b National Engineering Research Center of Low-Carbon Processing and Utilization of Forest Biomass, Nanjing Forestry University, Nanjing 210037, China
Sulfur-containing compounds [1–8], especially organic dithiocarbamates, are ubiquitous in various bioactive compounds [9–11]. For example, they are commonly used in medicinal chemistry and have been used in cancer treatment [12–14]. Their biological properties and key role in agriculture have led to the development of synthetic methods for these compounds. In addition, they also serve as useful synthetic intermediates [15–17], linkers in solid-phase organic synthesis [18]. Therefore, the development of efficient and novel methods to synthesize this important sulfur-containing compound will be of great value for the screening of bioactive molecules.
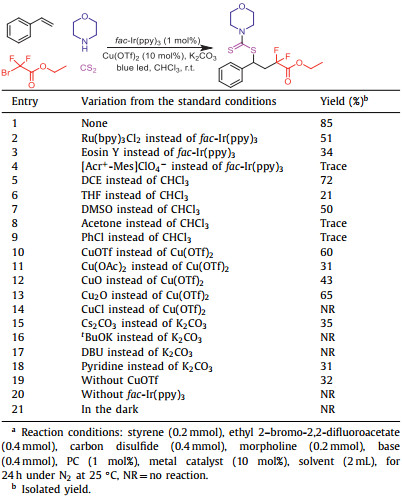
The difluoroalkyl motif is an important class of fundamentally important scaffolds. A variety of biologically active drugs containing CF2 moiety have been reported [19–21]. Ethyl difluorobromoacetate is an important reagent for introducing difluoroalkyl groups into organic compounds [22–27]. At present, various method have been discovered for introducing difluoroalkyl groups. Han group realized a photocatalytic cascade reaction of bromodifluoroacetate esters with indole-derived alkenes, providing an unknown type of tetracyclic tetrahydro-γ-carboline derivatives [28]. The palladium-catalyzed difluoroalkylative carbonylation of aryl olefins with ethyl bromodifluoroacetate was described by Wu group [29]. The nickel-catalyzed tandem reaction by difluoroalkylation−alkylation of N-vinyl-2-pyrrolidinone with difluoroalkyl bromides and dialkylzinc reagents was successfully conducted by Zhang group [30]. In 2019, the perfluoroalkylative pyridylation of alkenes via 4-cyanopyridine-boryl radicals was developed by Li's group (Scheme 1a) [31]. The 4-cyanopyridine-boryl radicals generated from 4-cyanopyridine and B2(pin)2 played an important role in the catalytic cycle. Studer and coworkers realized the three-component Minisci reaction of quinolines or pyridines with α-bromo carbonyl compounds and enamides mediated by dual photoredox and chiral Brønsted acid catalysis (Scheme 1b) [32]. Li and Han's group demonstrated that N-heterocyclic carbene could also catalyzed radical difluoroalkylation of olefins (Scheme 1c) [33]. The dearomative difunctionalization of indoles could be readily achieved via this reaction. In line with our interesting in visible light induced reactions [34]; [35] and radical reactions [36–40], herein, the visible light induced multicomponent reaction of styrene, carbon disulfide, amine and ethyl difluorobromoacetate for the synthesis of thiodifluoroesters is disclosed.
|
Download:
|
| Scheme 1. The difluoroalkylation reaction of olefins. | |
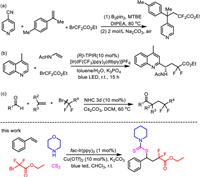
Initially, a template reaction of styrene, morpholine, ethyl 2-bromo-2,2-difluoroacetate and carbon disulfide was chosen to screen the reaction conditions. In the presence of fac-Ir(ppy)3 as photocatalyst, the reaction can be smoothly conducted to provide the corresponding product in 85% yield irradiated by blue led (Table 1, entry 1). Other photocatalyst such as Ru(bpy)3Cl2, Eosin Y and [Acr+-Mes]ClO4− were not effective for this reaction (Table 1, entries 2–4). Screening of common organic solvents revealed that CHCl3 was suitable for this multicomponent reaction (Table 1, entries 5–9). Among the copper sources tested, it is noteworthy that only moderate yields was obtained when Cu(OAc)2, CuOTf, CuO or Cu2O was subjected to this reaction (Table 1, entries 10–13). CuCl was not suitable for this reaction (Table 1, entry 14). The base also plays an important role in this reaction. Lower yield was obtained when K2CO3 was replaced by other bases (Table 1, entries 15–18). Further optimization of the reaction conditions revealed that Cu(OTf)2, Ir(ppy)3 and visible light is indispensable (Table 1, entries 19–21).
