b College of Public Health, Zhengzhou University, Zhengzhou 450001, China
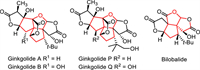
Terpenoids such as Ginkgolides and Bilobalide (Fig. 1) are unique components of the Ginkgo biloba tree, which displayed diverse bioactivities such as antioxidant, anti-aging, blood pressure lowering, blood circulation promotion and anti-platelet activating factors [1-4]. Therefore, synthesis of Ginkgolides, Bilobalide and their derivatives having multicyclic skeleton are very significant and have attracted much attention [5]. However, assembly of contiguous quaternary carbons in these terpenoid natural products remains a big challenge. New synthetic methods for these structures are highly desirable.
|
Download:
|
| Fig. 1. Terpenoid natural products. | |

|
Download:
|
| Fig. 2. X-ray crystal structure of the product 3af. | |
Pd-catalyzed decarboxylative cycloadditions are one of the most powerful tools for synthesis of carbo- and hetero-cyclic compounds from readily available starting material [6-20]. Generally, these (m + n) cycloadditions were furnished through stepwise reactions of in situ generated zwitterionic π-allyl palladium intermediates from decarboxylation of precursors with electron-deficient reaction partner [6-20]. Obviously, the type and reactivity of the precursors for formation of π-allyl palladium intermediate played a key role in Pd-catalyzed decarboxylative cycloadditions. In 2021, we designed and synthesized the allenylethylene carbonates (AECs) as the precursor of π-allyl palladium intermediates, which may act as 1,3 or 1,5-dipoles undergoing cycloadditions with dipolarophiles [21]. In our early exploration, we studied the cycloaddition reactions of AECs with electron-deficient reaction partner and successfully developed a palladium-catalyzed (3 + 3) annulation of AECs with nitrile oxides, producing 3,6-disubstituted-5-hydro-1,4,2-dioxazines in high yields (Scheme 1a) [21]. In this example, AECs worked as three-membered synthons and the allene moiety in AECs did not participate in the construction of six-membered ring. As continuous efforts in the exploration on the application of AECs in Pd-catalyzed cycloadditions, we anticipated to achieve formation of ring system involving the allenyl moiety. In 2019, Shao reported an elegant Pd-catalyzed (4 + 1) cycloaddition of allenyl acetates and pyrazolones, in which allenic esters worked as C4 synthons to be fused in cyclopentene rings (Scheme 1b) [22]. This inspired us to think about transformations of allene moiety of AECs for construction of cyclic framework. The 1,3-indandiones were often used in cascade/domino reactions to construct various cyclic compounds because the protons of methylene group are very acidic and prone to isomerize into their enol forms [23,24]. Considering the high reactivity of 1,3-indandiones, we explored their reaction with AECs under palladium catalysis. Herein we report palladium-catalyzed cascade cyclization of AECs with 1,3-indandiones, giving tetracyclic dihydrocyclopentaindenofuranone derivatives having three contiguous quaternary carbon centers (Scheme 1c).
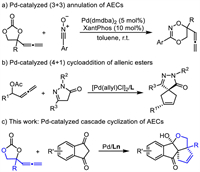
|
Download:
|
| Scheme 1. Pd-catalyzed decarboxylative annulations of AECs. | |
We chose the reaction of 1,3-indandione 1a and AEC 2a to begin the investigation (Table 1), which was performed in CH2Cl2 at 25 ℃. When the combination of Pd2dba3·CHCl3 with PPh3 was used, no product was observed (Table 1, entry 1). We then screened a series of ligands (entries 2−6) and found that when XantPhos was used as the ligand, and the desired product 3aa was isolated in the highest 35% yield (entry 2). Subsequently, with XantPhos as the ligand, different palladium catalysts were tested and no better yield was obtained (entries 7−9). We then screened several other solvents, including ClCH2CH2Cl (DCE), CHCl3, toluene, THF and CCl4 (entries 10−14). Among them, CHCl3 exhibited the best performance, and the product 3aa was obtained in 44% yield (entry 11). Effect of temperature to the reaction was also investigated (entries 15−17). The reaction at 60 ℃ led to the product in 55% yield (entry 16). During the optimization process, we found that the AEC underwent self-polymerization to form side products, so that we increased the loading amount of AEC and the yield of 3aa was thus increased to 64% (entry 18). In further optimization, various bases were examined as additives (detailed in Supporting information). To our great delight, when diisopropylethylamine was used as the base, the yield of product 3aa was improved to 77% (entry 19). In order to develop asymmetric catalytic reaction, we screened various types of chiral ligands (see Supporting information for details). Unfortunately, only chiral ligand L* displayed catalytic activity, delivering the product in 60% yield but with 0% ee (entry 20).
