Tumor angiogenesis has a critical influence on the occurrence and development of tumors. Anti-tumor angiogenesis therapy, a kind of targeted therapy, targets endothelial cells (ECs) and inhibits their growth and migration, tumor blood vessel formation, and the supply of tumor nutrition to inhibit tumor growth and metastasis. Tumor angiogenesis involves vascular endothelial growth factor (VEGF) [1,2], hypoxia-inducible factor (HIF) [3], cytokines and cell growth factors [4], platelet-derived growth factor (PDGF) [5], and other factors. Currently, several representative angiogenesis inhibitors have been adopted for clinical application. For example, Bevacizumab [6,7], a VEGF inhibitor, can eliminate angiogenesis-promoting factors, inhibit the formation and metastasis of ECs, and slow down the degradation of the basement membrane. Furthermore, small molecule receptor tyrosine kinase inhibitors (TKI), such as Sorafenib [8] and Sunitinib [9] are used to compete for the adenosine triphosphate binding sites of the intracellular receptor tyrosine kinase domain in order to inhibit tumor angiogenesis. In addition, PI3K/AKT/mTOR pathway inhibitors are also applied to target anti-angiogenesis, such as Everolimus [10]. Endogenous angiogenesis inhibitors have the ability to inhibit tumor vascular ECs by reducing VEGF activity, e.g., rh-endostatin/Endostar [11,12]. However, although the above angiogenesis inhibitors have shown good efficacy in tumor treatment, their clinical effects are limited, they lead to drug resistance, and they have inherent toxicity [13]. Therefore, it is necessary to develop tumor vascular inhibitors with high efficiency and low toxicity.
The trace element selenium (Se) is essential to the health of organisms. It has important biological functions. For instance, it is the active site of a variety of enzymes to maintain the balance of redox reactions in the bodies of organisms [14]. Seleno-compounds are mainly divided into three speciation: inorganic Se, organic Se, and Se nanoparticles (SeNPs). A number of studies have confirmed that seleno-compounds have important anti-tumor [15,16], antibacterial [17,18], anti-inflammatory [19], anti-oxidation effects [20] and enhance immune function [21,22]. SeNPs have attracted much attention in the field of anti-tumor studies due to their low toxicity, rapid absorption, and biological activity [23]. Recent studies have demonstrated that SeNPs, as drug carriers or a therapeutic drug, exhibit good biocompatibility and have high potential for treating cancer [24-28]. The inhibition of angiogenesis may be one of the mechanisms by which seleno-compounds inhibit tumor growth [29]. The current research status of the anti-angiogenic effect and mechanism of organic Se, inorganic Se and SeNPs is as follows, methylseleninic acid can inhibit angiogenesis, and their mechanism is closely related to cell cycle arrest, apoptosis, the hyperphosphorylation of p38MAPK, and the dephosphorylation of ERK1/2 and AKT [30,31]; selenadiazole derivatives (SeDs) inhibit VEGF-VEGFR2/ERK/AKT signaling by activating reactive oxygen species (ROS) to cause DNA damage [32]; SeNPs trigger ROS-mediated DNA damage to inhibit the VEGF-VEGFR2-ERK/AKT signaling axis and induce apoptosis and cell cycle arrest in human umbilical vein endothelial cells (HUVECs) to inhibit angiogenesis [33]. However, the influence of different Se speciation on anti-angiogenesis and its mechanisms has not been reported.