|
|
Table 1 Optimization of the reaction conditions.a |
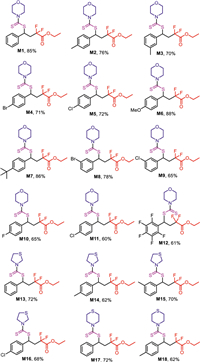
With the optimized reaction conditions in hand, the substrate scope and limitations of the homodimerization reaction were examined (Scheme 2). For substituted styrene, both electron-donating groups and electron-withdrawing groups were all well tolerated in this reaction, and the corresponding products can be isolated in moderate to high yields (M1-M11). It should be mentioned that ethyl 2,2-difluoro-4-((morpholine-4-carbonothioyl)thio)-4-(perfluorophenyl)butanoate was also obtained smoothly when 1,2,3,4,5-pentafluoro-6-vinylbenzene was subjected to this system (M12). In addition to morpholine, other amines were also screened. To our delight, thiomorpholine and thiazolidine were also found to be suitable for this procedure (M13-M18), affording the desired product in moderate yields.
|
Download:
|
| Scheme 2. Structures of the target products. Reaction conditions: styrene (0.2 mmol), ethyl 2-bromo-2,2-difluoroacetate (0.4 mmol), carbon disulfide (0.4 mmol), morpholine (0.2 mmol), K2CO3 (0.4 mmol), fac-Ir(ppy)3 (1 mol%), Cu(OTf)2 (10 mol%), CHCl3 (2 mL), for 24 h under N2 at 25 ℃. Isolated yield. | |
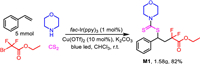
To demonstrate the practicability of the photocatalytic reaction, a scale-up reaction (5 mmol) was conducted (Scheme 3). To our delight, the isolated yield was similar to that from the small-scale reaction. The scalability of the developed process made this protocol very attractive for organic synthesis and industry production.
|
Download:
|
| Scheme 3. Gram-scale reaction. | |
To shed some light on the mechanism of this reaction, some control experiments were performed. When 2 equiv. free radical scavengers (TEMPO or BHT) are added to the photocatalytic reaction system, the desired conversion is completely inhibited (Scheme 4). The intermediate ethyl 2-(2,6-di-tert-butyl-4-methylphenoxy)-2,2-difluoroacetate was confirmed by HRMS. These results suggest that current photocatalytic reactions are carried out by free radical pathways.
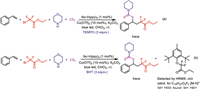
|
Download:
|
| Scheme 4. Control experiments. | |
According to the aboved mentioned observations and previous reports [41–47], a proposed reaction mechanism was described in Scheme 5. The excited photocatalyst Ir(Ⅲ)* was produced from the irradiation of Ir(Ⅲ) by visible light. Then oxidative quenching of the excited Ir(Ⅲ)* catalyst by ethyl 2-bromo-2,2-difluoroacetate occurred to give Ir(Ⅳ) and difluoromethyl radical Ⅰ. Difluoromethyl radical Ⅰ was captured by styrene to deliver benzyl radical Ⅳ. Meanwhile, catalyst Cu(Ⅰ) coordinates with thiocarbamate anion to generated intermediate Ⅱ, which is oxidized by Ir(Ⅳ) to give Cu(Ⅱ) comples Ⅲ, with the concurrent liberation of Ir(Ⅲ) catalyst. Then Cu(Ⅲ) species Ⅴ was formed via the reaction of benzyl radical Ⅳ and Cu(Ⅱ) comples Ⅲ. Finally, the desired product is delivered after reductive elimination from Cu(Ⅲ) intermediate Ⅴ, and the Cu(Ⅰ) catalyst is released to maintain the copper catalytic cycle.
