b National Engineering Research Center of Immunological, Department of Microbiology and Biochemical Pharmacy, College of Pharmacy and Laboratory Medicine, Third Military Medical University, Chongqing 400037, China;
c State Key Laboratory of Trauma, Burn and Combined Injury, Third Military Medical University, Chongqing 400037, China
Cytokines are highly active and multifunctional small molecular polypeptides secreted by active cells, which mainly mediate and regulate immune responses and inflammatory responses [1]. Among all chicken cytokines, chicken interleukin (ChIL-4) and chicken interferon gamma (ChIFN-γ) are the most important cytokines and have been extensively studied at present, which has been proved to play vital roles in inflammation, rapid allergic reaction, immunomodulatory properties and growth factors in chickens [2–4]. Currently, determination of the two cytokines is mainly conducted by biological methods, such as flow cytometry [5], in situ hybridization [6] and reverse transcriptase polymerase chain reaction [7], but these methods were hindered by high experimental cost and lack of accuracy [8–10]. Enzyme-linked immunoassay has also been developed to detect ChIL-4 and ChIFN-γ [11,12], but it was limited by single component detection and the insufficient sensitivity. Therefore, it is essential to explore a sensitive multi-component immunoassay with advantages of simplicity, rapidity and accuracy for simultaneous detection of multiple chicken cytokines.
Chemiluminescence (CL) imaging immunoassay, regarded as a promising analysis strategy, has attracted extensive attention in clinical diagnosis, food safety and life science owing to its multi-component detection, high analysis sensitivity, simple device and no scattered light interference [13–15]. Recently, CL immunoassay was developed to detect chicken cytokines, and but only detect one chicken cytokine in one assay turn. However, immune array has the advantages of high detection throughput and fast analysis, especially in integration, miniaturization and automation, makes it become the mainstream trend of multicomponent determination [16–19]. However, traditional CL usually utilizes natural enzyme labels to obtain enhanced CL detection. The natural enzyme molecules are suffered from poor stability and limited sources, which hinder the further development of CL immunoassay [20–25]. In recent years, various peroxidase (POD) mimetics including hemin, hemoglobin, porphyrin, hemin/G-quadruplex DNAzyme and nanoparticles have been explored in immunoassay applications [26–29]. The utilization of peroxidase mimetics with high catalytic activity improves the sensitivity of immunoassay [30–32]. Since the discovery of Fe3O4 nanoparticles (NPs) with inherent POD-mimetic activity [33], considerable attempts have been devoted to exploring nanomaterials with POD-like activity [34–36]. Various nanomaterials, including noble metal, metal oxides, metal sulfide and carbon are introduced as peroxidase mimetics to apply in bioanalysis [37–39]. Copper sulfide nanoparticles (CuSNPs) as an important semiconductor material, has been explored as excellent peroxidase mimetics for colorimetric and CL biosensing fields [40,41]. Recently, CuSNPs have been reported as excellent peroxidase mimetics for CL immunoassay application [42]. However, the research about CuSNPs-based CL immunoassay is very few, and the relatively low mimic catalytic activity of the CuSNPs limited the further application. Moreover, DNAzyme has attracted considerable attention in development of several sensitive biosensors due to high catalytic ability [43,44]. However, the single peroxidase mimetics show limited POD-like activity. So the design of composite peroxidase mimetics materials offer a new opportunity to gain high catalytic ability.
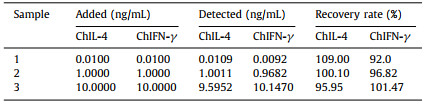
Herein, DNAzyme was modified onto CuSNPs with carboxyl functionalization to form DNAzyme@CuSNPs dual mimic enzyme (Scheme 1A), which was explored as peroxidase mimetics probe to develop ultrasensitive CL imaging array immunosensor for simultaneous detection of multiple chicken cytokines (Scheme 1B). The DNAzyme@CuSNPs mimic enzyme probe shows high POD-like activity, and significantly produced CL imaging signals due to the synergistic effect, which remarkably enhanced the detection sensitivity. The enhancement of CL imaging signals was linearly related to concentration of the two cytokines with very wide linear range and low detection limit. Compared to the previous research, the proposed CL immunosensor possesses wider linear range and much lower detection limit for ChIL-4 and ChIFN-γ. The constructed dual mimic enzyme-based CL imaging array immunosensor opens up a novel method, which can make up for the shortcomings of the traditional CL immunosensor, and provides a promising avenue for the development of sensitive multicomponent CL immunoassay and clinical diagnosis of chicken diseases.