In our study, we investigated the different anti-angiogenesis activities of organic Se, inorganic Se and SeNPs (Fig. 1A). The results indicate that organic Se, especially SeD, can more significantly inhibit the proliferation, migration, invasion, and angiogenesis ability in HUVECs than inorganic Se or SeNPs. The inhibitory effect may be caused by inducing apoptosis and cell cycle arrest. In addition, we found that organic Se can inhibit thioredoxin reductase (TrxR) to trigger ROS overproduction in HUVECs, which disturb the normal redox balance in cells. Of all the Se speciation, the anti-tumor angiogenesis effect of organic Se is the most significant, which shows the influence of Se speciation on the anti-tumor angiogenesis effect and mechanism.
|
Download:
|
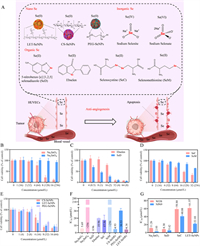
| Fig. 1. (A) The structure of seleno-compounds from different Se speciation. (B-E) Cytotoxicity of seleno-compounds from different Se speciation at different concentrations against HUVECs after 72 h of incubation. (F) The IC50 values of 9 seleno-compounds acting on HUVECs for 72 h. (G) The corresponding IC50 values of WI-38 cells and NP69 cells treated with Na2SeO3, SeD, SeC and LET-SeNPs for 72 h. | |
To evaluate the anti-angiogenic effects of different Se speciation in vitro, a MTT assay was conducted on nine seleno-compounds to test their cytotoxicity on HUVECs, including the inorganic seleno-compounds sodium selenite (Na2SeO3) and sodium selenate (Na2SeO4); the organic seleno-compounds 5-nitrobenzo[c][1,2,5]selenadiazole (SeD), Ebselen, selenocysteine (SeC), and selenomethionine (SeM), the SeC and SeM are belong to Se-containing amino acids; and the SeNPs Cs-SeNPs, LET-SeNPs, and PEG-SeNPs. As shown in Figs. 1B-E and Figs. S1A-D (Supporting information), after 72 h of treatment with different seleno-compounds, the survival rate of HUVECs treated with Na2SeO4 and SeM was more than 70%. This means that Na2SeO4 and SeM rarely inhibit cell proliferation. The other seven seleno-compounds could inhibit the proliferation of HUVECs to different degrees, and the inhibitory effect was more obvious with an increase in concentration. SeD had the most obvious inhibitory effect on cell proliferation. IC50 can reflect the toxic effect of seleno-compounds on cells, and the smaller the number the greater the toxicity. By measuring the cell survival rate after seleno-compounds treatment, we can calculate the corresponding IC50 of the seleno-compounds. As shown in Fig. 1F, the toxic effects of different seleno-compounds in the same speciation on HUVECs were different. Na2SeO3, SeD, SeC and LET-SeNPs showed more significant inhibitory effects on cell proliferation than other seleno-compounds in the same speciation.
In addition, we evaluated the effect of seleno-compounds treatment on the survival rate of WI-38 cells and NP69 cells via the MTT method and calculated the IC50 of the seleno-compounds. As shown in Fig. 1G and Table S1 (Supporting information), the toxicity of the four seleno-compounds to WI-38 cells and NP69 cells is very small, and the safety index (SI) is greater than 1, indicating that the safety of the seleno-compounds to normal cells is different, i.e., LET-SeNPs > SeD > SeC > Na2SeO3. To sum up, most seleno-compounds have anti-angiogenesis activity and no obvious side effects, and the inhibitory effects of different seleno-compounds of the same speciation are different. Therefore, Na2SeO3, SeD, SeC, and LET-SeNPs were selected for further investigation into their anti-angiogenesis effects and mechanisms of action on HUVECs.
In order to investigate the mechanism of the inhibitory activity of different seleno-compounds on the proliferation of HUVECs, we wondered whether it was due to the difference in cell absorption of different Se speciation. Therefore, we detected the absorption of different seleno-compounds by HUVECs at different time points. As shown in Figs. S2A-D (Supporting information), the absorption value of Na2SeO3, SeD, SeC and LET-SeNPs by HUVECs increased with an increase in time. The absorption value of SeD by HUVECs reached the maximum at 8 h, and the absorption value of the other three seleno-compounds reached the maximum at 12 h. Moreover, the absorption of Na2SeO3 by HUVECs was the strongest, and the intracellular Se content reached 452.7 ng/106 cells at 12 h. The absorption of LET-SeNPs was the weakest, and the intracellular Se content was maintained at about 6 ng/106 cells. Combined with the IC50 of the seleno-compounds, HUVECs have the weakest absorption of LET-SeNPs with strong toxicity and the strongest absorption of Na2SeO3 with relatively weak toxicity. Therefore, it can be seen that the difference in the inhibitory effect of different Se speciation on the proliferation of HUVECs may not be caused by affecting the absorption of Se by cells, and the specific reasons for this need to be further studied.