|
|
Table 1 Optimization of reaction conditions.a |
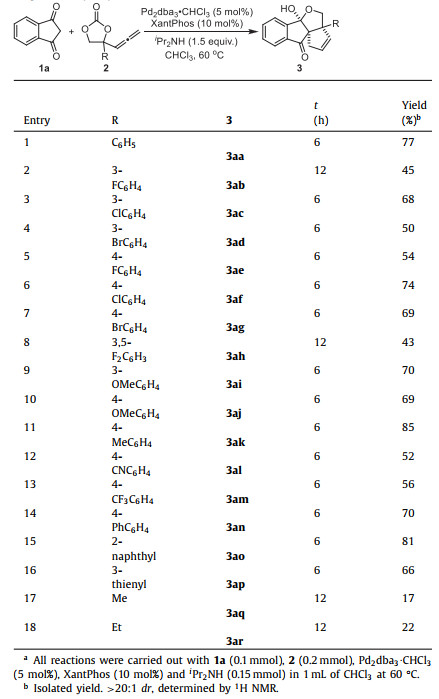
Having established the optimal conditions, we tested a variety of AECs to explore the reaction scope. The results were summarized in Table 2. The reactions of AECs 2 bearing diverse substituents with different electronic properties with 1,3-indandione 1a were carried out under standard conditions (entries 1−18). The AECs with halogen atoms at the meta or para position of the phenyl group were tolerated, producing the desired products (3ab−3ah) in 43%−74% yield (entries 2−8). The AECs having electron-donating (Me and OMe) and electron-withdrawing (CN and CF3) groups on the phenyl ring at the meta and para position were also favourable (entries 9−13), and the corresponding products were obtained in 52%−85% yields. The AECs with biphenyl moieties also performed well in the reaction, leading to the formation of the corresponding product 3an in 70% yield (entry 14). In addition, AECs containing fused or heterocyclic aromatic groups were also suitable substrates. The AEC containing a 2-naphthyl group afforded the corresponding product 3an in 81% yield (entry 15) while the 3-thienyl substituted one afforded the corresponding product 3ap in 66% yield (entry 16). Unfortunately, the AECs containing methyl and ethyl groups were challenging substrates, affording the corresponding products 3aq and 3ar in poor 17% and 22% yield respectively (entries 17 and 18). The structure of the product 3af was confirmed by the results of single crystal X-ray diffraction analysis (Fig. 2, CCDC 2,195,449, detailed in Supporting information).
