Hydrogen peroxide (H2O2) is one of the most valuable multifunctional chemicals with widespread applications. Currently, the well-established industrial anthraquinone-based method for H2O2 production is energy-intensive and environmentally unfriendly [1]. In addition to eco-friendly and economical electrochemical methods [2], artificial photosynthetic H2O2 generation is also considered as a clean and energy-saving approach that has attracted significant interest. Substantial efforts have been devoted to developing new photocatalysts with high efficiency, good selectivity, and stability [3]. Visible-light-responsive organic polymers [4], such as polymeric carbon nitrides, have been widely investigated as potential catalysts for H2O2 photosynthesis because of their high physical and chemical stability, low cost, and adjustable electronic bandgaps.
In 2014, Shiraishi et al. first reported that graphitic carbon nitride (g-C3N4) could produce H2O2 under photoirradiation using alcohol as an electronic donor [5]. Inspired by this seminal work, numerous polymeric carbon nitride-based photocatalysts have emerged in recent years for the photocatalytic production of H2O2, especially for solar-driven H2O2 production without any sacrificial agents [6]. However, most carbon nitride materials cannot produce effective holes or form active surface sites to directly oxidize H2O into H2O2, and tend to undergo the thermodynamically favorable 4e− WOR process [7]. Molecular engineering of carbon nitrides, including heteroatom doping (e.g., P or Sb) [8], modifying heteromolecules (e.g., pyromellitic diimide or biphenyl diimide) [9], surface defect engineering (e.g., C≡N groups and N vacancies) [10], and modulating triazine/heptazine based frameworks (e.g., by spatially separated redox centers) [11,12], plays a key role in the formation of architectures with great topologies [13]. Indeed, some substantial significant advances have been made to improve the non-sacrificial ORR and WOR efficiency for H2O2 photosynthesis; however, the efficiency and stability of current state-of-the-art photocatalysts are still low.
Each half-reaction includes different reaction pathways determined by the electron transfer number (Fig. 1a) [14-16]. For ORR half-reaction, the direct two-electron process exhibits a thermodynamically more favorable energy level (+0.695 V vs. NHE) and the indirect stepwise single-electron reduction firstly reduce O2 to form hydroperoxyl radicals (·OOH, −0.05 V vs. NHE) which then couple with protons to generate H2O2 (+1.44 V vs. NHE). For the WOR half-reaction, H2O can be directly oxidized by two holes to form H2O2 (+1.76 V vs. NHE), and the stepwise single-electron WOR process requires high concentrations of hydroxyl radicals (·OH, +2.73 V vs. NHE) to form the desired H2O2. Theoretically, based on the combination of different ORR and WOR pathways, there are four different pathways for the overall photosynthesis of H2O2: 2e− ORR and 4e− WOR pathways [8], stepwise 1e− ORR and 4e− WOR pathways [10], stepwise 1e− ORR and 1e− WOR pathways [17], and 2e− ORR and 2e− WOR pathways [11,12,18,19], which provide potent guidance for the design of photocatalytic processes (Fig. 1b) [14].

|
Download:
|
| Fig. 1. (a) Reaction scheme for photocatalytic H2O2 production and the corresponding energy diagrams for the ORR and WOR pathways. (b) Four classical combinations of ORR and WOR pathways for the overall photosynthesis of H2O2. Reprint with permission [14,15]. Copyright 2022, ACS. | |
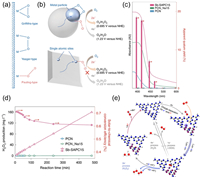
The creation of heteroatom-doped photocatalysts is an essential strategy in the pursuit of advanced photocatalytic processes. In an elegant study, Ohno et al. reported a Sb single-atom carbon nitride photocatalyst (Sb-SAPC) for non-sacrificial photocatalytic H2O2 synthesis under ambient conditions [8]. The introduction of highly active and selective Sb sites for the 2e− ORR to consume the O2 generated from 4e− WOR in the photocatalytic system offers a high yield and selectivity for H2O2 photosynthesis (Figs. 2a and b), with an apparent quantum yield of 17.6% at 420 nm (Fig. 2c), together with a solar-to-chemical conversion (SCC) efficiency of 0.61% (Fig. 2d) for H2O2 synthesis. The d10 electronic configuration of Sb single-atom sites produces a new O—O stretching mode in the Sb—OOH species with an end-on adsorption configuration appearing under photo-irradiation, which reduces the possibility of O—O bond breaking and enhances the trapping of photogenerated electrons for the 2e− ORR. Moreover, these sites are energetically unfavorable for the side hydrogen evolution reactions. In contrast, the photogenerated holes were concentrated at the N atoms in the s-heptazine moiety next to the Sb site to catalyze the 4e− WOR. Both the 2e− ORR and 4e− WOR pathways formed a prime overall photosynthesis of H2O2 (Fig. 2e). Further examples of Ga- and Co-doped carbon nitrides also proved that the single atoms in carbon nitride certainly tailor the electronic structure and induce charge redistribution. It was supposed to not only promote the separation/transfer of charge carriers and the absorption/activation of O2 in the 2e− ORR reaction but also effectively enhance the 2e−/4e− WOR reaction [19,20].
