b College of Public Health, Shanxi Medical University, Taiyuan 030001, China;
c School of Basic Medical Science, Health Science Center, Xi'an Jiaotong University, Xi'an 710061, China;
d School of Public Health, Health Science Center, Xi'an Jiaotong University, Xi'an 710061, China;
e College of Chemistry and Life Science, Sichuan Provincial Key Laboratory for Structural Optimization and Application of Functional Molecules, Chengdu Normal University, Chengdu 611130, China
Cancer is one of the major diseases that threaten human health worldwide [1]. Although significant efficacy has been observed in some conventional treatments modality such as surgery, chemotherapy, radiotherapy, molecular targeted therapy [2,3], gene therapy [4], magnetic hyperthermia therapy [5], sonodynamic therapy [6] and especially phototherapy [7], the challenges of tumor metastasis and recurrence still limit the clinical application of these treatment strategies. With a better understanding of cancer and immunity, cancer treatment has witnessed a transition from conventional therapies to advanced immunotherapy.
Cancer immunotherapy utilizes the body's own immune system to suppress tumor metastasis and recurrence via stimulating a systemic immune response and activating immune cells [8]. Its impregnable status was acknowledged and reinforced by James P. Allison and Tasuku Honjo, who received the Nobel Prize in physiology or medicine in 2018 for their discovery of cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1/programmed cell death protein ligand 1 (PD-1/PD-L1), respectively [9]. Until now, immunotherapy can be implemented via the following five strategies: antibody therapy [10], immunomodulators [11], tumor vaccines [12], adoptive cell therapy [13] and oncolytic immunotherapy [14]. Owing to its remarkable clinical efficacy, excellent patient tolerance, minimal side effects, and broad application prospects, cancer immunotherapy has been rapidly progressing and gradually become the fourth standard anticancer regimen after surgery, radiotherapy, and chemotherapy. However, despite immunotherapy can induce systemic antitumor immunity, its ablation efficiency against some solid primary tumors is still unsatisfactory. Meanwhile, the efficacy of immunotherapy is also limited by several major obstacles, such as poor monotherapy response rate (~20%) [15], intricate immune signaling pathway and immunosuppressive microenvironment [16], and broad target-associated side effects [17]. It is worth mentioning that the nanobiotechnology has opened up new opportunities to develop novel immunotherapy strategies to overcome these deficiencies, thereby ablating primary tumors and preventing distal metastasis.
In phototherapy, the utilization of light absorption to achieve ablation of cancer is the core concept of this treatment modality, which mainly consists of photothermal therapy (PTT) and photodynamic therapy (PDT) [18]. In this therapeutic strategy, PTT agents can capture near-infrared (NIR) laser irradiation and then convert it into heat, thereby increasing the local temperature of the tumor microenvironment (TME) to destroy the cancer cells [19]. Similarly, when non-toxic PDT photosensitizers (PSs) are enriched at the tumor site and irradiated by a specific wavelength of the laser, the PSs can undergo a series of photochemical reactions involving oxygen at the TME, followed by instantaneous production of large amounts of cytotoxic reactive oxygen species (ROS) to destroy the tumor cells. In general, PTT and PDT can effectively induce cell necrosis and apoptosis. Moreover, with further understanding of phototherapy, it is now realized that one of the more important phenomena produced by phototherapy is immunogenic cell death (ICD), which releases tumor-associated antigens (TAAs) to elicit a positive antitumor immune response. Of note, with additional spatial allostery of original tumor-associated epitopes, these "in situ vaccines" induce the transformation of "cold" TME to "hot" immunogenic TME. Meanwhile, these epitopes are recognized by antibodies and complements generated in vivo and trigger the proliferation and differentiation of specific cytotoxic T lymphocytes (CTLs), activating the host immune system and enhancing cellular immunity [20]. Thus, combining phototherapy with immunotherapy may be an opportunity to improve antitumor efficacy.
Although photoimmunotherapy has great potential advantages in cancer treatment, many small-molecule phototherapeutic agents are rapidly metabolized after systemic administration, making it difficult to achieve the desired therapeutic effect at the tumor site. Therefore, the proper design of advanced phototherapeutic agent delivery systems or phototherapeutic nano biomaterials may be necessary to provide efficient cancer photoimmunotherapy [21]. Currently, the rapid development of nano-drug delivery systems has demonstrated usefulness in combined theranostics against cancer because of the several benefits of nanoarchitecture [22-26]. For photoimmunotherapy, various micro/nano biomaterials have been constructed as PSs to enhance the efficiency of phototherapy and as carriers to deliver immunoadjuvants as well as amplify the phototherapy-stimulated immune response [27]. Furthermore, multifunctional biomaterials can deliver immunotherapeutic elements to remodel the tumor immune microenvironment and facilitate the encounter of immune cells with malignant cells, antigens, stimulators, and other immune cells [28]. Thus, nanocomplexes have played an important role in cancer theranostics [29-32], especially in photo-induced immunomodulation-related tumor treatment.
In this review, we focus on the construction of nanocomplexes and their mechanisms along with the classification of biomaterials for maximizing phototherapeutic efficiency and inducing immune responses. Furthermore, the recent advances in biomaterial-based photoimmunotherapy for improving the efficiency of cancer theranostics have been illustrated. Finally, the clinical challenges in biomaterial-assisted photoimmunotherapy have been discussed to promote a better understanding of this field and its promising future.
2. Photothermal immunotherapy for cancer managementPTT is a local thermal ablation strategy that employs photothermal agents (PTAs) to convert light energy to thermal energy, thereby increasing the tumor focal temperature to kill cancer cells [33]. An ideal PTA for safe and efficient PTT therapy should have the following characteristics: exhibiting a strong absorption in the tissue-transparent window (750–1350 nm), having a high photothermal conversion efficiency, specific identification and predisposition enrichment at tumor sites. Generally speaking, PTAs are divided into inorganic materials and organic materials. The inorganic biomaterials mainly include noble metal materials [34], transition metal chalcogenides [35], carbon-based nanomaterials [36], quantum dots (QDs) (Fig. 1) [37], and other two-dimensional (2D) material systems [38]. In parallel, the organic PTAs mainly consist of NIR-sensitive organic small molecules [39] and semiconducting polymer nanoparticles [40].