HUVECs migration, invasion, and capillary structure formation are indispensable steps in the process of neovascularization and have a key impact on tumor cell metastasis and invasion. Therefore, an in vitro model was used to study the migration, invasion, and formation of capillaries by HUVECs to verify the anti-angiogenic effects of different seleno-compounds. First of all, we used scratch tests to investigate the migration abilities of HUVECs based on different seleno-compounds. Before the experiment started, we screened the drug concentration by measuring the survival rate of HUVECs with a concentration of 2.5 × 105 cells/mL. As illustrated in Figs. S3A-D (Supporting information), the cell survival rate of HUVECs is more than 70%, indicating that the 24 h toxicity of HUVECs treated with seleno-compounds is low and has little effect on the normal biological function of the cells. As shown in Figs. S4A-D (Supporting information) and Fig. 2A, compared to the control group, the healing degree of the scratch area of HUVECs was significantly reduced after being treated with different concentrations of seleno-compounds for 24 h, indicating that HUVECs migration was inhibited concentration-dependently by the seleno-compounds. Na2SeO3 and SeD can inhibit HUVECs cell migration with 4 µmol/L drug concentrations, indicating that they have strong anti-angiogenesis activities, while SeC and LET-SeNPs with 32 µmol/L drug concentrations can inhibit HUVECs cell migration and also have good anti-angiogenesis activity. Na2SeO3 and SeD have the stronger inhibitory effect on the cell migration of HUVECs in vitro according to the quantitative analysis of HUVECs cell migration with statistical significance.

|
Download:
|
| Fig. 2. The effect of different Se speciation on migration, cell invasion and tube formation ability of HUVECs. Quantitative analysis of HUVECs migration (A), cell invasion ability (B) and tube formation ability (C). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the control group. | |
The Transwell assay is generally used to evaluate the invasion ability of cells. As shown in Figs. S5A-D (Supporting information) and Fig. 2B, after HUVECs were treated for 24 h with different concentrations of seleno-compounds, an abundance of invasive cells could be observed in the control group’s lower chamber while only 11.13%−21.18% invasive cells existed in the lower chamber of the drug group. HUVECs invasion was inhibited significantly by Na2SeO3 and SeD, and it increased as the concentration of drug increased. SeC and LET-SeNPs also showed a good inhibitory effect on HUVECs cell invasion in a concentration-dependent manner. It shows that the four seleno-compounds can inhibit the invasion ability of HUVECs and exert anti-angiogenesis activity well, and Na2SeO3 and SeD have a stronger inhibitory effect on the invasion ability of HUVECs than SeC and LET-SeNPs. The quantitative analysis of HUVECs cell migration had statistical significance.
The Matrigel tube formation experiment can restore the process of capillary formation in vivo as much as possible and is similar to the actual process of angiogenesis in vivo. The effect of seleno-compounds on angiogenesis was evaluated by detecting how HUVECs’ tube formation ability was influenced by different seleno-compounds. Figs. S6A-D (Supporting information) and Fig. 2C show the situation, the HUVECs of the control group treated with culture medium can form multiple complete tube-like structures in Matrigel, while the number of tube structures was significantly reduced in the drug treatment group. With an increase in drug concentration, the tube structure was incomplete, broken, and fragmented. Na2SeO3 and SeD can significantly inhibit the tube formation ability of HUVECs at a low drug concentration, and the number of tubules formed by intercellular aggregation was only 15% and 10%, respectively. SeC and LET-SeNPs could significantly inhibit the tube formation of HUVECs at a higher drug concentration. Overall, in comparison with the control group, all four seleno-compounds inhibited the formation of the tube statistically significantly. Na2SeO3 and SeD were stronger than SeC and LET-SeNPs and could significantly inhibit tube formation at low concentrations.