|
|
Table 2 Scope of allenylethylene carbonates.a |
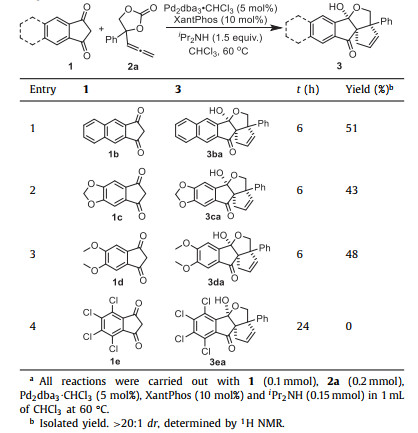
We further evaluated various 1,3-indandione in this cascade cyclization of AECs 2a. As indicated in Table 3, the effect of different substituents on the phenyl group of indandione 1 was tested. Delightedly, 1H-cyclopenta[b]naphthalene-1,3(2H)–dione (1b) was workable, producing the product (3ba) in 51% yield (entry 1). The reaction of 1,3-indandione 1c having dioxolane moiety on the benzene ring resulted in the formation of the corresponding product 3ca in 43% yield (entry 2). The reactivity of 5,6-dimethoxy-1H-indene-1,3(2H)–dione (1c) was moderate, giving 48% yield of the product 3 da (entry 3). Unfortunately, the 4,5,6,7-tetrachcloro-1,3-indene–dione (1e) did not work (entry 4). The big steric hindrance may suppress the reaction.
|
|
Table 3 Scope of indandiones.a |
In order to demonstrate the practical applicability of this reaction, we carried out a scale-up reaction under standard reaction conditions (Scheme 2). This cyclization reaction at 2 mmol of scale still proceeded smoothly to give the product 3aa in 65% yield. Subsequently, the transformation of product 3aa was conducted. As shown in Scheme 2, under the catalysis of palladium, the product 3aa was reduced by hydrogen to form the product 4 in 78% yield (CCDC: 2195450, detailed in Supporting information). The double bond of 3aa underwent addition reaction with Br2 to afford the corresponding product 5 in a yield of 75% (CCDC: 2195452, detailed in Supporting information). In the presence of Et3N, the hydroxyl in the product 3aa reacted with 3,5-dinitrobenzoyl chloride to form the product 6.
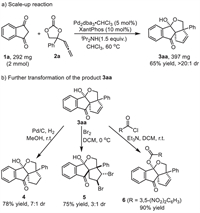
|
Download:
|
| Scheme 2. Scale-up reaction and further transformation of the product. | |
As shown in Scheme 3, a plausible reaction mechanism was proposed. In the presence of palladium catalyst, decarboxylation of AEC 2a afforded the zwitterionic intermediate A, which subsequently deprotonated the 1,3-indandione 1a to produce both the reaction partners B and C. They performed allenylic alkylation to produce the intermediate D. Subsequent isomerization generated the intermediate E, which perform hemiacetalization/intramolecular cyclization to produce the product 3aa with regeneration of the catalyst. According to the data in Table 1, the catalytic cycle could proceed without needing a base. Therefore, the role of iPr2NH probably was to assist deprotonation.
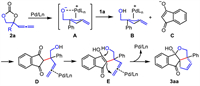
|
Download:
|
| Scheme 3. A plausible reaction mechanism. | |
In summary, we have developed a palladium-catalyzed cascade cyclization of allenylethylene carbonates with 1,3-indandiones. Under the standard conditions, a variety of AECs worked well with indandiones, producing biologically interesting tetracyclic dihydrocyclopentaindenofuranone derivatives having three contiguous quaternary carbon centers in moderate to high yields with excellent diastereoselectivities. Additionally, the scale-up reaction and late-stage transformations of the product 3aa were also demonstrated. In this reaction, the allene moiety of AECs was fused into the ring system for the first time. The cascade cyclization reaction indicates that AECs may work as a versatile synthon and find more applications in construction of ring system.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the Natural Science Foundation of China (Nos. 21871293 and 22071264).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108297.