|
Download:
|
| Fig. 1. (A) Precise and controllable preparation of clinically transformable Ag2S@human serum albumin nanodots. (B) Schematic description of nanodots for NIR-Ⅱ fluorescence/photoacoustic imaging and PTT in vivo. (C) In vivo NIR-Ⅱ fluorescence imaging in 4T1 tumor-bearing mice after injection of Ag2S nanodots with Ag dose of 50.0 µmol/kg for 48 h. (D) The calculated intensity corresponding to C. (E) In vivo photoacoustic imaging (PAI) in 4T1 tumor-bearing mice after injection of Ag2S nanodots with Ag dose of 50.0 µmol/kg for 24 h. (F) The calculated intensity corresponding to E. (G, H) Infrared thermography (G) and temperature elevations (H) of tumors in 4T1 tumor-bearing mice treated with different doses of Ag2S nanodots during 5 min irradiation (785 nm, 1.5 W/cm2). (I) Tumor growth curves of 4T1 tumor-bearing mice treated with Ag2S nanodots with or without 5 min irradiation (785 nm, 1.5 W/cm2). (J) Digital images of mice tumors at 30 days after irradiation. (K) Blood alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase and urea levels of mice treated with Ag2S nanodots with Ag dose of 50.0 µmol/kg at different times. Reproduced with permission [37]. Copyright 2017, American Chemical Society. | |
Previous studies have shown that PTT-induced dying tumor cells can in situ release TAAs, self-antigens, various immunogenic intracellular substances such as damage-associated molecular patterns (DAMPs), the exposure of calreticulin on the surface of cancer cells, and pro-inflammatory cytokines including tumor necrosis factor α (TNF-α), interleukin (IL)−1β, IL-6, IL-8, and IL-10, thus activating the immune system and triggering the antitumor immune effect [41]. Despite PTT treatment releases many immunostimulatory agents, nearly all investigations have indicated that phototherapeutic agents could not inhibit the growth of distant tumors and sometimes fail to decrease the primary tumor itself. Thus, this immune response needs to be amplified by the introduction of various immunoadjuvants and provides sufficient evidence for the synergism of PTT with other immunotherapies such as immune checkpoint blockade (ICB), chimeric antigen receptor T cell therapy, and cytokine therapy to completely improve the anticancer outcomes. Given this widely recognized importance and developmental momentum, photothermal immunotherapy for cancer will be discussed according to the material characteristics of photoabsorbers.
2.1. Inorganic nanoparticle-mediated photothermal immunotherapy 2.1.1. Noble metal nanomaterial-based photothermal immunotherapyNoble metal materials, such as gold (Au), silver (Ag), platinum (Pt) and palladium (Pd), are the most widely studied elements in inorganic photothermal therapeutics. Ever since Au NPs were first reported as a PTA in 2003, scientists have devoted great efforts to the structure casting of Au nanomaterials and their applications in cancer PTT [42,43]. In addition, nano-Au can induce notable photothermal immune responses, effectively carry immunotherapeutic components, and produce a long-lasting antitumor vaccine effect [44]. For instance, Zhou et al. [45] designed bovine serum albumin (BSA)-bioinspired Au nanorods loaded with immunoadjuvant imiquimod (R837) via electrostatic adsorption to highly kill B16F10 melanoma cells in vitro and in vivo and stimulate the secretion of immune-related cytokines such as TNF-α, IL-6, and IL-12 due to the involvement of R837. Tang et al. [46] developed a photothermal genome-editing technology to target PD-L1 and converted the second NIR window (NIR-Ⅱ) light into mild hyperthermia to stimulate ICD and gene expression of Cas9 to reduce PD-L1 expression under NIR irradiation. To overcome the frequent administration in clinical practice of PD-1 therapy, the anti-PD-1 peptide (APP) and hollow Au nanoshells were co-encapsulated into biodegradable poly(lactic-co-glycolic acid) (PLGA) NPs. The obtained nanocomplex exhibited robust photothermal effect under NIR irradiation and sustained release of APP and long-acting anti-PD-1 immunotherapy to concurrently eliminate distal neoplastic foci [47]. Luo et al. [48] conjugated the thiolated cytosine-phosphate-guanine (CpG) on the surface of the hollow Au nanospheres (HGNs) (named CpG-HGN). The increased enrichment of CpG-HGN in RAW 264.7 cells could greatly stimulate immune activity by secreting more pro-inflammatory cytokines and differentiating myeloid-derived suppressor cells (MDSC) into macrophages with antitumor activity directly or indirectly. Additionally, the size-dependent absorption wavelength variation of Au NPs and their elicited immune response were compared, and the underlying mechanisms were analyzed (Fig. 2) [49].

|
Download:
|
| Fig. 2. Near-infrared Ⅱ PTT-induced immunogenic cell death stimulates innate and adaptive antitumor immune response and further potentiates PD-1/PD-L1 immunoblockade for inhibiting tumor growth and metastasis. Reproduced with permission [49]. Copyright 2019, American Chemical Society. | |
Ag nanostructures, especially nanoprisms, also serve as potential nanoplatforms for PTT because of their several inherent advantages such as strong localized surface plasmon resonance (LSPR) peak in the NIR region, excellent photothermal conversion efficiency (PCE) and X-ray attenuation [50]. For instance, Ag-polydopamine (PDA) core-shell nanoprism equipped with doxorubicin (DOX) and arginine-glycine-aspartic acid peptide (RGD) were presented for CT/photoacoustic (PA)/ultrasonic/infrared thermal quadruple-modal imaging-mediated targeted photochemotherapy of cancer. This therapeutic strategy promoted the migration of DCs to lymph nodes and stimulated their maturation by presenting the major histocompatibility complex (MHC), which provides the feasibility of implementing photochemoimmunotherapy in combination with anti-PD-1 therapy to inhibit breast cancer growth and prevent its metastasis and relapse [51].
Pt nanostructures can absorb light in the biological range and convert the electromagnetic energy into hyperthermia for PTT of various cancers [52]. In an example by Yang et al. [53], coupled Au@Pt NPs and LMDP antagonist peptide of PD-L1 to generate matrix metalloproteinase-2 (MMP-2)-responsive multifunctional nanotherapeutics (Au@Pt-LMDP) that can be actively enriched in the tumor region and effectively eliminated primary breast cancer via NIR-excited PTT. Simultaneously, a substantial antitumor immune response was elicited, characterized by remarkably enhanced tumor infiltration of CD8+ T cells and CD4+ T cells, reduced regulatory T cells (Tregs), increased secretion of pro-inflammatory cytokines, and decreased anti-inflammatory cytokine IL-10.
Pd nanostructures are a class of nontoxic cell-permeable plasmonic PSs that absorb NIR radiation and induce hyperthermia to evoke PTT-induced immune responses to locally eliminate labeled cancer cells [54]. In a previous study, Pd-DOX NPs were proposed to perform photochemotherapeutic killing of colorectal cancer (CRC). This synergistic effect enhanced the ICD efficiency by inducing the release of DAMPs while boosting DCs maturation and CTLs infiltration. Moreover, the combination of anti-PD-L1 antibodies promoted the immune response, reversed the immunosuppressive TME, and eliminated the primary and abscopal lung metastasis [55].
2.1.2. Metal chalcogenide nanomaterial-based photothermal immunotherapyIn the family of metal oxides, iron oxide nanoparticles (IONPs) are considered as distinctive and appropriate PTAs with both magnetism and NIR absorption properties. Based on the excellent photothermal activity and intrinsic immunogenicity, superparamagnetic IONPs with different geometries and chemical compositions have been used for PTT-induced immune activation in cancer. Typically, superparamagnetic IONPs were co-loaded with CpG oligodeoxynucleotides (ODNs) into immunostimulator nanosystems for PA/magnetic resonance imaging (MRI)-guided tumor thermal ablation and autovaccine generation under NIR irradiation [56]. Furthermore, Zhang et al. [57] designed a core-shell nanointegrations containing IONPs, PD-1 antibody and perfluoropentane, which induced photothermal immunotherapy against melanoma through phase-transformation-enhanced antibody release and increased CD8+ T cell infiltration into tumors.
