b Hubei Key Laboratory of Novel Reactor and Green Chemical Technology, Wuhan Institute of Technology, Wuhan 430205, China;
c Pharmaceutical Research Institute, Wuhan Institute of Technology, Wuhan 430205, China;
d Key Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering & Pharmacy, Wuhan Institute of Technology, Wuhan 430205, China
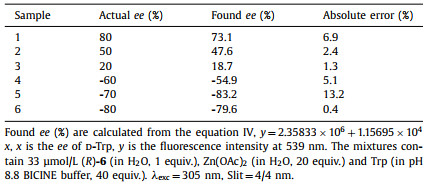
Chiral amino acids play essential and diverse roles in biological systems and serve as important synthons and chiral sources of diverse functional organic molecules [1–5]. Therefore, the chiral analysis of amino acids has attracted much attention in recent years [6–8]. As one of the chiral molecular recognition methods, the fluorescent probe has the advantages of rapid analysis and allowing direct imaging of the chiral substrates [6,7,9,10]. A number of research laboratories have focused on studies on the enantioselective fluorescent recognition of free amino acids [11,12]. Previously, Pu reported the 1,1′-bi-2-naphthol (BINOL)-based compound (S)-1 (Fig. 1) as a chiral fluorescent probe in the presence of Zn(Ⅱ) for the enantioselective recognition of functional amines including free amino acids [13]. In the following years, chemically diverse BINOL-based chiral fluorescence probes emerged [4,14–18].
|
Download:
|
| Fig. 1. Structures of (S)-1 and (S,S)-2. | |
In 2019, we reported a fluorescent probe (S,S)-2 (Fig. 1) with high enantioselectivity toward various free amino acids in the presence of Zn(Ⅱ) in the mixed solvent of CH3CN/H2O, which can be applied to determine the enantiomeric composition of the amino acids [19]. However, this fluorescent probe could not be applied to recognize chiral amino acids in the single solvent H2O due to its poor water solubility. Obviously, the development of water-soluble fluorescent probes is significant. On the one hand, it is conducive to expanding the application range of chiral recognition of amino acids, because of the excellent water solubility of most amino acids. On the other hand, it could effectively eliminate the interference of water-insoluble impurities in fluorescence tests.
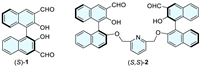
In order to improve the water solubility of BINOL-based chiral fluorescent probes, three strategies have been adopted as follows. Firstly, various hydrophilic side chains have been installed at the 6,6′-position of (S)-1 by Suzuki coupling reactions, such as (S)-3 (Fig. 2) [14]. Secondly, hydrophilic micelles have been applied to encapsulate (S)-1 in aqueous media [20,21]. Thirdly, the probes have been modified as water-soluble salts, including the installment of imidazolium at 3,3′-position, such as (R,R)-4 (Fig. 2) [22], or incorporation of sulfonate fragments at 6,6′-position, such as (R)-5 (Fig. 2) [23]. Among these strategies, the third one is more fascinating for concise synthetic methods and great improvement of water solubility.
|
Download:
|
| Fig. 2. Structures of representative water-soluble fluorescent probes. | |
Meanwhile, in the study of chiral recognition of amino acids by BINOL-based fluorescent probes, the fast fluorescence response and continuous stability are always the ambition of investigators. However, the fluorescent response of the reported BINOL-based probes toward chiral amino acids always reach stability after at least 2–3 h, and some even need to be quenched at low temperature or by other ways before fluorescence testing [14,20,22,23].