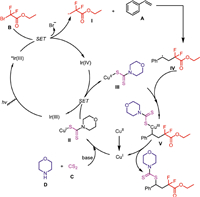
|
Download:
|
| Scheme 5. Proposed reaction mechanism. | |
In summary, the multicomponent reaction of styrene, carbon disulfide, amine and ethyl difluorobromoacetate for the synthesis of thiodifluoroesters induced by visible light is developed. Various of valuable thiodifluoroesters in moderate to good yields were generated smoothly. Preliminary mechanistic studies revealed that a radical process might be involved in this transformation. Further mechanistic studies and applications of this strategy to more complicated materials and drug candidates are underway in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful for financial support from National Natural Science Foundation of China (No. 21772107), Shandong Province Key Research and Development Plan (No. 2019GSF108017), Youth Innovation Team Project for Talent Introduction and Cultivation in Universities of Shandong Province (2021).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108131.
| [1] |
K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500. DOI:10.1039/D0GC02663H |
| [2] |
G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258. DOI:10.1016/j.cclet.2020.03.007 |
| [3] |
D. Chen, Y. Sun, D. Dong, Q. Han, Z. Wang, Chin. J. Org. Chem. 40 (2020) 4267-4273. DOI:10.6023/cjoc202006025 |
| [4] |
K.J. Liu, J.H. Deng, J. Yang, et al., Green Chem. 22 (2020) 433-438. DOI:10.1039/C9GC03713F |
| [5] |
L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70. DOI:10.1016/j.cclet.2019.05.041 |
| [6] |
X.Y. Li, Y. Liu, X.L. Chen, et al., Green Chem. 22 (2020) 4445-4449. DOI:10.1039/C9GC04445K |
| [7] |
Z. Wang, N. Meng, Y. Lv, et al., Chin. Chem. Lett. 34 (2023) 107599. DOI:10.1016/j.cclet.2022.06.022 |
| [8] |
D.Q. Dong, Q.Q. Han, S.H. Yang, et al., ChemistrySelect 5 (2020) 13103-13134. DOI:10.1002/slct.202003650 |
| [9] |
J. García Fernández, C. Ortiz Mellet, J. Jiménez Blanco, et al., Carbohydr. Res. 268 (1995) 57-71. DOI:10.1016/0008-6215(94)00312-4 |
| [10] |
Q.C. Liu, T.T. Guo, Z. Fan, D. Li, W.H. Li, Chin. Chem. Lett. 22 (2011) 801-803. DOI:10.1016/j.cclet.2010.11.036 |
| [11] |
G. Li, Q. Yan, Z. Gan, et al., Org. Lett. 21 (2019) 7938-7942. DOI:10.1021/acs.orglett.9b02921 |
| [12] |
L. Ronconi, C. Marzano, P. Zanello, et al., J. Med. Chem. 49 (2006) 1648-1657. DOI:10.1021/jm0509288 |
| [13] |
X. Hou, Z. Ge, T. Wang, et al., Bioorg. Med. Chem. Lett. 16 (2006) 4214-4219. DOI:10.1016/j.bmcl.2006.05.085 |
| [14] |
R.D. Li, X. Zhang, Q.Y. Li, Z.M. Ge, R.T. Li, Bioorg. Med. Chem. Lett. 21 (2011) 3637-3640. DOI:10.1016/j.bmcl.2011.04.096 |
| [15] |
E.B. Ozer, C. Caglayan, S. Bayindir, Tetrahedron 120 (2022) 132896. DOI:10.1016/j.tet.2022.132896 |
| [16] |
T. Bi, Y. Xu, X. Xu, et al., Chin. Chem. Lett. 33 (2022) 2015-2020. DOI:10.1016/j.cclet.2021.10.043 |
| [17] |
Y.J. Chen, R.Y. Bao, X.D. Zhang, Y.F. Tang, Chin. Chem. Lett. 24 (2013) 953-956. DOI:10.1016/j.cclet.2013.07.015 |
| [18] |
P. Morf, F. Raimondi, H.G. Nothofer, et al., Langmuir 22 (2006) 658-663. DOI:10.1021/la052952u |
| [19] |
S. Abel, D.J. Back, M. Vourvahis, Antivir. Ther. (Lond.) 14 (2009) 607-618. DOI:10.1177/135965350901400514 |
| [20] |