Other promising binary photoabsorbers, such as Ag2S, have also shown satisfactory efficacy in PTT-induced TAA release and synergistically enhanced immunotherapies. In an example by Han and co-workers [58], the size of Ag2S NPs was modulated to screen their NIR-Ⅱ/PAI ability and PTT efficacy. The optimized nanosystem induced tumor necrosis and completely removed primary breast cancer through multiple PTT cycles and their produced in situ vaccine effect to promote the maturation and differentiation of DCs. In another case, Hou et al. [59] designed a polypeptide-based injectable hydrogel (Ag2S QD/DOX/Bestatin@PC10ARGD) with a high PCE of 28.7%. This nanohydrogel demonstrated strong photochemotherapeutic activity and immune enhancement effect correlated with the increase of costimulatory factors, such as CD80, CD86 and MHC class Ⅱ. It could effectively inhibit lung metastasis and recurrence of breast cancer by a photochemoimmunosynergy.
In addition, other metallic chalcogenide-based nanoplatforms, such as ovalbumin and copper sulfide (CuS) co-loaded PLGA NPs [60], maleimide PEG-modified CuS NPs [61], CuS NP-encapsulated mesoporous silica nanoparticles (MSNs) camouflaged by homogeneous tumor cell membranes (Fig. 3) [62], core-shell CuS@MSN-PFP (pentafluorophenyl ester)-PEG nanocomposites [63], CuS@MSN-PDMAEMA (poly(2-(dimethylamino)ethyl methacrylate)) polycation [64], FePt NPs, CpG ODNs and folic acid-anchored molybdenum sulfide (MoS2) nanosheets loaded with CTLA-4 antibody [65], bioinspired red blood cell-camouflaged 2D molybdenum selenide (MoSe2) nanosheets [66], PEG-modified bismuth selenide (Bi2Se3) nanocages loaded with immunoadjuvant imiquimod and PD-L1 antibody [67], have also been shown to play an important role in PTT-induced immune remodeling and synergistic cancer inhibition.

|
Download:
|
| Fig. 3. The preparation (A) and synergistic effects (B) of homogeneous tumor cell membrane-camouflaged CuS NP-encapsulated MSNs in PTT combined with immune remodeling against triple negative breast cancer. Reproduced with permission [62]. Copyright 2020, American Chemical Society. | |
Carbon nanomaterials, such as graphenes, graphene oxides (GOs), carbon nanotubes (CNTs) and carbon nanodots (CDs), have attracted considerable attention for their application in PTT-involved immunotherapy because of the excellent optical absorption and photothermal effects. Graphene, a 2D nanomaterial composed of carbon atoms forming a one-atom-thick honeycomb lattice, has a strong light absorption capacity in the NIR region and can convert laser energy into hyperthermia through plasma photothermal effect [68]. Recently, considering the unique 2D nanogeometry and photothertapy of graphene, especially the achievements of immunotherapy in assisting the removal of phototherapy-triggered residual lesions, graphene-guided photothermal immunotherapy has gained increasing attention. For instance, Yan et al. [69] designed a multifunctional indoleamine 2, 3-dioxygenase (IDO) inhibitor (IDOi)-loaded reduced GO (IDOi/rGO) nanosheets that could directly destroy tumor cells under NIR irradiation while stimulated immune response, such as the excessive release of cytokines of interferon-gamma (IFN-γ), enhanced the intratumoral infiltration of lymphocytes including CD45+ leukocytes, CD4+ T cells, CD8+ T cells and natural killer (NK) cells, as well as effective inhibition of the immunosuppressive activity of Tregs. In parallel, rGO was selected to co-carry mitoxantrone and transforming growth factor-beta (TGF-β) inhibitor. The prepared nanohybrids coupled with laser irradiation afforded a synergistic chemo-immuno-photothermal effect against metastatic triple-negative breast cancer (TNBC) through in situ vaccination [70]. In another study, Wang et al. [71] developed a PEGylated Fe3O4-rGO electrostatically connected nanoassembly that could enhance the activity of the host antitumor immune response efficiently in metastatic breast cancer by reducing tumor-associated macrophages, eliciting danger-associated cytokines by PTT induced ICD, and activating DCs in lymph nodes.
As another carbon-based one-dimensional nanomaterial, CNT possesses excellent light absorption, cargo loading, and remarkable photothermal ability, has been widely utilized for thermal therapy of cancer [72]. For example, single-walled CNTs (SWNTs) modified with immunoadjuvant glycated chitosan (GC) were capable of local photocautery of primary breast cancer under NIR irradiation and induced in situ autologous vaccinization effects. Meanwhile, this provoked systemic antitumor immunity can be further amplified by GC and coordinated with anti-CTLA-4 antibody to resist lung metastasis and extend the subjects' overall survival [73]. Using the same 4T1 cells and BALB/c tumor-bearing mice, another team by Wang et al. [74] obtained analogous success in preventing lung metastasis of breast cancer and improving survival time of animals via an immunologic photothermal agent comprising of PEGylated SWNTs with anti-CTLA-4 antibody therapy. In parallel, another carbon structure such as biodegradable CDs-incorporated MSNs was developed and concentrated in tumors for enhanced PTT effects. The degraded nanodebris were able to in situ stimulate TAAs to activate NK cells and macrophages for photothermal-immunotherapy collaboration [75].
MXenes are another rapidly emerging class of graphene-like PTAs. Generally, MXenes have a common formula: Mn+1Xn, where M stands for transition metal elements [for example, molybdenum (Mo), titanium (Ti), niobium (Nb), vanadium (V), tantalum (Ta) and zirconium (Zr)] and X stands for carbon (C) or nitrogen (N) [36]. Notably, MXenes have higher electronic conductivity and light absorption capacity than rGO, enabling them to be thoroughly explored in PTT-induced multimodal cancer treatment. Among them, Ti3C2, Ta4C3 and Nb2C have been widely studied for photothermal cancer therapy. Bai et al. [76] constructed a 2D multifunctional nanocomposites (Ti3C2@Met@CP) via layer by layer adsorbing of metformin (Met) and compound polysaccharide (CP) onto Ti3C2 nanosheets. The resulting Ti3C2@Met@CP nanosystems exhibited synergistic efficacy of PTT/PDT/chemotherapy under NIR irradiation and activated host immunity to eradicate breast cancer and prevent tumor metastasis and recurrence. Manganese oxide (MnOx) NPs deposited in situ on Ta4C3 nanosheets and further functionalized by soybean phospholipid (SP) for contrast-enhanced CT/PAI/T1-weighted MRI-visualized photothermal ablation of breast cancer [77]. Similarly, Ta4C3–IONP–SP MXenes nanocomposites were developed for contrast-enhanced CT/T2-weighted MRI-mediated radical eradication of breast cancer and suppression of tumor metastasis and recurrence [78]. In parallel, Lin et al. [79] fabricated 2D polyvinylpyrrolidone-modified Nb2C MXene nanosheets with precisely tunable compositions and excellent PCE and photostability for enzyme-responsive PAI-induced photothermal eradication of breast cancer under both NIR-Ⅰ and NIR-Ⅱ exposure.