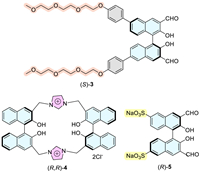
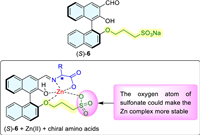
Herein, we design a novel fluorescent probe (S)-6 (Fig. 3) featuring a sodium sulfonate fragment at the 2′-position, which could complex with Zn(Ⅱ) with improved stability and might avail the chiral recognition of amino acids as the possible action mode of Fig. 3. To our delight, the fluorescent response of probe (S)-6 toward amino acids only needs 30 min at room temperature to reach the maximum and remain stable for at least 5.5 h, which is time-saving and conducive to operability and repeatability for chiral fluorescence analysis.
|
Download:
|
| Fig. 3. Structure of (S)-6 and possible action mode of chiral recognition. | |
The fluorescent probe (S)-6 were synthesized via a concise and high-efficiency route as Scheme 1. The intermediate (S)-8 was readily prepared via the sulfonation of the starting material (S)-7 using 1,3-propanesulfonic lactone as the sulfonating reagent, which was milder and more atom-economic than the method based on concentrated sulfuric acid in literature [23]. Then (S)-8 was deprotected to remove methoxymethoxy (MOM) group in the presence of HCl/H2O/CHCl3, followed by alkalization with aqueous NaHCO3 to obtain the target probe (S)-6.
|
Download:
|
| Scheme 1. Synthesis of (S)-6. | |
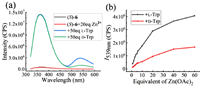
The synthesized probe (S)-6 exhibited excellent water solubility. Aqueous solutions of (S)-6 with or without Zn(Ⅱ) both showed no fluorescence response. However, after the addition of L- or D-tryptophan (Trp) in the presence of Zn(Ⅱ), the solutions showed enantioselective fluorescent enhancement at 539 nm, as shown in Fig 4a. The influence of Zn(Ⅱ) on the enantioselectivity of the probe toward amino acid was studied. Fig. 4b displayed that the fluorescent intensity at 539 nm of the probe solutions contained L-Trp or D-Trp strengthened obviously with the increase of Zn(Ⅱ) among 1–20 equiv. It also can be found that the solutions containing L-Trp presented more obvious strengthening trend than that containing D-Trp. It demonstrated that the complexation of Zn(Ⅱ) is conducive to improving the enantioselectivity of the probe toward amino acids.
|
Download:
|
| Fig. 4. (a) Fluorescence spectra of (S)-6 (33 µmol/L in H2O, 1 equiv.) with D- and L-Trp (in pH 8.8 BICINE buffer, 50 equiv.) in the presence of Zn(OAc)2 (in H2O, 20 eq); (b) Fluorescent intensities toward D- and L-Trp at 539 nm versus the equivalent of Zn(OAc)2. The above mixtures firstly standed at r.t. for 2 h at the conditions of 0.2 mmol/L (S)-6, then were diluted to 33 µmol/L, and finally standed for 30 min before the fluorescence test. λexc = 305 nm, slit = 4/4 nm. | |
It is well known that fluorescence has a self-quenching phenomenon at high concentrations, therefore the fluorescence measurement is usually carried out at micromolar or nanomolar concentrations. However, good reactivity between the fluorescent probe and chiral substrates is liable to happen at high concentrations. Therefore, in our previous studies [17,19], the procedure of diluting the reaction mixtures at micromolar or nanomolar concentrations was necessary. To our delight, the probe (S)-6 displays good reactivity toward Trp at micromolar concentrations which is suitable for the fluorescence test. In Fig. 4 and Fig. S1 (Supporting information), we compared the results of the two different methods, (a) the mixtures with 0.2 mmol/L probe firstly reacted with the amino acid for 2 h, then were diluted to 33 µmol/L and standed for 30 min before the fluorescence test (Fig. 4), (b) the mixtures with 33 µmol/L probe stand at room temperature for 30 min directly followed by the fluorescence test without dilution (Fig. S1 in Supporting information). The results exhibited that the fluorescent responses were very similar between the two methods. Obviously, the latter method is more efficient.
