conradi, T. Junkers, J. Photochem. Photobiol. A 259 (2013) 41-46. DOI:10.1016/j.jphotochem.2013.02.024 |
| [22] |
T. Fukuyama, Y. Kajihara, Y. Hino, I. Ryu, J. Flow. Chem. 1 (2011) 40-45. |
| [23] |
A. Talla, B. Driessen, N.J.W. Straathof, et al., Adv. Synth. Catal. 357 (2015) 2180-2186. DOI:10.1002/adsc.201401010 |
| [24] |
K. Donnelly, M. Baumann, J. Flow. Chem. 11 (2021) 223-241. DOI:10.1007/s41981-021-00168-z |
| [25] |
T. Fukuyama, T. Kasakado, M. Hyodo, I. Ryu, Photochem. Photobiol. Sci. 21 (2022) 761-775. DOI:10.1007/s43630-021-00151-6 |
| [26] |
K.P.L. Kuijpers, M.A.H. van Dijk, Q.G. Rumeur, et al., React. Chem. Eng. 2 (2017) 109-115. DOI:10.1039/C7RE00024C |
| [27] |
Y. Su, N.J.W. Straathof, V. Hessel, T. Noel, Chem. Eur. J. 20 (2014) 10562-10589. DOI:10.1002/chem.201400283 |
| [28] |
D. Cambié, C. Bottecchia, N.J.W. Straathof, V. Hessel, T. Noël, Chem. Rev. 116 (2016) 10276-10341. DOI:10.1021/acs.chemrev.5b00707 |
| [29] |
L. Buglioni, F. Raymenants, A. Slattery, S.D. Zondag, T. Noël, Chem. Rev. 122 (2021) 2752-2906. |
| [30] |
T. Noël, Photochemical Processes in Continuous-Flow Reactors, 1st ed., World Scientific, 2017.
|
| [31] |
H. Lv, Z. Yang, J. Zhang, et al., Micromachines 12 (2021) 883. DOI:10.3390/mi12080883 |
| [32] |
H. Lv, J. Wang, Z. Shu, et al., Chin. Chem. Lett. 34 (2023) 107710. DOI:10.1016/j.cclet.2022.07.053 |
| [33] |
M.E. Budén, V.A. Vaillard, S.E. Martin, R.A. Rossi, J. Org. Chem. 74 (2009) 4490-4498. DOI:10.1021/jo9006249 |
| [34] |
A. Albini, M. Fagnoni, Handbook of Synthetic Photochemistry, 1st ed., Wiley, 2009.
|
 2024, Vol. 35
2024, Vol. 35