2.1.4. Other inorganic nanomaterial-based photothermal immunotherapyApart from the substances listed above, many other inorganic nanomaterials, such as black phosphorus (BP), possess the adjustable bandgap and optical absorption, stable structure and biocompatibility, non-toxic biodegradation process and products after reacting with water and oxygen, and excellent photothermal properties. Inspired by the immunity regulate ability to therapy cancer, a therapeutic nanovaccine was developed by loading BPQDs into the serum exosomes of hyperthermal treated mice, and the nanovaccine could greatly elevate the intratumoral infiltration of T lymphocytes and activate the immune response against lung cancer [80]. In another example, Wan et al. [81] co-loaded PEG-protected BP nanosheets and the immunoadjuvant R837 to achieve photothermally induced in situ generations of TAAs and R837-enhanced immunotherapy outcomes against B16 melanoma.
In addition to the aforementioned nanomaterials, a variety of inorganic nanoplatforms have been widely favored for their usability in photothermal-activated cancer immunotherapy [82-84].
2.2. Organic small molecule nanomaterial-mediated photothermal immunotherapyCurrently, the most widely studied organic PTAs are organic small molecules and semiconductor polymers [85]. Within these, organic small molecule-derived PTAs, mainly including cyanine, porphyrin, phthalocyanine (Pc), boron dipyrromethene (BODIPY) and croconaine, have been widely used for light-induced cancer diagnosis and treatment. As a thriving field, various organic small molecule-based nano-PTAs have been rationally designed for theoretical and clinical PTT-induced cancer immunotherapy owing to their excellent structural variability and bioactivity.
2.2.1. Cyanine-based photothermal immunotherapyCyanine dyes are structurally consisting of two aromatic nitrogen-containing heterocycles conjugated through a polymethine chain, also known as push-pull configuration, and their properties are determined by the number of aromatic rings and length of polyene chains [86]. Notably, such dyes have a flexible molecular structure that allows modification at multiple sites on the carbon backbone to adjust their absorption and emission spectra from visible to NIR region, making them feasible as PTAs. A series of cyanine molecules, including indocyanine green (ICG), cypate, IR-780, IR-820 and IR-825 have been used as key candidates for cancer bioimaging and PTT therapy.
Among these, ICG is the only one approved by the U.S. Food and Drug Administration (FDA) for clinical NIR imaging and as a PTA [87]. Although ICG-mediated PTT could activate the immune system and recruit multiple immune cells, it was not powerful enough to elicit a robust antitumor immune response. Inspired by the ability of combinatorial immunostimulatory molecules to amplify PTT-triggered immunity, Xu et al. [88] developed a thermally responsive nanoliposome system encapsulating ICG and other immune stimulators, polyinosinic: polycytidylic acid for achieving direct photothermal ablation of CRC, melanoma, and the tumor antigen-specific immune clearance of lung metastasis. Chen et al. [89] developed a eukaryotic–prokaryotic vesicles derived from melanoma cytomembrane and attenuated the Salmonella outer membrane. This nanoarchitecture could be used not only as a prophylactic vaccine to against tumorigenesis but also as a therapeutic vaccine in combination with ICG to accelerate the photothermal immune eradication of primary and metastatic melanoma. Similarly, Zhao et al. [90] designed a breast cancer cell membrane core-shell biomimetic nanoparticles with ICG and decitabine that assembled at tumors and triggered chemotherapy and PTT-initiated programmed pyroptosis, leading to further secretion of pro-inflammatory cytokines and activation of antitumor immunity against the primary and distal tumors. In a representative study, in collaboration with PD-1 and mucin domain-containing protein 3 blockade, ICG-liposome nanoassembly demonstrated the complete ablation of CRC metastasis [91].
IR820 is a successfully modified product of ICG that has been widely investigated to mediate potent PTT effects and induce notable immune enhancement. In a previous report, IR820 was encapsulated into polycaprolactone GC: poloxamer nanoblends for photothermal and ROS-mediated phototherapy as well as GC-stimulated immune clearance of hyperthermia-resistant breast cancer [92]. CpG ODNs self-crosslinked NPs loaded IR820-conjugated hydrogel were rationally developed to activate TAA release and immune cell infiltration for dual autofluorescence-driven photothermal immune ablation of refractory melanoma [93]. Similarly, Huang et al. [94] co-encapsulated IR820 and PD-L1 antibody into a thermo-responsive injectable lipid gel. Under laser irradiation, IR820 produced mild hyperthermia and prompted in situ solations of the lipid gel to release the PD-L1 antibody. Meanwhile, the tumor immune microenvironment was remodeled for excellent synergistic efficiency in immunologically "cold" breast cancer and melanoma. Another strategy to achieve cold to hot immunity in TME was to integrate IR820 and an IDO inhibitor, 1-methyl-tryptophan (1MT), into a nanocomplex, which considerably reversed immune inertia of some breast cancers and melanomas and prevented tumor metastasis and recurrence [95]. Some more versatile nanohybrids, such as MMP-2-responsive IR820-docetaxel-d-peptide nanotherapeutics [96] and IR820-R837-1MT spatiotemporally cooperated zeolitic imidazolate framework-8-based metal-organic framework (MOF) NPs [97], have also been designed for photothermally induced immune synergy.
Briefly, cyanine-based nanointegration has shown promising therapeutic outcomes in PTT-induced systemic antitumor immunity. Nonetheless, some fundamental limitations, such as maintaining the balance between optical properties and solubility, as well as limited stability and tumor specificity, require further attention.
2.2.2. Other small molecule-based photothermal immunotherapyTypically, methylene blue with maximum absorption at 660 nm and Toll-like-receptor 7 agonist R837 were encapsulated into naturally sourced collagen/alginate hydrogels. The nanohybrids photothermally ablated primary breast cancer under laser irradiation and released TAAs, which were immunologically enhanced by R837 to suppress tumor metastasis and recurrence [98].
Some researchers have explored the potential of porphyrin and its derivatives, such as chlorins and bacteriochlorins, as PTAs in recent years. In an exploratory study, enzymatically biodegradable porphysomes were developed by self-assembling porphyrin bilayers. These nanosystems demonstrated remarkable photothermal efficiency and comparable efficacy to Au NPs in FI/PAI dual-mode imaging-tracked photothermal ablation of human oral epidermal carcinoma [99]. The further comparison revealed that porphysome NPs could overcome the hypoxic TME and showed superior photothermal ability to eliminate tumors and extended the overall survival in experimental animals when compared with their PDT effects [100].
Excellent light-absorption and photostable properties have prompted researchers to explore the photothermal potential of Pc and its derivatives. For instance, Pc-based polymer micelles with broadened NIR absorption were synthesized and demonstrated to have high PCE and good photothermal suppression of TNBC [101]. In one study, Yu et al. [102] designed BSA-protected zinc Pc (ZnPc) and sorafenib nanocomposites for PTT/PDT/chemotherapy of orthotopic hepatocellular carcinoma (HCC). In another study, Li et al. [103] further developed several Pc derivatives for photothermal ablation of HCC and revealed that the photothermal potential of Pc-based PSs can be more favorably investigated through modifying molecular structures rather than forming aggregates.