The normal operation of the cell cycle is an indispensable condition in the process of cell proliferation. When cell growth is inhibited, it may be a result of the suspension of the cell cycle or the apoptosis of the cells. Therefore, further research into the intrinsic mechanisms of action is required. We used flow cytometry to detect the cell cycle of HUVECs after drug action. As depicted in Figs. S7A-D (Supporting information) and Figs. 3A-D, HUVECs treated with different seleno-compounds for 72 h could induce cell cycle arrest, which mainly in the G2/M phase, and the degree of cell cycle arrest increased with an increase in drug concentration. Na2SeO3 could induce obvious cell cycle arrest at a low drug concentration. The G2/M phase at 2 µmol/L increased by 29.5% compared with the control group. A total of 13.67% of the cells in the sub-G1 phase could be detected at 4 µmol/L, indicating that Na2SeO3 has a strong toxic effect on HUVECs. After the cells were treated with 4 µmol/L SeD and LET-SeNPs for 72 h, the G2/M phase increased by 13.29% and 25.3% compared with the control group, showing obvious cell cycle inhibition. Additionally, the proportion of cells in the sub-G1 phase increased with an increase in drug concentration. However, SeC could significantly inhibit the cell cycle at higher drug concentrations in a concentration-dependent manner. Therefore, it can be seen that different Se speciation can inhibit the proliferation of HUVECs by inducing cell cycle arrest at the G2/M phase. However, the proportion of sub-G1 phase cells in the drug treatment group increased, indicating that different seleno-compounds may induce the apoptosis of HUVECs.

|
Download:
|
| Fig. 3. Flow cytometry was used to measure the effects of different Se speciation on the HUVECs cell cycle distribution. (A-D) Quantitative analysis of HUVECs cell cycle arrest. HUVECs were detected through flow cytometry following the induction of apoptosis by different Se speciation. (E-H) Quantitative analysis of HUVECs apoptosis. | |
Apoptosis is another mechanism by which seleno-compounds inhibit the proliferation of HUVECs. Therefore, we detected the effect of different speciation of seleno-compounds on the induction of HUVECs cell apoptosis by flow cytometry. As shown in Figs. S8A-D (Supporting information) and Figs. 3E-H, HUVECs were treated with four seleno-compounds for 72 h and they had different degrees of apoptosis. They could induce HUVECs to suffer from early and late apoptosis. Compared with the control group, SeD and LET-SeNPs could induce significant apoptosis in HUVECs. SeD mainly induced early apoptosis in HUVECs, there are 4.94%−37.6% early apoptosis cells induced with 1, 2 and 4 µmol/L seleno-compounds concentrations. LET-SeNPs induced 52.44%−57.46% and 15.3%−18.72% early and late apoptosis cells with 8, 16 and 32 µmol/L seleno-compounds concentrations. Na2SeO3 and SeC also induced apoptosis in HUVECs, mainly early apoptosis, but the proportion of overall apoptotic cells was small, and the effect of inducing apoptosis was less than that of SeD and LET-SeNPs. Based on the above results, we can conclude that different Se speciation can induce apoptosis in HUVECs, and the inducing ability is different. SeD and LET-SeNPs have strong inducing effects. It is thus shown that different Se speciation can exert anti-angiogenic effects by inducing HUVECs apoptosis.