|
Download:
|
| Scheme 1. Schematic of preparation of DNAzyme@CuSNPs signal probe (A) and CL array immunosensor for detection of chicken cytokines of ChIL-4 and ChIFN-γ (B). | |
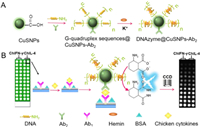
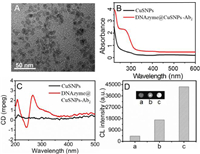
It is can be seen from the high-resolution transmission electron microscope (TEM) image (shown in the Fig. 1A), the prepared carboxyl-functionalized CuSNPs have regular morphology and good dispersion with uniform size of 6–8 nm. These features are favorable to load more DNAzyme and second antibodies (Ab2) to obtain excellent dual mimic enzyme probe. The UV–vis spectra of CuSNPs and DNAzyme@CuSNPs-Ab2 were utilized to verify the successful immobilization of DNAzyme and Ab2. As shown in Fig. 1B, there was no obvious absorption peak of CuSNPs at 260–280 nm. While DNAzyme@CuSNPs-Ab2 showed a clear absorption peak at 278 nm, which can be attributed to the characteristic absorption of proteins. According to the obvious research, a typical parallel G-quadruplex structure shows a positive peak (265 nm) and a negative peak (240 nm) [45]. Circular dichroism (CD) spectrum of DNAzyme@CuSNPs-Ab2 (Fig. 1C) shows a positive peak at 267 nm and a negative peak at 242 nm, proving that DNAzyme is successfully modified on the surface of CuSNPs. These results indicate that both DNAzyme and Ab2 have been successfully modified on the surface of CuSNPs.
|
Download:
|
| Fig. 1. (A) High-resolution TEM image of CuSNPs. (B) UV–vis spectra of DNAzyme@CuSNPs-Ab2. (C) CD spectra of DNAzyme@CuSNPs-Ab2. (D) CL imaging signals of CuSNPs-Ab2 (a), HRP@AuNPs-Ab2 (b), and DNAzyme@CuSNPs-Ab2 (c) for detection of 0.1 ng/mL ChIL-4. | |
In order to evaluate the catalytic property of the DNAzyme@CuSNPs-Ab2 probe, different label probes were incubated on the same immune array for detection of 0.1 ng/mL ChIL-4 via sandwich immunoreaction. As shown in Fig. 1D, the CL imaging intensity of CuSNPs-Ab2, horseradish peroxidase@AuNPs-Ab2 (HRP@AuNPs-Ab2) and DNAzyme@CuSNPs-Ab2 probes is 4222, 17,066 and 43,255, respectively. It can be significantly seen that the DNAzyme@CuSNPs-Ab2 signal probe designed in this work produces the stronger CL signal than single CuSNPs-Ab2 due to its synergetic enzyme-mimic catalytic activity. Natural enzyme HRP-coated AuNPs has been popular signal probe for biosensing application due to excellent catalytic property [14]. Compared with HRP@AuNPs-Ab2 probe, DNAzyme@CuSNPs-Ab2 signal probe also shows much stronger CL signal, further confirming excellent performance of dual mimic enzyme probe. In this research, the dual signal amplification of the CL array immunosensor is realized on the basis of dual mimic enzyme probe, which greatly improves the sensitivity for two chicken cytokines detection.
Scanning electron microscope (SEM) and electrochemical impedance spectroscopy were used to characterize construction of the chicken cytokines array immunosensor. The glass slides were functionalized with epoxy groups by silanization action, and then printed with micropore array for capturing cytokines antibody. As illustrated in Fig. 2A, the SEM image of epoxy group-treated array slides show smooth and uniform surface. Compared to Fig. 2A, Fig. 2B exhibits much rougher surface, which results from combination of antibody (Ab1) molecules onto the array slides. The electron transfer resistance is quantified by the semicircle diameter of the Nyquist impedance spectrum. Fig. 2C shows the electrochemical impedance spectra (EIS) of different modified electrodes from 0.05 kHz to 10 kHz. Due to the gradual modification of molecules, the impedance values of bare GCE (curve a) Ab1-chitosan/GCE (curve b), and BSA/Ab1-chitosan/GCE (curve c) increased gradually due to the hindrance of electron transfer, demonstrating the successful fabrication of the CL array immunosensor. When the array immunosensor captured ChIL-4 antigen, impedance value of the ChIL-4/Ab1-chitosan/GCE (curve d) further increased. After further incubation with DNAzyme@CuSNPs-Ab2 probe, it is worth noting that the impedance value of DNAzyme@CuSNPs-Ab2/ChIL-4/Ab1-chitosan/GCE (curve e) decreased obviously, which is owing to the excellent conductivity of CuSNPs.