In another nanoarchitecture, reduced BSA-encapsulated heptacyclic B, O-chelated BODIPY nanostructures showed a high NIR PCE of 58.7% and demonstrated potent photothermal ablation of TNBC without tumor metastasis or recurrence [104]. To further enhance PCE, Xi et al. [105] introduced CF3 "barrier-free" rotating components at the meso‑position of BODIPY structure to synthesize an effective PTA with up to 88.3% of PCE and explored its photothermal anticancer potency. Moreover, croconaine dye-based nanoPSs, such as PEG-modified CR780 [106], PEG-linked CR760 and αvβ3 integrin ligand [107] were also exquisitely designed for PA/NIR FI-mediated PTT and active targeting-enhanced PAI-guided photothermal treatment of breast cancer, respectively.
In brief, thanks to the excellent structural variability and biological functionality, organic nanoPSs based on various materials and construction strategies have achieved valuable performance in the theoretical and clinical research of photothermal immunity against cancer. Continuously increasing results will not be described here individually [108-110].
2.3. Polymer nanomaterial-mediated photothermal immunotherapyCurrently, the most popular types of polymer PTAs are polyaniline (PANI), polypyrrole (PPy), PDA, poly(3, 4-ethylenedioxythiophene)-poly(styrenesulfonate) and other conjugated polymers. Tian et al. [111] constructed a pH-driven BSA-PANI nanoassembly for amplified PAI imaging and enhanced PTT therapy in acidic TME, through acid-base interaction between BSA and PANI. Liu et al. [112] further synthesized the photothermal agent poly(p-phenylenediamine) which exhibited efficient photothermal conversion and TAA induction. Under the coordination of anti-PD-L1 ICB, the nanocomplexes exerted a PTT/PDT/chemodynamic therapy/immunotherapy synergistic inhibition of CRC growth and metastasis (Fig. 4).
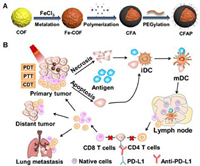
|
Download:
|
| Fig. 4. (A) Construction of multifunctional covalent organic framework-based nanodrugs and (B) their application in PTT/PDT/chemodynamic therapy-synergized immunotherapy to inhibit tumor growth and metastasis. Reproduced with permission [112]. Copyright 2020, American Chemical Society. | |
Given the excellent optical properties, conductivity, and biocompatibility of PPy, Sun et al. [113] designed a nanocomposite with PPy as the core and a NIR dye IRDye800CW and camptothecin-conjugated hyaluronic acid (HA) as the shell. Under 808 nm laser, the PPy-induced photothermal effect promoted camptothecin release and exhibited chemo-PTT-sensitized enhancement and intratumoral infiltration of CD8+ T cells and concurrently suppressed CD4+, CD25+ tumor-infiltrating Tregs, and CD11b+ Gr-1+ MDSCs for chemo-photothermal-immunotherapy against TNBC. To improve PD-L1 crackdown in TNBC, a bromodomain and extra-terminal inhibitor, JQ1, was loaded into PDA NPs. The self-degradability of nanoagents enabled them to steadily release JQ1 while exhibiting strong molecular targeting efficacy, PTT-enhanced CTL presentation, and immune memory generation [114]. Creatively, microfluidic technology was adopted to develop uniformly controlled cRGD-decorated conjugated polymer NPs with appearance-dependent PTT efficacy and its induced pro-inflammatory immune activation against cancer [115]. In addition, a variety of other polymer photothermal reagents have also been nanostructured and used for synergistic immune ablation of malignant tumors [116].
Briefly, polymer-based photothermal agents have multiple advantages, such as easy modification, good biocompatibility, self-carrier capacity, and universal nanocrystallization. Nevertheless, the relatively complex preparation, unmanageable uniformity, and ambiguous photothermal-immune response relationship hinder their theoretical and applied research, which need to be addressed to facilitate their progress in PTT-immunotherapy synergistic anticancer.
2.4. Other biomaterial-mediated photothermal immunotherapyOther chemical or biological materials capable of photothermal transformation have also been developed for immune-enhancing cancer treatment [117]. To overcome the imprecision of PTT and the heat tolerance of cancer cells, a novel 2D Mn-coordinated tetrahydroxyanthraquinone was constructed for T1-MRI-traced PTT. In collaboration with HCC-specific aptamer-modified NK cells, the residual microlesions after PTT were thoroughly cleared, and tumor recurrence was blocked [118]. Some naturally sourced photothermal substances, such as cuttlefish ink extract NPs that are rich in melanin, were developed as photothermal-induced immunomodulators to achieve photothermal-enhanced immune clearance of CRC [119]. Without PS, 980 nm laser irradiation for 2 h following intratumoral injection of immunostimulant N-dihydrogalactochitosan resulted in the PTT-enhanced tumor ablation and neutrophil infiltration for effectively preventing melanoma lung metastasis by raising tumor-infiltrating lymphocytes [120].
2.5. Clinical progress of photothermal immunotherapyIn a preliminary clinical study for the treatment of breast cancer patients in South America, ICG was used as a photosensitizer and N-dihydrogalactochitosan as an immunoadjuvant. The treatment was administered every 4 weeks in a cycle of 4 times. Physical examination and biochemical tests were performed to assess therapeutic toxicity following photothermal immunotherapy in 10 patients with advanced breast cancer for which no other treatment options were available. The results showed no grade 3 or 4 side effects other than photothermal damage and local injection of the immunoadjuvant to the treated area. The objective response rate was 62.5% and the clinically beneficial response rate was 75% in the eight patients available for assessment [121]. Since Naylor et al. [122] initiated in situ photoimmunotherapy treatment of two melanoma patients in 2006, several clinical cases of photothermal immunoablation of metastatic melanoma with poor prognosis have been reported. A previous study had shown that in situ photoimmunotherapy combined with a checkpoint inhibitor ipilimumab was feasible and effective in the treatment of patients with advanced metastatic melanoma. After combination therapy, all lung metastases were reduced and eventually disappeared, and the patients were tumor-free for 7 years [123]. In another clinical practice, PTT combined with Dinitrophenyl hapten observably increased interferon-γ secretion and decreased IL-10, TGF-β1 and TGF-β2 production in patients with malignant melanoma, resulting in a significant extension of overall and disease-free survival [124].
In a word, PTT is an effective anticancer modality for the rapid development of the synergistic effect of PTT and immunotherapy in the anticancer paradigm. To maximize the synergistic effect, some constraints need to be addressed. For instance, the design, preparation, photothermal properties, function assignment, biocompatibility and toxicity of new photothermal materials are the primary factors to be considered. The types and quantification of photothermally induced antitumor immune markers must be identified. Moreover, the influence of patient constitution and tumor type on antitumor immune response must be analyzed and summarized.