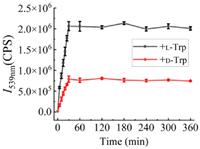
In addition, the fluorescent stability was also studied at this low concentration of 33 µmol/L (S)-6 in 3 mL aqueous solution with 50 equiv. amino acids and 20 equiv. Zn(OAc)2. We monitored the fluoresence intensity at 539 nm versus the mixing time range from 0 to 6 h. As shown in Fig. 5, in the initial 30 min, the fluorescence of (S)-6 is greatly enhanced toward both L-Trp and D-Trp, and reached the maximum after 30 min, then tends to be stable in the following 5.5 h. Rapid fluorescence response and continuous stability are conducive to improving the timeliness and repeatability of fluorescence chiral recognition.
|
Download:
|
| Fig. 5. Fluorescence intensity at 539 nm versus the mixing time range from 0 to 6 h. The mixtures contain 33 µmol/L (S)-6 (in H2O, 1 equiv.), L-Trp or D-Trp (in pH 8.8 BICINE buffer, 50 equiv.), and Zn(OAc)2 (in H2O, 20 equiv.). The error bars were obtained from three independent experiments. λexc = 305 nm, slit = 4/4 nm. | |
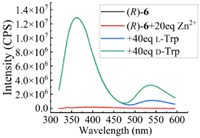
The enantiomer (R)-6 of (S)-6 was prepared from (R)-7 according to the same route (Scheme 1). The fluorescence response of (R)-6 toward D- and L-Trp was studied under the same condition, as shown in Fig. 6. As same as the spectra of (S)-6 + Zn(Ⅱ) toward chiral Trp, fluorescence intensity at 539 nm presented enantioselective enhancement at the addition of enantiomer of Trp, but relative intensity toward L-Trp and D-Trp just reversed. By comparing Fig. 6 with Fig. 4a, the mirror relationship of fluorescence response of (R)-6 and (S)-6 toward D- and L-Trp could be found, which confirmed the enantioselective recognition of chiral Trp.
|
Download:
|
| Fig. 6. Fluorescence spectra of (R)-6 (33 µmol/L in H2O, 1 equiv.) with D- and L-Trp (in pH 8.8 BICINE buffer, 40 equiv.) in the presence of Zn(OAc)2 (in H2O, 20 equiv.). λexc = 305 nm, slit = 4/4 nm. | |
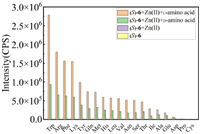
The fluorescence response of (S)-6 toward other 18 common amino acids was investigated. As shown in Fig. S2 (Supporting information), under the same conditions as those for tryptophan, except for proline, aspartic acid and cysteine, obvious enantioselective enhancement was found for the other 15 amino acids, such as arginine, serine, valine, asparagine, lysine, phenylalanine, glutamine, leucine, methionine, histidine, threonine, alanine, glutamine acid, tyrosine and isoleucine. Relative fluorescence intensity at 550 nm of (S)-6, (S)-6 + Zn(Ⅱ), (S)-6 + Zn(Ⅱ) + L-/D-amino acid was shown in Fig. 7. For the above 15 amino acids, fluorescence intensity toward L-enantiomers was stronger than that of the corresponding D-enantiomers. This phenomenon indicates that the probe has universal enantioselectivity for amino acids in aqueous solutions and can be widely used for chiral recognition of various amino acids.
|
Download:
|
| Fig. 7. Fluorescence response at 550 nm for the mixtures of (S)-6 (33 µmol/L in H2O, 1 equiv.) + Zn2+ (in H2O, 20 equiv.) with 19 pairs of D-/L-amino acids, including Trp (in pH 8.8 BICINE buffer, 40 equiv.). λexc = 305 nm, slit = 4/4 nm. | |
Concentration and optical purity are two important parameters of chiral substances. Therefore, we explore the dependence of these two factors on the fluorescence intensity of the probe. The equivalent of Trp to (S)-6 and the enantiomeric excess value of D-Trp (ee = [D − L]/[D + L]) are used to describe concentration and optical purity, respectively. Fig. 8 shows the dependence of the equivalent of Trp on fluorescence intensity at 360 nm and 539 nm, respectively. The fluorescence spectra were displayed in Fig. S3 (Supporting information). The fluorescence emission peak at 360 nm is assigned to Trp. In the range of 0–20 eq, the linearity between its intensity at 360 nm and the concentration of Trp is maintained without being interfered by the interaction with the probe and Zn(Ⅱ). The linear equation of I360nm versus the equivalent of Trp to (S)-6 is y = 1.89200 × 105 + 2.28271 × 105 x (Eq. Ⅰ). The peak at 539 nm shows enantioselective enhancement within the studied equivalent range, and there is a good linear relationship between its intensity and the amount of Trp in the range of 0–20 equiv. The linear equations are y = 2.90647 × 105 + 1.63419 × 105 x (Eq. Ⅱ, I539nm versus equivalent of L-Trp), and y = 8.78935 × 104 + 5.38397 × 104 x (Eq. Ⅲ, I539nm versus equivalent of D-Trp).