| [1] |
T.K. Mohanta, Y. Tamboli, P.K. Zubaidha, Nat. Prod. Res. 28 (2014) 746-752. DOI:10.1080/14786419.2013.879303 |
| [2] |
C. Sarkar, C. Quispe, S. Jamaddar, et al., Biomed. Pharmacother. 132 (2020) 110908. DOI:10.1016/j.biopha.2020.110908 |
| [3] |
P.M. Manisha, S.M. Anuja, N.D. Prajakta, et al., GSC Biol. Pharm. Sci. 16 (2021) 229-240. |
| [4] |
J. Lu, L. Xie, K. Liu, et al., Phytother. Res. 35 (2021) 6114-6130. DOI:10.1002/ptr.7220 |
| [5] |
T. Busch, A. Kirschning, Nat. Prod. Rep. 25 (2008) 318-341. DOI:10.1039/b705652b |
| [6] |
J.D. Weaver, A. Recio Ⅲ, A.J. Grenning, J.A. Tunge, Chem. Rev. 111 (2011) 1846-1913. DOI:10.1021/cr1002744 |
| [7] |
A. Khan, Y.J. Zhang, Synlett 26 (2015) 853-860. DOI:10.1055/s-0034-1380170 |
| [8] |
B.D.W. Allen, C.P. Lakeland, J.P.A. Harrity, Chem. Eur. J. 23 (2017) 13830-13857. DOI:10.1002/chem.201702486 |
| [9] |
W.S. Guo, J.E. Gómez, À. Cristòfol, J.N. Xie, A.W. Kleij, Angew. Chem. Int. Ed. 57 (2018) 13735-13747. DOI:10.1002/anie.201805009 |
| [10] |
N. De, E.J. Yoo, ACS Catal. 8 (2018) 48-58. DOI:10.1021/acscatal.7b03346 |
| [11] |
L. Zuo, T. Liu, X. Chang, W. Guo, Molecules 24 (2019) 3930-3946. DOI:10.3390/molecules24213930 |
| [12] |
J.E. Gómez, A.W. Kleij, Adv. Organomet. Chem. 71 (2019) 175-226. |
| [13] |
J. James, M. Jackson, P.J. Guiry, Adv. Synth. Catal. 361 (2019) 3016-3049. DOI:10.1002/adsc.201801575 |
| [14] |
Q.Z. Li, Y. Liu, M.Z. Li, et al., Org. Biomol. Chem. 18 (2020) 3638-3648. DOI:10.1039/d0ob00458h |
| [15] |
B.M. Trost, G. Mata, Acc. Chem. Res. 53 (2020) 1293-1305. DOI:10.1021/acs.accounts.0c00152 |
| [16] |
J. Wang, S.A. Blaszczyk, X. Li, W. Tang, Chem. Rev. 121 (2021) 110-139. DOI:10.1021/acs.chemrev.0c00160 |
| [17] |
B. Niu, Y. Wei, M. Shi, Org. Chem. Front. 8 (2021) 3475-3501. DOI:10.1039/d1qo00273b |
| [18] |
B. Yan, W. Guo, Synthesis 54 (2022) 1964-1976. DOI:10.1055/a-1715-7413 |
| [19] |
Y. You, Q. Li, Y.P. Zhang, et al., ChemCatChem 14 (2022) e202101887. DOI:10.1002/cctc.202101887 |
| [20] |
M.M. Zhang, B.L. Qu, B. Shi, W.J. Xiao, L.Q. Lu, Chem. Soc. Rev. 51 (2022) 4146-4174. DOI:10.1039/d1cs00897h |
| [21] |
T. Pan, X. Gao, S. Yang, et al., Org. Lett. 23 (2021) 5750-5754. DOI:10.1021/acs.orglett.1c01921 |
| [22] |
L. Li, P. Luo, Y. Deng, Z. Shao, Angew. Chem. Int. Ed. 58 (2019) 4710-4713. DOI:10.1002/anie.201901511 |
| [23] |
J. Sun, J. Cao, Y. Han, C.G. Yan, Chin. J. Org. Chem. 40 (2020) 4122-4146. DOI:10.6023/cjoc202005003 |
| [24] |
S. Asadi, G.M. Ziarani, Mol. Divers. 20 (2016) 111-152. DOI:10.1007/s11030-015-9589-z |
 2023, Vol. 34
2023, Vol. 34