|
Download:
|
| Fig. 2. (A) SEM of epoxy group-modified slide. (B) SEM of Ab1 modified array slide. (C) Nyquist plots of EIS for bare GCE (a), Ab1-chitosan/GCE (b), BSA/Ab1-chitosan/GCE (c), ChIL-4/Ab1-chitosan/GCE (d), and DNAzyme@CuSNPs-Ab2/ChIL-4/Ab1-chitosan/GCE (e). | |
The kinetic behavior of the dual mimic enzyme catalytic CL reaction of the cytokines array immunosensor was investigated in this research. As seen in Fig. S1 in Supporting information, CL reaction immediately was triggered after adding CL substrate, and the CL intensity reached maximum value and then reduced after 1.0 min. In addition, 80% of the maximum CL intensity can be maintained at 300 s. In order to achieve high sensitive detection, CCD imaging requires a longer exposure time to collect a weaker CL intensity, thereby, the total time of CL dynamic integration needs 300 s. The incubation time, a key constraint of the entire immunoassay speed, was tested here. This experiment includes two-step incubation. The first step can be regarded as the incubation of the Ab1 and cytokine antigen to form a stable immune complex. The second step is determined as immune complex incubating with DNAzyme@CuSNPs-Ab2 probe to form sandwich immune complex. As shown in Fig. S2 (Supporting information), taking ChIL-4 as an example, when adding 10 ng/mL ChIL-4 antigen and DNAzyme@CuSNPs-Ab2 probe on array immunosensor, the CL imaging intensity increased correspondingly with the increase of incubation time and two step-incubation tended to be stable at 25 min. It shows that the binding reaction of array immunosensor to DNAzyme@CuSNPs-Ab2 probe and cytokines antigen can reach saturation in 25 min and forming a stable sandwich immune complex, so the two-step optimal incubation time selected as 25 min.
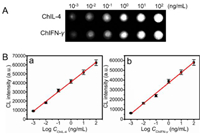
Under the optimal analysis conditions, the CL imaging intensity increased with the increase of the concentration of ChIL-4 and ChIFN-γ antigen (Fig. 3A), demonstrating a linear correlation with CL imaging value versus logarithm of cytokines analyte concentration (Fig. 3B). The linear curves show linear ranges of 10−3–102 ng/mL (R2 = 0.9949) and 10−3–102 ng/mL (R2 = 0.9972) for ChIL-4 and ChIFN-γ, respectively. In addition, the detection limits of the proposed chicken cytokines array immunosensor for ChIL-4 and ChIFN-γ was calculated to be 0.41 pg/mL and 0.36 pg/mL, which are far lower than the reported detection limit 0.05 ng/mL, 2 pg/mL, 3.2 pg/mL for ChIL-4 and 3 pg/mL, 2.9 pg/mL for ChIFN-γ [2,14,42]. The high sensitivity of array immunosensor may result from the excellent POD-like activity of DNAzyme@CuSNPs and dual mimic enzyme signal amplification strategy. In addition, the dual mimic enzyme signal amplification and multiplex immunoassay strategy can simultaneously detect two or more samples, which greatly shortens analysis time and detection cost, offering a sensitive, reliable, fast and high-throughput immunoassay method for clinical diagnosis.