|
Download:
|
| Fig. 8. Fluorescence intensity at (a) 360 nm (I360nm) and (b) 539 nm (I539nm) versus equivalent of D- and L-Trp (in pH 8.8 BICINE buffer). All the mixtures contain 33 µmol/L (S)-6 (in H2O, 1 equiv.) and Zn(OAc)2 (in H2O, 20 equiv.). Error bars are from three independent experiments. λexc = 305 nm, slit = 4/4 nm. | |
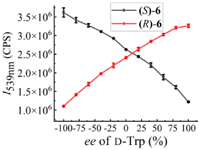
Fig. 9 shows the fluorescence response of each enantiomeric probe versus ee value of D-Trp. The mirror-image relation between the fluorescence responses of this enantiomeric probe pair was observed, and both present linear relationships. The linear equations are y = 2.35833 × 106 + 1.15695 × 104 x [Eq. Ⅳ, I539nm of (R)-6 versus ee value] and y = 2.62021 × 106 − 1.30391 × 104 x [Eq. Ⅴ, I539nm of (S)-6 versus ee value]. These plots and linear equations can be applied to determine the optical purities of the amino acid.
|
Download:
|
| Fig. 9. Fluorescence intensity of (S)-6 and (R)-6 (33 µmol/L in H2O, 1 equiv.) at 539 nm versus the ee value of D-Trp (in pH 8.8 BICINE buffer, 40 equiv.) in the presence of Zn(OAc)2 (in H2O, 20 equiv.). Error bars are from three independent experiments. λexc = 305 nm, slit = 4/4 nm. | |
We have applied equations Ⅰ and Ⅳ to analyze the concentration and enantiomeric composition of Trp, respectively. As the results summarized in Tables 1 and 2, the measured equivalent of Trp to (S)-6 and ee values of D-Trp had good consistency with the actual data. The maximum absolute errors of equivalent and ee value are 0.7 and 13.2%, respectively.
|
|
Table 1 Concentration determination of Trp samples through the fluorescent probe (S)-6. |
|
|
Table 2 Enantiomeric excess measurement of Trp samples through the fluorescent probe (R)-6. |
Chiral recognition mechanism of the probe (S)-6 to Trp was investigated by 1H nuclear magic resonance (1H NMR). 1H NMR results (Fig. S4 in Supporting information) of the 4 samples: (S)-6, (S)-6 + Zn(OAc)2, (S)-6 + Zn(OAc)2 with L-Trp, (S)-6 + Zn(OAc)2 with D-Trp, displayed that (S)-6 gave two singlets with one at δ 10.17 for its aldehyde protons and another at δ 8.63 for the naphthyl proton ortho to the aldehyde groups. When Zn(OAc)2 was added, no obvious change was found for these two singlets, while addition of L-Trp or D-Trp brought about the disappearance of the singlet at δ 10.17, which can be speculated that aldehyde group participates the reaction with Trp.