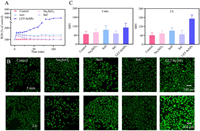
Researchers have found that cell apoptosis is associated with the intracellular production of ROS [34]. When cells are disturbed by external factors, a large amount of ROS are produced, which destroys the redox balance in cells and leads to apoptosis. Therefore, we used a 2,7-dichlorodihydrofluoresceindiacetate (DCFH-DA) probe to detect whether the production of ROS in HUVECs is related to the mechanism of apoptosis. As showcased in Fig. 4A and Fig. S9 (Supporting information), when compared to the control group, the ROS levels of HUVECs treated with LET-SeNPs increased over time and reached a peak at about 2 h. The ROS of cells treated with the other seleno-compounds increased at first, reached the highest value at 5 min, and then decreased slowly. SeC and LET-SeNPs induced ROS production in a concentration-dependent manner. The results showed that different Se speciation could induce HUVECs to produce a large amount of ROS, among which LET-SeNPs had the most obvious effect on the increase in intracellular ROS. At the same time, we captured DCF fluorescence images under a fluorescence microscope, which can be seen in Figs. 4B and C. The green fluorescence intensity of HUVECs treated with seleno-compounds was significantly higher when compared to the control group. After 2 h of drug treatment, the green fluorescence intensity of HUVECs groups other than the LET-SeNPs group was slightly higher than the control group, while treatment with LET-SeNPs significantly increased the green fluorescence intensity of HUVECs compared to the other groups. Overall, LET-SeNPs and SeD can induce HUVECs to produce a large amount of ROS, resulting in disrupting the normal redox balance to induce HUVECs apoptosis, which finally achieve the effect of anti-angiogenesis.
|
Download:
|
| Fig. 4. Effects of different Se speciation on ROS levels in HUVECs. (A) After HUVECs were treated with 4 µmol/L Na2SeO3, 4 µmol/L SeD, 32 µmol/L SeC, and 32 µmol/L LET-SeNPs, intracellular ROS production was observed for 2 h. (B) DCF fluorescence images of HUVECs treated with different seleno-compounds. (C) Mean fluorescence intensity (MFI) of DCF generated by HUVECs treated with seleno-compounds. | |
The TrxR and glutathione peroxidase (GPx) systems play important roles in maintaining oxidation–reduction balance by offsetting excessive ROS, making them promising cancer pharmacological targets [35]. Therefore, in order to further study the targets of different Se speciation when inhibiting angiogenesis, we detected the effect of the extracellular and intracellular activity of TrxR and GPx after HUVECs were treated with organic Se and SeNPs. As shown in Fig. S10A (Supporting information), with an increase in drug concentration, the TrxR activity of HUVECs in vitro decreases gradually after treatment with SeD, while the TrxR activity with LET-SeNPs treatment decreased only slightly. Fig. S10B (Supporting information) shows that the activity of GPx measured in vitro increased slightly after HUVECs were treated with SeD and LET-SeNPs. As shown in Table S2 (Supporting information), the IC50 value of TrxR is 41.07 µmol/L, and the IC50 value of GPx is greater than 40 µmol/L of SeD. However, the IC50 value of TrxR and GPx of LET-SeNPs is higher than 40 µmol/L. The results show that organic Se has higher extracellular TrxR inhibitory activity, and different Se speciation has little effect on the extracellular GPx activity in HUVECs. These results are consistent with the cytotoxic effects of Se speciation on HUVECs. In order to further confirm the roles of TrxR and GPx on the inhibitory effect of different Se speciation on angiogenesis, we also examined their inhibitory kinetics on intracellular TrxR and GPx in HUVECs’ cell lysates. As shown in Figs. S10C and D (Supporting information), SeD significantly inhibited TrxR activity in a time-dependent manner, which was much higher than that of LET-SeNPs, while SeD and LET-SeNPs had little effect on the inhibitory kinetics of GPx in HUVECs. Overall, Se speciation has different effects on the TrxR activity of HUVECs. Organic Se can significantly inhibit the activity of TrxR, resulting in redox imbalance in vivo, which gives rise to the intracellular production of ROS. Thereby, TrxR may be the target of organic Se when inhibiting angiogenesis.
Taken together, in our study, we found that different anti-angiogenesis effects and mechanism between different Se speciation, including organic Se, inorganic Se and SeNPs. The results indicate that organic Se, especially SeD, can more significantly inhibit HUVECs proliferation, migration, invasion and angiogenesis ability than inorganic Se and SeNPs. Among them, these results demonstrate that organic Se can significantly inhibit tumor angiogenesis though the inhibition of TrxR, which induces apoptosis and cell cycle arrest and increases ROS production in HUVECs.