3. Photodynamic immunotherapy for cancer managementPDT is a spatiotemporal selective, minimally invasive, and FDA-approved drug-device combination therapeutic procedure towards various types of malignant tumors [125]. Recent advances in photodynamic immune effects and nanotechnology-assisted synergies have been systematically analyzed based on the classification of PDT material categories, see Supporting information.
4. Other photoimmunotherapy for cancer managementOther photoimmunotherapeutic approaches focus on the construction of PS-antibody photo-immunoconjugates and their nanocrystallization for cancer diagnosis and treatment. This is beyond the scope of this review and will not be explored in detail.
5. Concluding remarks and future perspectivesPhototherapy, including PTT and PDT, is a non-invasive and effective light-activated strategy for local ablation of malignancies by causing an ICD effect. This highlights the rationality and necessity of immunotherapy to improve the efficiency of phototherapy and prevent metastasis. Immunotherapy is a revolutionary and extremely important therapeutic approach in recent years and has gained promising clinical progress. The vigorous development of nanotheranostic technology and the strong demand for tumor eradication through precision medicine have further promoted researchers to explore photo-induced immunological mechanisms and the efficacy of photoimmunotherapy for stubborn malignant tumors. In this review, phototherapy-synergized immunoenhancement and anticancer performance have been explored according to the classification of PSs based on material properties.
Despite these breakthroughs, the following are the major limitations of clinical advances in photoimmunotherapy: (1) difficulties in predicting and quantifying photo-induced immune response caused by tumor heterogeneity; (2) the unclear mechanism of phototherapy-triggered immune response and host tolerance to immunotherapy; (3) the lack of optimized and personalized clinical protocol design to eliminate the immunosuppressive factors in TME and target CTLs to the tumor site to assess efficacy; (4) obstacles in component screening and integration, performance optimization, and large-scale synthesis of nanotheranostic probes; (5) the availability of simple current tumor models to simulate the complex human TME make it impossible to replicate some of the interesting animal results in humans; (6) the stability, in vivo distribution, pharmacokinetics, biotoxicity, and safety of nanocomposites must be carefully evaluated; and (7) the lack of industrial standards for cancer immunotherapy and theranostic synergy. Therefore, further study requires unremitting efforts to explore the intervention mechanism of phototherapy on the host immune system, construct sophisticated and clinically translatable nanohybrids to validate their efficacy in patient-derived tumor models and develop professional standards for photoimmune nanotheranostics of cancer through scientific and reasonable experimental results.
In conclusion, the advantages of phototherapy, including simple procedures and effective ablation, make it valuable in the local and exclusive removal of tumors. Moreover, phototherapy can improve the host's immune response by interfering with the TME, thus making it feasible for use in combination with immunotherapy, an emerging and revolutionary anticancer paradigm. The explosion of bionanotechnology and the potential of personalized precision medicine have yielded notable outcomes in fundamental research and clinical practice, thus creating an urgency to construct photo-induced immunotherapeutic nanocomplexes. However, some obstacles such as the imprecise internal mechanism of phototherapy in interfering with the immune system, complex material design, industrialization process, potential biosafety issues of nanoprobes and their interaction pathway with organisms, and differences between experimental models and clinical cases impede the widespread application of nanosystems. Therefore, overcoming these challenges through multidisciplinary approaches will accelerate the rapid development of photoimmunotherapy and pave the way for creating a new generation of precise cancer theranostics.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81903662, 51903201), the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0428), the Natural Science Foundation of Shaanxi Province (No. 2023-YBSF-270), and the Startup Foundation for Doctors of Shanxi Medical University (No. XD1824).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2023.108180.
| [1] |
C. Fitzmaurice, C. Allen, R.M. Barber, et al., JAMA Oncol. 3 (2017) 524-548. DOI:10.1001/jamaoncol.2016.5688 |
| [2] |
Y. Li, Y. Cheng, M. Zhang, et al., iScience 23 (2020) 101254. DOI:10.1016/j.isci.2020.101254 |
| [3] |
Y. Li, Q. Dong, Y. Cui, Cancer Biol. Med. 16 (2019) 415-434. DOI:10.20892/j.issn.2095-3941.2019.0137 |
| [4] |
L. Liu, X. Du, Chin. Chem. Lett. 32 (2021) 1942-1946. DOI:10.1016/j.cclet.2020.12.061 |
| [5] |
Y. Li, H. Zhang, Nanomedicine 14 (2019) 1493-1512. DOI:10.2217/nnm-2018-0346 |
| [6] |
Y. Dai, Y. Ding, L. Li, Chin. Chem. Lett. 32 (2021) 2715-2728. DOI:10.1016/j.cclet.2021.03.036 |