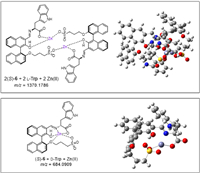
In order to further explore the mechanism of the fluorescent enantioselective enhancement of probe (S)-6 toward Trp, high resolution mass spectrometry (HR-MS) of the mixtures of (S)-6 and D- or L-enantiomer of Trp in the presence of Zn(OAc)2 were studied. Figs. S5a and b (Supporting information) are from the mixture of (S)-6, L-Trp and Zn(OAc)2, and Figs. S5c and d (Supporting information) from another mixture of (S)-6, D-Trp and Zn(OAc)2. Figs. S5a and c (Supporting information) are both showed the molecular ion peak (m/z = 436.0981) of the protonated (S)-6, that can be from the incompletely reacted probe. Meanwhile, we also found several newly produced molecular ion peaks at m/z = 1370.1786 showed in Fig. S5b (Supporting information), which could be ascribed to the complex of 2 (S)-6 + 2 L-Trp + 2 Zn(Ⅱ), and m/z = 684.0909 showed in Fig. S5d (Supporting information), which might belong to the complex of (S)-6 + D-Trp + Zn(Ⅱ). Fig. 10 gives the molecular structures of these two deduced complexes and the corresponding modeling structures based on density functional theory (DFT) calculations by Gaussian 09 program. The computational results of the two pairs of complexes, 2 (S)-6 + 2 L-Trp + 2 Zn(Ⅱ) & 2 (S)-6 + 2 D-Trp + 2 Zn(Ⅱ), (S)-6 + D-Trp + Zn(Ⅱ) & (S)-6 + L-Trp + Zn(Ⅱ), are shown in Figs. S6 and S7 (Supporting information). The energy of 2 (S)-6 + 2 L-Trp + 2 Zn(Ⅱ) is 11.2 kcal/mol lower than 2 (S)-6 + 2 D-Trp + 2 Zn(Ⅱ), and the energy of (S)-6 + D-Trp + Zn(Ⅱ) is 3.4 kcal/mol lower than (S)-6 + L-Trp + Zn(Ⅱ). We thus propose that when the probe (S)-6 is treated with the enantiomeric amino acid in the presence of Zn(Ⅱ) in aqueous solution, different structures of products and different stability of the Zn(Ⅱ) complexes formed from the D- and L-enantiomers of the amino acid, bring about the enantioselective fluorescent response.
|
Download:
|
| Fig. 10. Deduced and modeling structures of the products from mixtures of (S)-6 and enantiomer of Trp in the presence of Zn(OAc)2. | |
In conclusion, we designed a water-soluble BINOL-based fluorescence probe (S)-6 featuring a sodium sulfonate fragment at the 2′-position, which could strength the stability of Zn complex. (S)-6 is readily available via simple synthetic procedures under mild reaction conditions, which facilitate its application in enantioselective recognition of amino acids in water. The probe (S)-6, at micromolar concentrations in aqueous solution, displays excellent enantioselectivity toward 15 common amino acids. (S)-6 and (R)-6 both can be used for enantiomeric excess determination of amino acids. The fluorescence response of (S)-6 toward amino acids reaches the maximum after standing for 30 min at room temperature, and remains stable in the following 5.5 h. The timeliness and outstanding fluorescent stability endow the (S)-6 with great potential in the application of chiral fluorescence analysis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe financial support from the National Natural Science Foundation of China (Nos. 21877087, 22074114), Natural Science Foundation of Hubei Province of China (Nos. 2020CFB623, 2021CFB556), Key Laboratory for Green Chemical Process of Ministry of Education (No. GCP20200201), Hubei Key Laboratory of Novel Reactor and Green Chemical Technology (No. 40201002), Wuhan Institute of Technology Graduate Education and Teaching Reform Research Project (Nos. 2022JYXM09, 2021JYXM07) and Wuhan Institute of Technology Graduate Innovation Fund (Nos. CX2022450, CX2022009, CX2022058) are greatly appreciated.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108345.