Recent decades, a number of studies have confirmed that seleno-compounds have important anti-tumor [15,16], antibacterial [17,18], anti-inflammatory [19], anti-oxidation effects [20], and immune promotion functions [21,22]. For example, Na2SeO3 was first used to prevent and treat Keshan disease [36]. With the indepth study of element Se, it was found that the regulation of redox reaction by Na2SeO3 can inhibit the proliferation of various tumor cell lines, such as prostate cancer, breast cancer, lung cancer, bladder cancer [37]. In general, most inorganic seleno-compounds exhibit high genotoxicity, and the safe dose is difficult to control, which limits the clinical application and development of inorganic Se [36]. Different speciation of Se as an anti-tumor drug has its own advantages. Studies have shown that the metabolism of organic Se in the body is different from that of inorganic Se. It has the characteristics of easy absorption, high biological activity, and relatively low toxicity [36]. Organic Se can regulate oxidative stress, induce cell apoptosis, and enhance chemotherapy and anti-tumor activity [14]. SeNPs are an emerging type of seleno-compounds in recent years. Compared with inorganic Se and organic Se, SeNPs have lower toxicity and higher bioavailability, which facilitates better control of the dosage [38]. The surface-modified SeNPs have multiple functions such as positioning, imaging, improving the accumulation rate of drugs in tumors, and anti-drug resistance [39-42]. It has been proved that seleno-compounds have anti-angiogenic effects and are potential anti-angiogenic drugs. However, it has not been reported that the influence of different Se speciation on anti-angiogenesis and its mechanism.
Traditional anti-tumor treatments include surgery, chemotherapy and radiotherapy [43,44], with the development of medicine, anti-angiogenesis has played an important role in modern anti-tumor therapy and has become the first-line treatment choice for many kinds of malignant tumors [45]. Previous studies have shown that certain seleno-compounds have anti-angiogenic effects and have potential in anti-angiogenic treatments. Nevertheless, the effects of different Se speciation on anti-tumor angiogenesis remain unknown. To figure out this, we determined the anti-angiogenic activity by different Se speciation. In this study, the anti-angiogenic activities of different Se speciation were determined. These results demonstrate that organic Se (SeD) can significantly inhibit tumor angiogenesis though the inhibition of TrxR, which induces apoptosis and cell cycle arrest and increases ROS production in HUVECs. Inorganic Se (Na2SeO3) can induce cell cycle arrest and increase ROS production in HUVECs, showing effective anti-angiogenic effects. SeNPs (LET-SeNPs) slightly inhibit tumor angiogenesis by inducing apoptosis and cell cycle arrest and increasing the production of ROS. In summary, this study elucidated that different Se speciation can affect the inhibiting angiogenesis effect of seleno-compounds. Compared with inorganic Se and SeNPs, the anti-tumor angiogenesis effect of organic Se is the most significant among all Se speciation, which shows the influence of Se speciation on the anti-tumor angiogenesis effects and mechanisms. We should design seleno-compounds to prevent tumor angiogenesis. A drug design based on organic Se can provide a good reference for the design of new types of angiogenesis inhibitors with high efficiency and low toxicity in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was funded by Science and Technology Projects in Guangzhou (No. 202102010083), National Natural Science Foundation of China (No. 32201062), Guangzhou Basic and Applied Basic Research Foundation (No. 202201010339).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108264.