| [7] |
F. An, Z. Yang, M. Zheng, et al., J. Nanobiotechnol. 18 (2020) 49. DOI:10.1186/s12951-020-00603-8 |
| [8] |
H. Liu, Z. Miao, Z. Zha, Chin. Chem. Lett. 33 (2022) 1673-1680. DOI:10.1016/j.cclet.2021.10.057 |
| [9] |
D.M. Altmann, Immunology 155 (2018) 283-284. DOI:10.1111/imm.13008 |
| [10] |
J. Wang, L. Zhuo, P. Zhao, et al., Chin. Chem. Lett. 33 (2022) 3502-3506. DOI:10.1016/j.cclet.2022.03.056 |
| [11] |
E.C. Lavelle, R.W. Ward, Nat. Rev. Immunol. 22 (2022) 236-250. DOI:10.1038/s41577-021-00583-2 |
| [12] |
H. Lin, H. Hong, L. Feng, Chin. Chem. Lett. 32 (2021) 4041-4044. DOI:10.1016/j.cclet.2021.04.034 |
| [13] |
C.H. June, R.S. O'Connor, O.U. Kawalekar, et al., Science 359 (2018) 1361-1365. DOI:10.1126/science.aar6711 |
| [14] |
A. Melcher, K. Harrington, R. Vile, Science 374 (2021) 1325-1326. DOI:10.1126/science.abk3436 |
| [15] |
J.D. Martin, H. Cabral, T. Stylianopoulos, R.K. Jain, Nat. Rev. Clin. Oncol. 17 (2020) 251-266. DOI:10.1038/s41571-019-0308-z |
| [16] |
G.L. Beatty, W.L. Gladney, Clin. Cancer Res. 21 (2015) 687-692. DOI:10.1158/1078-0432.CCR-14-1860 |
| [17] |
W. Sang, Z. Zhang, Y. Dai, X. Chen, Chem. Soc. Rev. 48 (2019) 3771-3810. DOI:10.1039/c8cs00896e |
| [18] |
S. Gai, G. Yang, P. Yang, et al., Nano Today 19 (2018) 146-187. DOI:10.1016/j.nantod.2018.02.010 |
| [19] |
S.G. Alamdari, M. Amini, N. Jalilzadeh, et al., J. Control. Release 349 (2022) 269-303. DOI:10.1016/j.jconrel.2022.06.050 |
| [20] |
T. Wang, D. Wang, H. Yu, et al., Nat. Commun. 9 (2018) 1532. DOI:10.1038/s41467-018-03915-4 |
| [21] |
W. Fan, B. Yung, P. Huang, X. Chen, Chem. Rev. 117 (2017) 13566-13638. DOI:10.1021/acs.chemrev.7b00258 |
| [22] |
Y. Li, C. Zhang, G. Li, et al., Acta Pharm. Sin. B 11 (2021) 2220-2242. DOI:10.1016/j.apsb.2021.01.017 |
| [23] |
Y. Li, L. Du, C. Wu, et al., Curr. Top. Med. Chem. 19 (2019) 74-97. DOI:10.2174/1568026619666190125144621 |
| [24] |
Y. Li, J. Xin, Y. Sun, et al., Cancer Biol. Med. 17 (2020) 307-327. DOI:10.20892/j.issn.2095-3941.2020.0072 |
| [25] |
Y. Li, H. Zhang, J. Biomed. Nanotechnol. 15 (2019) 1-27. DOI:10.1166/jbn.2019.2670 |
| [26] |
Y. Li, T. Mei, S. Han, et al., Chin. Chem. Lett. 31 (2020) 3027-3040. DOI:10.1016/j.cclet.2020.05.027 |
| [27] |
Q. Chen, M. Chen, Z. Liu, Chem. Soc. Rev. 48 (2019) 5506-5526. DOI:10.1039/c9cs00271e |
| [28] |
R. Guo, S. Wang, L. Zhao, et al., Biomaterials 282 (2022) 121425. DOI:10.1016/j.biomaterials.2022.121425 |
| [29] |
Y. Li, Q. Dong, T. Mei, et al., Curr. Drug Targets 21 (2020) 228-251. DOI:10.2174/1389450120666190807143245 |
| [30] |
Y. Li, Y. Chang, X. Lian, et al., J. Biomed. Nanotechnol. 14 (2018) 1515-1542. DOI:10.1166/jbn.2018.2614 |
| [31] |
Y. Li, Y. Yang, F. An, et al., Nanotechnology 24 (2013) 015103. DOI:10.1088/0957-4484/24/1/015103 |
| [32] |
J. Zhang, Y. Li, F.F. An, et al., Nano Lett. 15 (2015) 313-318. DOI:10.1021/nl503598u |
| [33] |
Q. Chen, J. Chen, Z. Yang, et al., Adv. Mater. 31 (2019) 1802228. DOI:10.1002/adma.201802228 |
| [34] |
C. Li, Y. Zhang, Z. Li, et al., Adv. Mater. 30 (2018) 1706150. DOI:10.1002/adma.201706150 |
| [35] |
M. Saeed, M.Z. Iqbal, W. Ren, et al., J. Mater. Chem. B 6 (2018) 3800-3810. DOI:10.1039/C8TB00745D |
| [36] |
H. Liu, L. Mo, H. Chen, et al., Adv. Healthc. Mater. 11 (2022) e2101448. DOI:10.1002/adhm.202101448 |
| [37] |
T. Yang, Y. Tang, L. Liu, et al., ACS Nano 11 (2017) 1848-1857. DOI:10.1021/acsnano.6b07866 |
| [38] |
K. Huang, Z. Li, J. Lin, G. Han, P. Huang, Chem. Soc. Rev. 47 (2018) 5109-5124. DOI:10.1039/C7CS00838D |
| [39] |
M.A. Rajora, J.W.H. Lou, G. Zheng, Chem. Soc. Rev. 46 (2017) 6433-6469. DOI:10.1039/C7CS00525C |
| [40] |
J. Shao, C. Ruan, H. Xie, et al., Adv. Sci. (Weinh) 5 (2018) 1700848. DOI:10.1002/advs.201700848 |
| [41] |
S. Toraya-Brown, M.R. Sheen, P. Zhang, et al., Nanomedicine 10 (2014) 1273-1285. DOI:10.1016/j.nano.2014.01.011 |
| [42] |
W. Tao, X. Cheng, D. Sun, et al., Biomaterials 287 (2022) 121621. DOI:10.1016/j.biomaterials.2022.121621 |
| [43] |
L.R. Hirsch, R.J. Stafford, J.A. Bankson, et al., Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 13549-13554. DOI:10.1073/pnas.2232479100 |
| [44] |
Y. Liu, B.M. Crawford, T. Vo-Dinh, Immunotherapy 10 (2018) 1175-1188. DOI:10.2217/imt-2018-0029 |
| [45] |
B. Zhou, J. Song, M. Wang, et al., Nanoscale 10 (2018) 21640-21647. DOI:10.1039/c8nr05323e |
| [46] |
H. Tang, X. Xu, Y. Chen, et al., Adv. Mater. 33 (2021) 2006003. DOI:10.1002/adma.202006003 |
| [47] |
L. Luo, J. Yang, C. Zhu, et al., J. Control. Release 278 (2018) 87-99. DOI:10.1016/j.jconrel.2018.04.002 |
| [48] |
J. Luo, Y. Cheng, X.Y. He, et al., Colloids Surf. B: Biointerfaces 175 (2019) 248-255. DOI:10.1016/j.colsurfb.2018.12.001 |
| [49] |
Y. Ma, Y. Zhang, X. Li, et al., ACS Nano 13 (2019) 11967-11980. DOI:10.1021/acsnano.9b06040 |
| [50] |
X. Bi, Q. Bai, M. Liang, et al., Small 18 (2022) e2104160. DOI:10.1002/smll.202104160 |
| [51] |
X. Zeng, S. Yan, C. Di, et al., ACS Appl. Mater. Interfaces 12 (2020) 11329-11340. DOI:10.1021/acsami.9b21166 |
| [52] |
E. Spyratou, M. Makropoulou, E.P. Efstathopoulos, et al., Cancers 9 (2017) 173. DOI:10.3390/cancers9120173 |
| [53] |
Q. Yang, J. Peng, K. Shi, et al., J. Control. Release 308 (2019) 29-43. DOI:10.1016/j.jconrel.2019.06.031 |