| [1] |
Y.P. Xue, C.H. Cao, Y.G. Zheng, Chem. Soc. Rev. 47 (2018) 1516-1561. DOI:10.1039/c7cs00253j |
| [2] |
M. Ke, Z. Liu, K. Zhang, et al., Green Synth. Catal. 2 (2021) 228-232. DOI:10.1016/j.gresc.2021.04.004 |
| [3] |
Y. Zhou, H. Chen, P. Lei, et al., Chin. Chem. Lett. 33 (2022) 4850-4855. DOI:10.1016/j.cclet.2022.02.029 |
| [4] |
J. Yang, L. Jiang, J. Tian, et al., Org. Lett. 24 (2022) 9327-9331. DOI:10.1021/acs.orglett.2c03962 |
| [5] |
L. Pu, Chem. Commun. 58 (2022) 8038-8048. DOI:10.1039/d2cc02363f |
| [6] |
R. Ge, Y. Zhu, H. Wang, et al., Chin. J. Org. Chem. 42 (2022) 424-433. DOI:10.6023/cjoc202108047 |
| [7] |
S.X. Gu, H.F. Wang, Y.Y. Zhu, et al., Pharm. Fronts 2 (2020) e79-e87. DOI:10.1055/s-0040-1713820 |
| [8] |
L. Dreisewerd, R.L.E.G. Aspers, M.C. Feiters, et al., J. Am. Chem. Soc. 145 (2023) 1518-1523. DOI:10.1021/jacs.2c11285 |
| [9] |
W. Li, X. Zhang, S. Chen, et al., Chin. Chem. Lett. 33 (2022) 4405-4410. DOI:10.1016/j.cclet.2021.12.026 |
| [10] |
Q.Q. Wang, R. Hu, Z.Q. Fang, et al., Chin. Chem. Lett. 33 (2022) 3782-3786. DOI:10.1016/j.cclet.2021.11.012 |
| [11] |
B.T. Herrera, S.L. Pilicer, E.V. Anslyn, et al., J. Am. Chem. Soc. 140 (2018) 10385-10401. DOI:10.1021/jacs.8b06607 |
| [12] |
L. Pu, Angew. Chem. Int. Ed. 59 (2020) 21814-21828. DOI:10.1002/anie.202003969 |
| [13] |
Z. Huang, S. Yu, K. Wen, et al., Chem. Sci. 5 (2014) 3457-3462. DOI:10.1039/C4SC00818A |
| [14] |
F. Zhao, Y. Du, J. Tian, et al., Eur. J. Org. Chem. 16 (2018) 1891-1895. DOI:10.1002/ejoc.201701773 |
| [15] |
Y.C. Wang, L.L. Hu, F. Zhao, et al., Chem. Eur. J. 23 (2017) 17678-17681. DOI:10.1002/chem.201704640 |
| [16] |
L. Hu, S. Yu, Y. Wang, et al., Org. Lett. 19 (2017) 3779-3782. DOI:10.1021/acs.orglett.7b01645 |
| [17] |
Y.Y. Zhu, X.D. Wu, M. Abed, et al., Chem. Eur. J. 25 (2019) 7866-7873. DOI:10.1002/chem.201900880 |
| [18] |
Y. Jiang, J. Tian, H. Guo, et al., J. Org. Chem. 88 (2023) 211-217. DOI:10.1021/acs.joc.2c02152 |
| [19] |
Y.Y. Zhu, X.D. Wu, S.X. Gu, et al., J. Am. Chem. Soc. 141 (2019) 175-181. DOI:10.1021/jacs.8b07803 |
| [20] |
G. Du, L. Pu, Org. Lett. 21 (2019) 4777-4781. DOI:10.1021/acs.orglett.9b01661 |
| [21] |
G. Du, Y. Mao, M.A. Abed, et al., Mater. Chem. Front. 4 (2020) 2384-2388. DOI:10.1039/d0qm00260g |
| [22] |
L. Yang, S. Qin, X. Su, et al., Org. Biolmol. Chem. 8 (2010) 339-348. DOI:10.1039/B908540H |
| [23] |
F. Zhao, Y. Wang, X. Wu, et al., Chem. Eur. J. 26 (2020) 7258-7262. DOI:10.1002/chem.202000423 |
 2023, Vol. 34
2023, Vol. 34