|
Download:
|
| Fig. 2. (a) Schematic structures of O2 adsorption on metal surface. (b) ORR mechanism on a metal particle (top) and isolated atomic site (bottom). (c) Action spectra of PCN, PCN_Na15, and Sb-SAPC15 towards H2O2 production in phosphate buffer solution. (d) Solar-to-chemical conversion efficiencies of PCN, PCN_Na15, and Sb-SAPC15 under AM 1.5, illumination in a phosphate buffer solution. (e) Reaction mechanism of CN with Sb sites for photocatalytic H2O2 production. The white, gray, blue, red and magenta spheres refer to hydrogen, carbon, nitrogen, oxygen and Sb atoms, respectively. Reprint with permission [8]. Copyright 2021, Nature. | |
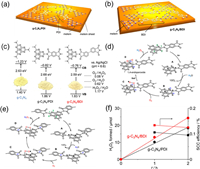
In addition to heteroatom doping, incorporating heteromolecules, such as aromatic diimides, an important class of n-type organic semiconductors with high electron mobility and stability, also positively adjusts the electronic structure and shifts the oxidation and reduction potentials, owing to their high electron affinity [9]. It has been demonstrated that the VB position of g-C3N4 is not thermodynamically feasible for the concurrent WOR. The introduction of pyromellitic diimide (PDI, Fig. 3a) and biphenyl diimide (BDI, Fig. 3b) into the g-C3N4 structure can generally positively shift the CB and VB positions and improve the thermodynamic driving force for catalyzing the O2 evolution reaction (Fig. 3c). Figs. 3d and e show the reaction pathways for the overall photosynthesis of H2O2 via the coupling of the 2e− ORR pathway with the 4e− WOR pathway on g-C3N4/PDI and g-C3N4/BDI. Accordingly, g-C3N4/PDI and g-C3N4/BDI exhibited considerable H2O2 production rates without sacrificial agents in a pure water system, and the average SCC efficiencies of the optimized g-C3N4/PDI and g-C3N4/BDI reached 0.10% and 0.13%, respectively (Fig. 3f).
|
Download:
|
| Fig. 3. (a) Molecular structure of g-C3N4/PDI. (b) Molecular structure of g-C3N4/BDI. (c) Electronic band structures of g-C3N4, g-C3N4/PDI, and g-C3N4/BDI. (d) Mechanism of g-C3N4/PDI for photocatalytic H2O2 production in pure water. (e) Mechanism of g-C3N4/BDI for photocatalytic H2O2 production in pure water system. (f) Change in the amount of H2O2 formed and SCC efficiency under simulated AM1.5 G sunlight (1 sun) with time. Reprint with permission [9]. Copyright 2016, ACS. | |
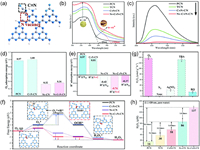
Furthermore, engineering defects on a photocatalyst surface is a universal and powerful method for improving the photocatalytic conversion efficiency. Recently, Zheng et al. sequentially introduced the –C≡N groups and N vacancies into g-C3N4 (Nv—C≡N—CN, Fig. 4a) [10]. The synergistic effect of the dual defect sites enhanced the visible-light absorption (Fig. 4b) and carrier separation capabilities (Fig. 4c). Besides, it has been verified that N vacancies can effectively adsorb and activate O2 (Fig. 4d), while the C≡N groups enhance the adsorption of H+ (Fig. 4e), which synergistically promotes the formation and hydrogenation of OOH* to H2O2 (Fig. 4f). Accordingly, the Nv—C≡N—CN group achieved overall photosynthesis of H2O2 in pure water via concurrent stepwise single-electron ORR and four-electron WOR pathways (Fig. 4g). Meanwhile, the Nv—C≡N—CN photocatalyst exhibited 8.5 times H2O2 production rates higher than pristine bulk carbon nitrides without sacrificial agents, leading to a significant SCC efficiency of 0.23% (Fig. 4h).