| [1] |
H.H. Pulkkinen, M. Kiema, J.P. Lappalainen, et al., Angiogenesis 24 (2021) 129-144. DOI:10.1007/s10456-020-09748-4 |
| [2] |
J.Q. Fan, S.F. Wen, J.P. Zhang, et al., Chin. Chem. Lett. 31 (2020) 3153-3157. DOI:10.1016/j.cclet.2020.03.077 |
| [3] |
J. Cheng, H.L. Yang, C.J. Gu, et al., Int. J. Mol. Med. 43 (2019) 945-955. |
| [4] |
W. Lin, S. Li, Y. Meng, et al., Front. Pharmacol. 12 (2021) 755394. DOI:10.3389/fphar.2021.755394 |
| [5] |
R. Gianni-Barrera, A. Butschkau, A. Uccelli, et al., Angiogenesis 21 (2018) 883-900. DOI:10.1007/s10456-018-9634-5 |
| [6] |
K. El Alaoui-Lasmaili, E.H. Djermoune, J.B. Tylcz, et al., Angiogenesis 20 (2017) 149-162. DOI:10.1007/s10456-016-9536-3 |
| [7] |
J. Garcia, H.I. Hurwitz, A.B. Sandler, et al., Cancer Treat. Rev. 86 (2020) 102017. DOI:10.1016/j.ctrv.2020.102017 |
| [8] |
M. Kudo, K. Ueshima, M. Ikeda, et al., Gut 69 (2020) 1492-1501. DOI:10.1136/gutjnl-2019-318934 |
| [9] |
R.J. Motzer, A. Ravaud, J.J. Patard, et al., Eur. Urol. 73 (2018) 62-68. DOI:10.1016/j.eururo.2017.09.008 |
| [10] |
J.A. Hellyer, M.M. Ouseph, S.K. Padda, H.A. Dennison, Lung Cancer 149 (2020) 97-102. DOI:10.1016/j.lungcan.2020.09.006 |
| [11] |
Y. Zhou, Y. Gao, N. Zhang, et al., J. Thorac. Dis. 13 (2021) 1100-1105. DOI:10.21037/jtd-20-1493 |
| [12] |
Z. Hu, S. Sun, X. Zhao, et al., Oncologist 27 (2022) 253-e312. DOI:10.1093/oncolo/oyab078 |
| [13] |
G.C. Jayson, R. Kerbel, L.M. Ellis, A.L. Harris, Lancet 388 (2016) 518-529. DOI:10.1016/S0140-6736(15)01088-0 |
| [14] |
S. Hariharan, S. Dharmaraj, Inflammopharmacology 28 (2020) 667-695. DOI:10.1007/s10787-020-00690-x |
| [15] |
E.G. Varlamova, E.A. Turovsky, Int. J. Mol. Sci. 22 (2021) 6614. DOI:10.3390/ijms22126614 |
| [16] |
T. Chen, Y. Yang, Y. Wang, L. Xu, Chin. Chem. Lett. 31 (2020) 1801-1806. DOI:10.1016/j.cclet.2020.03.004 |
| [17] |
H.W. Han, K.D. Patel, J.H. Kwak, et al., Biomolecules 11 (2021) 1028. DOI:10.3390/biom11071028 |
| [18] |
M. Vahdati, T.Tohidi Moghadam, Sci. Rep. 10 (2020) 510. DOI:10.1038/s41598-019-57333-7 |
| [19] |
C.L. Bi, S.J. Zhang, Y.Z. Shen, et al., Biol. Trace. Elem. Res. 199 (2021) 604-610. DOI:10.1007/s12011-020-02166-z |
| [20] |
Y. Huang, Y. Fu, M. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 4406-4414. DOI:10.1002/anie.201910615 |
| [21] |
Y. Hu, T. Liu, J. Li, et al., Biomaterials 222 (2019) 119397. DOI:10.1016/j.biomaterials.2019.119397 |
| [22] |
Z. Song, W. Luo, H. Zheng, et al., Adv. Healthc. Mater. 10 (2021) e2100149. DOI:10.1002/adhm.202100149 |
| [23] |