|
Download:
|
| Fig. 3. (A) CL imaging signals for various concentration of ChIL-4 and ChIFN-γ. (B) Calibration curves for immunoassay of ChIL-4 (a) and ChIFN-γ (b). | |
The cross-reaction between target analytes and non-specific antibodies was studied in Fig. 4A. Taking ChIL-4 immune array micropore as a model, blank solution (column a), ChIFN-γ (10 ng/mL, column b), ChIL-4 (10 ng/mL, column c), and mixture (column d) of ChIFN-γ (10 ng/mL), IgG (10 ng/mL), BSA (10 ng/mL), and ChIL-4 (10 ng/mL) were added respectively into the immune array sensor. As expected, the ChIL-4 immune array micropores only responded to the target antigen ChIL-4 (column c) and the mixture containing ChIL-4 antigen (column d) and exhibited strong CL signals. Conversely, there was weak CL signal with the addition of blank solution (column a) and ChIFN-γ (10 ng/mL) (column b) without presence ChIL-4. Similarly, ChIFN-γ immune array micropore presents similar phenomenon, which indicates the non-specific binding of interfering antigens can be negligible. Taken together, every immune micropore is independent and immune response only occurs in the corresponding immune micropores, which can well avoid the cross-reaction between the adjacent immune micropores.

|
Download:
|
| Fig. 4. (A) Effect of addition of varying concentration of competing cytokine on CL imaging intensity for immunoassay of 10.0 ng/mL ChIL-4 and ChIFN-γ (a: blank solution; b: 10 ng/mL competing cytokine of ChIFN-γ or ChIL-4; c: 10 ng/mL target cytokine of ChIL-4 or ChIFN-γ; d: 10 ng/mL of ChIFN-γ, IgG, BSA and ChIL-4). (B) CL imaging signals of the array immunosensor after the storage of 0 d, 7 d, 14 d, 21 d and 28 d at the concentration of 10.0 ng/mL cytokine antigens. | |
The reproducibility of the chicken cytokine array immunosensor can be evaluated by intra- and inter variation coefficients. The variation coefficient of the intra-group can be determined by relative standard deviation (RSD) of the same sample detected repeatedly five times with the same immunosensor. The variation coefficient of inter-group can be determined as RSD of the same sample detected parallelly with five different immunosensors. Herein, ChIL-4 and ChIFN-γ (1.0 ng/mL) samples were applied, intra- and inter variation coefficients of ChIL-4 were 2.2% and 5.8%. Intra and inter variation coefficients of ChIFN-γ were 2.3% and 4.5%. These data indicate that the constructed chicken cytokines array immunosensor possesses good detection reproducibility. Fig. 4B presents the storage stability of the array immunosensor via detecting the CL imaging signals of ChIL-4 and ChIFN-γ by storing array immunosensor in PBS solution at 4 ℃ for 4 weeks. It can be found that the CL imaging signal changes of ChIL-4 and ChIFN-γ on array immunosensor are less than 1.9% and 2.4%. The above analysis shows that the immune sensing arrays have good reproducibility and acceptable stability.
In order to investigate the accuracy and actual application value of the CL imaging array immunosensor, recovery experiments were carried out by adding standard ChIL-4 and ChIFN-γ antigens to clinical chicken serum samples (Table 1). The recovery values of ChIL-4 and ChIFN-γ were calculated, and the corresponding ranges are 95.95%−109.0% and 92.00%−101.5%, respectively, illustrating the applicability in practical serum sample.
|
|
Table 1 The recovery experiments of CL array immunosensor for ChIL-4 and ChIFN-γ (n = 5). |
In this work, a DNAzyme@CuSNPs dual mimic enzyme signal amplification probe strategy was proposed to develop a novel and ultrasensitive chemiluminescent imaging array immunosensor for detecting a variety of chicken cytokines. DNAzyme@CuSNPs owns excellent peroxidase property, and was modified with second antibody (Ab2) to prepare DNAzyme@CuSNPs-Ab2 detection probe. Compared to the single CuSNPs and HRP labels, DNAzyme@CuSNPs probe demonstrated much higher catalysis CL imaging signal due to the synergistic catalysis action. Combining excellent DNAzyme@CuSNPs mimic enzyme signal probe, the CL imaging array immunosensor was successfully applied to detect ChIL-4 and ChIFN-γ, and exhibits a wide linear range, low detection limit and high throughput. Moreover, The chicken cytokines array immunosensor also has high specificity, good accuracy, acceptable reproducibility and stability, indicating that the proposed dual mimic enzyme signal-amplified strategy offer a promising method to develop sensitive CL imaging immunoassay for chicken diseases detection.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21575125 and 21475116), the Natural Science Foundation of Jiangsu Province (Nos. BK20221370 and BK20191434), Key University Natural Science Foundation of Jiangsu-Province (No. 20KJA150004), Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD), Project for Science and Technology of Yangzhou (No. YZ2022074), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX22_3462).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108791.