|
Download:
|
| Fig. 4. (a) Defect structure in g-C3N4 heptazine units. Inset: C≡N groups and N vacancies. (b) UV–Vis DRS of all samples. Inset: Photographs of PCN and Nv—C≡N—CN. (c) Photoluminescence (PL) spectra of all samples. (d) O2 adsorption energy and (e) H+ adsorption energy on g-C3N4 with different defect models. (f) Free energy profiles for photocatalytic H2O2 evolution reactions over PCN, C≡N—CN, Nv—CN, and Nv—C≡N—CN. (g) Photocatalytic H2O2 generation rates of Nv—C≡N—CN under different reaction gasses or sacrificial agents. (h) Comparison of photocatalytic H2O2 generation activity of different samples under the same conditions in a pure water system. Reprint with permission [10]. Copyright 2022, RSC. | |
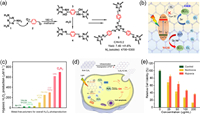
Expect for the above-mentioned engineering of g-C3N4, the modulation of triazine/heptazine-based frameworks has great potential for the photocatalytic synthesis of H2O2. Rationally designed monomers for pre-assembly and precise regioselective coupling polymerization play key roles in the formation of architectures with separated redox centers. Very recently, Zhang et al. modulated a triazine-based framework with spatially separated redox centers and reported well-defined C5N2 with a conjugated C═N linkage to photosynthesize H2O2 from H2O without sacrificial agents under hypoxic conditions (Fig. 5a) [12]. Compared with the tertiary N linkage in g-C3N4 breaking the conjugation over the heptazine rings, the conjugated linkers were redistributed in the C5N2 units, resulting in the strengthened delocalization of π-electrons, which downshifted the CB and VB positions. C5N2 with unusually low conduction ( > 0 V vs. NHE) and valence band positions promoted realistic selectivity in thermodynamics and high activity in kinetics for overall H2O2 photosynthesis through synergistic 2e− ORR and 2/4e− WOR (Fig. 5b), and C5N2 demonstrated the highest H2O2 photocatalytic activity (698 µmol L−1 h−1) under hypoxic conditions among the polymeric photocatalysts (Fig. 5c). For the first time, C5N2 was successfully applied to hypoxic/normoxic tumor photodynamic therapy/chemodynamic therapy (PDT/CDT) (Fig. 5d) and exhibited competitive performance under hypoxic and normoxic conditions (Fig. 5e). These results indicate that C5N2 offers an opportunity to potentially cut the cost of H2O2 production in a large scale and opens new avenues for highly efficient and selective H2O2 photosynthesis, particularly under hypoxic conditions in biomedical therapy systems.
|
Download:
|
| Fig. 5. (a) Conjugated linker formation in the synthesis of C5N2 (5) from melamine (1) and p-phthalaldehyde (2) via Schiff base reactions. (b) Bandgap position of C5N2 and mechanism of photocatalytic H2O2 production by C5N2. Inset: C═N linkage in C5N2. (c) Summary of the photocatalytic activity for H2O2 production in a hypoxic microenvironment using different metal-free polymers in the literature. (d) Therapeutic processes using C5N2 to generate ·OH via the Fenton reaction under light irradiation in hypoxic environments. (e) Cell viability assay of C5N2−Fe−NS-treated 4T1 cells under light irradiation in hypoxic and normoxic environments. Reprint with permission [12]. Copyright 2022, Wiley. | |
Although previous studies have been devoted to enhancing the SCC efficiency toward solar-driven H2O2 production via the design of polymer photocatalysts, the efficiency of non-sacrificial H2O2 generation has not yet fulfilled the industrial requirements. Thus, the technical and economic feasibility of H2O2 photosynthesis should be rigorously analyzed. Apart from the wide use of H2O2 in the energy field owing to its high energy density, its strong oxidation power, and endogenous nature may achieve a potential application in environmental and biomedical fields, such as coupling solar-driven H2O2 production with dye degradation [18], conversion of organic molecules [21], bacterial disinfection [22], and cancer therapy [12], which could potentially lower the cost of large-scale production of H2O2.
In summary, we highlight the significant advances in H2O2 photosynthesis in the molecular engineering of carbon nitrides, a deep understanding of the photocatalysis process for the rational design and reaction pathways of carbon nitrides, and the technical and economical emerging applications of classical examples to address the growing H2O2 demand. The high energy density, strong oxidation power, and endogenous nature of H2O2 make it potentially applicable in energy, environmental, and biomedical fields by coupling solar-powered technologies for H2O2 production with carbon nitride. Furthermore, other potential catalysts, such as organic conjugated polymer resorcinol formaldehyde resins with a low bandgap, also exhibit outstanding efficiency without sacrificial reagents for the overall photosynthesis of H2O2 [3,23].