Y.J. Roh, Y.G. Park, S. Kang, S.Y. Kim, J.I. Moon, Graefe's Arch. Clin. Exp. Ophthalmol. 250 (2012) 1765-1775. DOI:10.1007/s00417-012-2125-2 |
| [21] |
D.Q. Dong, S.H. Yang, P. Wu, et al., Molecules 27 (2022) 8461. DOI:10.3390/molecules27238461 |
| [22] |
W. Xiong, W.B. Qin, Y.S. Zhao, K.Z. Fu, G.K. Liu, Org. Chem. Front. 9 (2022) 2141-2148. DOI:10.1039/D2QO00192F |
| [23] |
A. Granados, R.K. Dhungana, M. Sharique, J. Majhi, G.A. Molander, Org. Lett. 24 (2022) 4750-4755. DOI:10.1021/acs.orglett.2c01699 |
| [24] |
Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021) 1907-1910. DOI:10.1016/j.cclet.2021.01.021 |
| [25] |
Y. Ouyang, F.L. Qing, Chin. J. Org. Chem. 40 (2020) 806-807. DOI:10.6023/cjoc202000011 |
| [26] |
D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575. DOI:10.1039/D0QO00567C |
| [27] |
X.F. Tao, R. Sheng, K. Bao, Y.X. Wang, Y.X. Jin, Chin. J. Org. Chem. 39 (2019) 2726-2734. DOI:10.6023/cjoc201903063 |
| [28] |
H. Mei, A. Liu, J. He, Y. Yu, J. Han, Org. Lett. 24 (2022) 2630-2635. DOI:10.1021/acs.orglett.2c00626 |
| [29] |
Z.P. Bao, Y. Zhang, X.F. Wu, Chem. Sci. 13 (2022) 9387-9391. DOI:10.1039/D2SC02665A |
| [30] |
C. Xu, Z.F. Yang, L. An, X. Zhang, ACS Catal. 9 (2019) 8224-8229. DOI:10.1021/acscatal.9b02488 |
| [31] |
J. Cao, G. Wang, L. Gao, et al., Chem. Sci. 10 (2019) 2767-2772. DOI:10.1039/C8SC05237A |
| [32] |
D. Zheng, A. Studer, Angew. Chem. Int. Ed. 58 (2019) 15803-15807. DOI:10.1002/anie.201908987 |
| [33] |
J.L. Li, Y.Q. Liu, W.L. Zou, et al., Angew. Chem. Int. Ed. 59 (2020) 1863-1870. DOI:10.1002/anie.201912450 |
| [34] |
Y.Y. Sun, J.C. Song, S.H. Yang, et al., New J. Chem. 45 (2021) 16438-16441. DOI:10.1039/D1NJ03579G |
| [35] |
S.H. Yang, J.C. Song, D.Q. Dong, et al., Chin. J. Org. Chem. (2022) 202207019. |
| [36] |
W.C. Yang, C.Y. Chen, J.F. Li, Z.L. Wang, Chin. J. Catal. 42 (2021) 1865-1875. DOI:10.1016/S1872-2067(21)63814-7 |
| [37] |
D.Q. Dong, J.C. Song, S.H. Yang, et al., Chin. Chem. Lett. 33 (2022) 1199-1206. DOI:10.1016/j.cclet.2021.08.067 |
| [38] |
Q.Q. Han, D.M. Chen, Z.L. Wang, et al., Chin. Chem. Lett. 32 (2021) 2559-2561. DOI:10.1016/j.cclet.2021.02.018 |
| [39] |
Q.Q. Han, Y.Y. Sun, S.H. Yang, J.C. Song, Z.L. Wang, Chin. Chem. Lett. 32 (2021) 3632-3635. DOI:10.1016/j.cclet.2021.04.019 |
| [40] |
D. Dong, S. Yue, Z. Wang, et al., Chin. J. Org. Chem. 41 (2021) 4651-4660. DOI:10.6023/cjoc202106006 |
| [41] |
F.D. Lu, L.Q. Lu, G.F. He, J.C. Bai, W.J. Xiao, J. Am. Chem. Soc. 143 (2021) 4168-4173. DOI:10.1021/jacs.1c01260 |
| [42] |
P. Zhang, W. Li, W. Qu, et al., Org. Lett. 23 (2021) 9267-9272. DOI:10.1021/acs.orglett.1c03608 |
| [43] |
Q.W. Gui, F. Teng, H. Yang, et al., Chem. Asian J. 17 (2022) e202101139. DOI:10.1002/asia.202101139 |
| [44] |
X.L. Li, W.D. Meng, X.H. Xu, Y.G. Huang, Chin. J. Org. Chem. 42 (2022) 1820-1830. DOI:10.6023/cjoc202201011 |
| [45] |
C.H. Ma, L. Zhao, X. He, Y.Q. Jiang, B. Yu, Org. Chem. Front. 9 (2022) 1445-1450. DOI:10.1039/D1QO01870A |
| [46] |
J.Y. Chen, J. Huang, K. Sun, W.M. He, Org. Chem. Front. 9 (2022) 1152-1164. DOI:10.1039/D1QO01504D |
| [47] |
Y.H. Lu, Z.T. Zhang, H.Y. Wu, et al., Chin. Chem. Lett. 34 (2023) 108036. DOI:10.1016/j.cclet.2022.108036 |
 2023, Vol. 34
2023, Vol. 34