| [1] |
A.W. Li, W.A. Lim, Science 370 (2020) 1034-1035. DOI:10.1126/science.abb5607 |
| [2] |
Z.J. Yang, M.M. Lu, J. Li, et al., Biosens. Bioelectron. 89 (2017) 558-564. DOI:10.1016/j.bios.2016.02.046 |
| [3] |
F.Y. Shi, G.F. Erf, J. Invest, Dermatol. 132 (2012) 643-649. |
| [4] |
K. Haq, I. Elawadli, P. Parvizi, Antiviral Res. 90 (2011) 218-226. DOI:10.1016/j.antiviral.2011.04.001 |
| [5] |
H. Mikami, M. Kawaguchi, C.J. Huang, et al., Nat. Commun. 11 (2020) 1162-1172. DOI:10.1038/s41467-020-14929-2 |
| [6] |
N. Ostromohov, D. Huber, M. Bercovici, G.V. Kaigala, Anal. Chem. 90 (2018) 11470-11477. DOI:10.1021/acs.analchem.8b02630 |
| [7] |
E. Oki, Y. Maehara, E. Tokunaga, et al., Cancer Lett. 188 (2002) 191-198. DOI:10.1016/S0304-3835(02)00057-5 |
| [8] |
C. Grabinski, N. Schaeublin, A. Wijaya, et al., ACS Nano 5 (2011) 2870-2879. DOI:10.1021/nn103476x |
| [9] |
R.T. Ranasinghe, M.R. Challand, K.A. Ganzinger, et al., Nat. Commun. 9 (2018) 655-664. DOI:10.1038/s41467-017-02714-7 |
| [10] |
J. Liao, B. Pan, X.B. Zhou, et al., Chin. Chem. Lett. 33 (2022) 4345-4349. DOI:10.1016/j.cclet.2021.12.065 |
| [11] |
Z.P. Sun, C. Liu, T.R. Pan, et al., Dev. Comp. Immunol. 77 (2017) 30-37. DOI:10.1016/j.dci.2017.07.018 |
| [12] |
Z.F. Hu, R. Xu, S.H. Yu, et al., Analyst 145 (2020) 7864-7869. DOI:10.1039/D0AN01553A |
| [13] |
H. Zhao, E.B. Su, L. Huang, et al., Chin. Chem. Lett. 33 (2022) 743-746. DOI:10.1016/j.cclet.2021.07.017 |
| [14] |
Y.H. Zhong, X.Y. Wu, J. Li, et al., Anal. Chim. Acta 1049 (2019) 213-218. DOI:10.1016/j.aca.2018.10.050 |
| [15] |
S.T. He, L.L. He, B.B. Liu, et al., Chin. Chem. Lett. 30 (2019) 1031-1034. DOI:10.1016/j.cclet.2019.03.013 |
| [16] |
J.W. Cao, P. Ouyang, S.H. Yu, et al., Chem. Commun. 57 (2020) 1766-1769. |
| [17] |
Z.J. Yang, Y. Cao, J. Li, et al., ACS Appl. Mater. Inter. 19 (2016) 12031-12038. |
| [18] |
C. Zong, F. Jiang, X.Y. Wang, et al., Biosens. Bioelectron. 177 (2021) 112998. DOI:10.1016/j.bios.2021.112998 |
| [19] |
A. Qileng, W.P. Liu, Z.Q. Sun, et al., Adv. Funct. Mater. 32 (2022) 2203244. DOI:10.1002/adfm.202203244 |
| [20] |
G. Gübitz, M.G. Schmid, H. Silviaeh, H.Y. Aboul-Enein, Crit. Rev. Anal. Chem. 31 (2010) 141-148. |
| [21] |
C. Shim, R. Chong, J.H. Lee, Talanta 171 (2017) 229-235. DOI:10.1016/j.talanta.2017.05.007 |
| [22] |
Q. Xiao, C.X. Xu, Trends Anal. Chem. 124 (2020) 115780. DOI:10.1016/j.trac.2019.115780 |
| [23] |
H.Z. Liang, A. Qileng, H.R. Shen, et al., Anal. Chem. 94 (2022) 4294-4302. DOI:10.1021/acs.analchem.1c04957 |
| [24] |