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis research was supported by the National Natural Science Foundation of China (Nos. 22174014 and 22074015).
| [1] |
C. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Science 366 (2019) 226-231. DOI:10.1126/science.aay1844 |
| [2] |
X. Hu, Z. Sun, G. Mei, et al., Adv. Energy Mater. 12 (2022) 2201466. DOI:10.1002/aenm.202201466 |
| [3] |
Y. Shiraishi, T. Takii, T. Hagi, et al., Nat. Mater. 18 (2019) 985-993. DOI:10.1038/s41563-019-0398-0 |
| [4] |
H. Yang, Z. Wang, S. Liu, Y. Shen, Y. Zhang, Chin. Chem. Lett. 31 (2020) 3047-3054. DOI:10.1016/j.cclet.2020.07.048 |
| [5] |
Y. Shiraishi, S. Kanazawa, Y. Kofuji, et al., Angew. Chem. Int. Ed. 53 (2014) 13454-13459. DOI:10.1002/anie.201407938 |
| [6] |
Y. Kofuji, Y. Isobe, Y. Shiraishi, et al., J. Am. Chem. Soc. 138 (2016) 10019-10025. DOI:10.1021/jacs.6b05806 |
| [7] |
H. Zhao, Q. Jin, M.A. Khan, et al., Chem. Catal. 2 (2022) 1720-1733. DOI:10.1016/j.checat.2022.04.015 |
| [8] |
Z. Teng, Q. Zhang, H. Yang, et al., Nat. Catal. 4 (2021) 374-384. DOI:10.1038/s41929-021-00605-1 |
| [9] |
Y. Kofuji, S. Ohkita, Y. Shiraishi, et al., ACS Catal. 6 (2016) 7021-7029. DOI:10.1021/acscatal.6b02367 |
| [10] |
X. Zhang, P. Ma, C. Wang, et al., Energy Environ. Sci. 15 (2022) 830-842. DOI:10.1039/D1EE02369A |
| [11] |
H. Cheng, H. Lv, J. Cheng, et al., Adv. Mater. 34 (2022) e2107480. DOI:10.1002/adma.202107480 |
| [12] |
J. Ma, X. Peng, Z. Zhou, et al., Angew. Chem. Int. Ed. 61 (2022) e202210856. DOI:10.1002/anie.202210856 |
| [13] |
H. Hou, X. Zeng, X. Zhang, Angew. Chem. Int. Ed. 59 (2020) 17356-17376. DOI:10.1002/anie.201911609 |
| [14] |
H. Cheng, J. Cheng, L. Wang, H. Xu, Chem. Mater. 34 (2022) 4259-4273. DOI:10.1021/acs.chemmater.2c00936 |
| [15] |
J.J. Gao, B. Liu, ACS Mater. Lett. 2 (2020) 1008-1024. DOI:10.1021/acsmaterialslett.0c00189 |
| [16] |
Y. Sun, L. Han, P. Strasser, Chem. Soc. Rev. 49 (2020) 6605-6631. DOI:10.1039/D0CS00458H |
| [17] |
Y. Liu, X. Zeng, X. Hu, et al., Catal. Today 335 (2019) 243-251. DOI:10.1016/j.cattod.2018.11.053 |
| [18] |
M. Kou, Y. Wang, Y. Xu, et al., Angew. Chem. Int. Ed. 61 (2022) 1521-3773. |
| [19] |
H. Tan, P. Zhou, M. Liu, et al., Nat. Synth. 2 (2023) 557-563. DOI:10.1038/s44160-023-00272-z |
| [20] |
W. Wang, Q. Song, Q. Luo, et al., Nat. Commun. 14 (2023) 2493. DOI:10.1038/s41467-023-38211-3 |
| [21] |
Y. Zhang, C. Pan, G. Bian, et al., Nat. Energy 8 (2023) 361-371. DOI:10.1038/s41560-023-01218-7 |
| [22] |
W. Wang, H. Xie, G. Li, et al., ACS ES&T Water 1 (2021) 1483-1494. |
| [23] |
C. Zhao, X. Wang, Y. Yin, et al., Angew. Chem. Int. Ed. 62 (2023) e202218318. DOI:10.1002/anie.202218318 |
 2023, Vol. 34
2023, Vol. 34 