Z. Zhang, Y. Du, T. Liu, K.H. Wong, T. Chen, Biomater. Sci. 7 (2019) 5112-5123. DOI:10.1039/c9bm01104h |
| [24] |
Y. Yang, Q. Xie, Z. Zhao, et al., ACS Appl. Mater. Interfaces 9 (2017) 25857-25869. DOI:10.1021/acsami.7b07167 |
| [25] |
T. Liu, C. Shi, L. Duan, et al., J. Mater. Chem. B 6 (2018) 4756-4764. DOI:10.1039/c8tb01398e |
| [26] |
F. Yang, J. Huang, H. Liu, et al., Nanoscale 12 (2020) 14494-14503. DOI:10.1039/d0nr02171g |
| [27] |
Y. Yang, Z. Zhang, Q. Chen, et al., Front. Bioeng. Biotechnol. 9 (2021) 758482. DOI:10.3389/fbioe.2021.758482 |
| [28] |
X. Liu, Z. Yuan, Z. Tang, et al., Biomater. Sci. 9 (2021) 4691-4700. DOI:10.1039/d1bm00348h |
| [29] |
G.N. Schrauzer, Crit. Rev. Biotechnol. 29 (2009) 10-17. DOI:10.1080/07388550802658048 |
| [30] |
Z. Wang, C. Jiang, J. Lu, Mol. Carcinog. 34 (2002) 113-120. DOI:10.1002/mc.10056 |
| [31] |
Z. Wang, C. Jiang, H. Ganther, J. Lu, Cancer Res. 61 (2001) 7171-7178. |
| [32] |
H. Lai, X. Fu, C. Sang, et al., Chem. Asian J. 13 (2018) 1447-1457. DOI:10.1002/asia.201800110 |
| [33] |
X. Fu, Y. Yang, X. Li, et al., Nanomedicine 12 (2016) 1627-1639. DOI:10.1016/j.nano.2016.01.012 |
| [34] |
Y. Liu, Y. Luo, X. Li, W. Zheng, T. Chen, Chem. Asian J. 10 (2015) 642-652. DOI:10.1002/asia.201403409 |
| [35] |
C. Chang, B.L. Worley, R. Phaeton, N. Hempel, Cancers 12 (2020) 2197. DOI:10.3390/cancers12082197 |
| [36] |
D. Colle, D.B. Santos, V. de Souza, et al., Mol. Biol. Rep. 46 (2019) 751-762. DOI:10.1007/s11033-018-4531-y |
| [37] |
D. Radomska, R. Czarnomysy, D. Radomski, K. Bielawski, Int. J. Mol. Sci. 22 (2021) 1009. DOI:10.3390/ijms22031009 |
| [38] |
A. Khurana, S. Tekula, M.A. Saifi, P. Venkatesh, C. Godugu, Biomed. Pharmacother. 111 (2019) 802-812. DOI:10.1016/j.biopha.2018.12.146 |
| [39] |
C. Ferro, H.F. Florindo, H.A. Santos, Adv. Healthc. Mater. 10 (2021) e2100598. DOI:10.1002/adhm.202100598 |
| [40] |
J. Ma, C. Wu, Exploration 2 (2022) 20210083. |
| [41] |
W. Ding, S. Wang, J. Gu, L. Yu, Chin. Chem. Lett. 34 (2023) 108043. DOI:10.1016/j.cclet.2022.108043 |
| [42] |
R. Liu, C. Luo, Z. Pang, et al., Chin. Chem. Lett. 34 (2023) 107518. DOI:10.1016/j.cclet.2022.05.032 |
| [43] |
M. Chen, X. Huang, J. Lai, L. Ma, T. Chen, Chin. Chem. Lett. 32 (2021) 158-161. DOI:10.1016/j.cclet.2020.11.050 |
| [44] |
R. Jin, Z. Liu, T. Liu, et al., Chin. Chem. Lett. 32 (2021) 3076-3082. DOI:10.1016/j.cclet.2021.03.084 |
| [45] |
C. Ziming, Z. Kuo, F. Jiaqi, et al., Chin. Chem. Lett. 31 (2020) 3107-3112. DOI:10.1016/j.cclet.2020.04.006 |
 2023, Vol. 34
2023, Vol. 34 