H.R. Shen, A. Qileng, H. Yang, et al., Anal. Chem. 93 (2021) 11816-11825. DOI:10.1021/acs.analchem.1c02395 |
| [25] |
Y.X. Liu, M.D. Cao, Z.X. Huang, et al., Chin. Chem. Lett. 33 (2022) 1855-1860. DOI:10.1016/j.cclet.2021.08.117 |
| [26] |
C. Wang, J. Wu, C. Zong, H.X. Ju, F. Yan, Analyst 136 (2011) 4295. DOI:10.1039/c1an15512a |
| [27] |
J. Li, T.X. Yuan, T.T. Yang, et al., Sensor. Actuat. B: Chem. 271 (2018) 239-246. DOI:10.1016/j.snb.2018.05.045 |
| [28] |
X. Deng, Y.S. Fang, S. Lin, et al., ACS Appl. Mater. Inter. 9 (2017) 3514-3523. DOI:10.1021/acsami.6b15637 |
| [29] |
J. Xu, J. Wu, C. Zong, H.X. Ju, F. Yan, Anal. Chem. 85 (2013) 3374-3379. DOI:10.1021/ac4000688 |
| [30] |
T.T. Song, W. Wang, L.L. Meng, et al., Chin. Chem. Lett. 28 (2017) 226-230. DOI:10.1016/j.cclet.2016.07.021 |
| [31] |
K. Aslan, C.D. Geddes, Chem. Soc. Rev. 38 (2009) 2556-2564. DOI:10.1039/b807498b |
| [32] |
H.H. Sun, Y. Gao, N. Hu, et al., J. Colloid Interf. Sci. 578 (2020) 366-378. DOI:10.1016/j.jcis.2020.06.001 |
| [33] |
L.Z. Gao, J. Zhuang, L. Nie, et al., Nat. Nanotechnol. 2 (2007) 577-583. DOI:10.1038/nnano.2007.260 |
| [34] |
M.M. Liang, X.Y. Yan, Acc. Chem. Res. 52 (2019) 2190-2200. DOI:10.1021/acs.accounts.9b00140 |
| [35] |
S.J. Cao, Z.Y. Zhao, Y.J. Zheng, et al., Adv. Mater. 34 (2022) 2200255. DOI:10.1002/adma.202200255 |
| [36] |
X.Y. Ma, W. Ding, C. Wang, et al., Sens. Actuators B: Chem. 331 (2021) 129422-129431. DOI:10.1016/j.snb.2020.129422 |
| [37] |
C. Zong, J. Wu, Y. Zang, H.X. Ju, Chem. Commun. 54 (2018) 11861-11864. DOI:10.1039/C8CC06356G |
| [38] |
L.Z. Zhao, Y.Z. Fu, S.W. Ren, et al., Biosens. Bioelectron. 171 (2021) 112729. DOI:10.1016/j.bios.2020.112729 |
| [39] |
T.T. Bezuneh, T.H. Fereja, S.A. Kitte, H.J. Li, Y.D. Jin, Talanta 248 (2022) 123611. DOI:10.1016/j.talanta.2022.123611 |
| [40] |
Y.J. Zhang, S.Q. Yang, J. Wang, et al., Talanta 233 (2021) 122594. DOI:10.1016/j.talanta.2021.122594 |
| [41] |
P. Borthakur, P.K. Boruah, M.R. Das, ACS Sustain. Chem. Eng. 9 (2021) 13245-13255. DOI:10.1021/acssuschemeng.1c04203 |
| [42] |
Y.H. Zhong, X. Tang, J. Li, et al., Chem. Commun. 54 (2018) 13813. DOI:10.1039/C8CC07779G |
| [43] |
C. Zong, D.D. Zhang, H. Yang, et al., Microchim. Acta 184 (2017) 3197-3204. DOI:10.1007/s00604-017-2331-z |
| [44] |
R. Zhang, J. Wu, H. Ao, et al., Anal. Chem. 93 (2021) 9933-9938. DOI:10.1021/acs.analchem.1c02229 |
| [45] |
J.T. Ren, J.H. Wang, J. Wang, N.W. Luedtke, E.K. Wang, Biosens. Bioelectron. 31 (2012) 316-322. DOI:10.1016/j.bios.2011.10.038 |
 2023, Vol. 34
2023, Vol. 34