| [54] |
A. Sathiyaseelan, K. Saravanakumar, P. Manivasagan, et al., Carbohydr. Polym. 280 (2022) 119021. DOI:10.1016/j.carbpol.2021.119021 |
| [55] |
Y. Wen, X. Chen, X. Zhu, et al., ACS Appl. Mater. Interfaces 11 (2019) 43393-43408. DOI:10.1021/acsami.9b17137 |
| [56] |
Y. Guo, Y. Ran, Z. Wang, et al., Biomaterials 219 (2019) 119370. DOI:10.1016/j.biomaterials.2019.119370 |
| [57] |
N. Zhang, J. Song, Y. Liu, et al., J. Control. Release 306 (2019) 15-28. DOI:10.1016/j.jconrel.2019.05.036 |
| [58] |
R. Han, Y. Xiao, Q. Yang, et al., Biomaterials 264 (2021) 120451. DOI:10.1016/j.biomaterials.2020.120451 |
| [59] |
X.L. Hou, X. Dai, J. Yang, et al., J. Mater. Chem. B 8 (2020) 8623-8633. DOI:10.1039/d0tb01370f |
| [60] |
Z. Chen, Q. Zhang, L. Zeng, et al., Pharmacol. Res. 158 (2020) 104902. DOI:10.1016/j.phrs.2020.104902 |
| [61] |
R. Wang, Z. He, P. Cai, et al., ACS Appl. Mater. Interfaces 11 (2019) 13964-13972. DOI:10.1021/acsami.9b01107 |
| [62] |
Y. Cheng, Q. Chen, Z. Guo, et al., ACS Nano 14 (2020) 15161-15181. DOI:10.1021/acsnano.0c05392 |
| [63] |
W. Zhang, C.C. Zhang, X.Y. Wang, et al., ACS Appl. Mater. Interfaces 12 (2020) 48420-48431. DOI:10.1021/acsami.0c16526 |
| [64] |
X. Lin, X. Wang, J. Li, et al., Nanoscale 13 (2021) 1745-1758. DOI:10.1039/d0nr06182d |
| [65] |
D. Zhang, P. Cui, Z. Dai, et al., Nanoscale 11 (2019) 19912-19922. DOI:10.1039/c9nr05684j |
| [66] |
L. He, T. Nie, X. Xia, et al., Adv. Funct. Mater. 29 (2019) 1901240. DOI:10.1002/adfm.201901240 |
| [67] |
Y. Song, Y. Wang, S. Wang, et al., Nano Res. 12 (2019) 1770-1780. DOI:10.1007/s12274-019-2341-8 |
| [68] |
A.M. Itoo, S.L. Vemula, M.T. Gupta, et al., J. Control. Release 350 (2022) 26-59. DOI:10.1016/j.jconrel.2022.08.011 |
| [69] |
M. Yan, Y. Liu, X. Zhu, et al., ACS Appl. Mater. Interfaces 11 (2019) 1876-1885. DOI:10.1021/acsami.8b18751 |
| [70] |
F. Zhou, M. Wang, T. Luo, et al., Biomaterials 265 (2021) 120421. DOI:10.1016/j.biomaterials.2020.120421 |
| [71] |
L. Wang, M. Wang, B. Zhou, et al., J. Mater. Chem. B 7 (2019) 7406-7414. DOI:10.1039/c9tb00630c |
| [72] |
L. Lin, L. Liu, B. Zhao, et al., Nat. Nanotechnol. 10 (2015) 465-471. DOI:10.1038/nnano.2015.28 |
| [73] |
Y. Li, X. Li, A. Doughty, et al., Nanomedicine 18 (2019) 44-53. DOI:10.1117/12.2538632 |
| [74] |
C. Wang, L. Xu, C. Liang, et al., Adv. Mater. 26 (2014) 8154-8162. DOI:10.1002/adma.201402996 |
| [75] |
M. Qian, L. Chen, Y. Du, et al., Nano Lett. 19 (2019) 8409-8417. DOI:10.1021/acs.nanolett.9b02448 |
| [76] |
L. Bai, W. Yi, T. Sun, et al., J. Mater. Chem. B 8 (2020) 6402-6417. DOI:10.1039/d0tb01084g |
| [77] |
C. Dai, Y. Chen, X. Jing, et al., ACS Nano 11 (2017) 12696-12712. DOI:10.1021/acsnano.7b07241 |
| [78] |
Z. Liu, H. Lin, M. Zhao, et al., Theranostics 8 (2018) 1648-1664. DOI:10.7150/thno.23369 |
| [79] |
H. Lin, S. Gao, C. Dai, et al., J. Am. Chem. Soc. 139 (2017) 16235-16247. DOI:10.1021/jacs.7b07818 |
| [80] |
Q. Liu, T. Fan, Y. Zheng, et al., Nanoscale 12 (2020) 19939-19952. DOI:10.1039/d0nr05953f |
| [81] |
S. Wan, B. Zhang, S. Li, B. He, Y. Pu, J. Mater. Chem. B 8 (2020) 2805-2813. DOI:10.1039/d0tb00434k |
| [82] |
L. Tang, A. Zhang, Z. Zhang, et al., Cancer Commun. 42 (2022) 141-163. DOI:10.1002/cac2.12255 |
| [83] |
J. Liu, Y. Song, Y. Wang, et al., ACS Appl. Mater. Interfaces 14 (2022) 40612-40623. DOI:10.1021/acsami.2c09978 |
| [84] |
F. Wang, J. Zhu, Y. Wang, J. Li, Nanomaterials 12 (2022) 1656. DOI:10.3390/nano12101656 |
| [85] |
H.S. Jung, P. Verwilst, A. Sharma, et al., Chem. Soc. Rev. 47 (2018) 2280-2297. DOI:10.1039/c7cs00522a |
| [86] |
B.Le Guennic, D. Jacquemin, Acc. Chem. Res. 48 (2015) 530-537. DOI:10.1021/ar500447q |
| [87] |
A. Vincy, N. Bhatia, R. Vankayala, A.C.S. Biomater, Sci. Eng. 12 (2022) 5119-5128. DOI:10.1021/acsbiomaterials.2c01135 |
| [88] |
L. Xu, W. Zhang, H.B. Park, et al., J. Immunother. Cancer 7 (2019) 220. DOI:10.1080/10641963.2018.1465076 |
| [89] |
Q. Chen, G. Huang, W. Wu, et al., Adv. Mater. 32 (2020) e1908185. DOI:10.1002/adma.201908185 |
| [90] |
P. Zhao, M. Wang, M. Chen, et al., Biomaterials 254 (2020) 120142. DOI:10.1016/j.biomaterials.2020.120142 |
| [91] |
T.Y. Huang, G.L. Huang, C.Y. Zhang, et al., Front. Chem. 8 (2020) 1. DOI:10.3389/fchem.2020.00001 |
| [92] |
P. Kumar, R. Srivastava, Mater. Sci. Eng. C: Mater. Biol. Appl. 57 (2015) 321-327. DOI:10.1016/j.msec.2015.08.006 |
| [93] |
X. Dong, J. Liang, A. Yang, et al., Biomaterials 209 (2019) 111-125. DOI:10.1016/j.biomaterials.2019.04.024 |
| [94] |
L. Huang, Y. Li, Y. Du, et al., Nat. Commun. 10 (2019) 4871. DOI:10.1038/s41467-019-12771-9 |
| [95] |
D. Zhang, J. Zhang, Q. Li, et al., ACS Appl. Mater. Interfaces 11 (2019) 32633-32646. DOI:10.1021/acsami.9b09568 |
| [96] |
J. Peng, Q. Yang, Y. Xiao, et al., Adv. Funct. Mater. 29 (2019) 1900004. DOI:10.1002/adfm.201900004 |
| [97] |
H. Zhang, J. Zhang, Q. Li, et al., Biomaterials 245 (2020) 119983. DOI:10.1016/j.biomaterials.2020.119983 |
| [98] |
E. Mei, S. Li, J. Song, et al., Colloids Surf. A: Physicochem. Eng. Asp. 577 (2019) 570-575. DOI:10.1016/j.colsurfa.2019.06.023 |
| [99] |
J.F. Lovell, C.S. Jin, E. Huynh, et al., Nat. Mater. 10 (2011) 324-332. DOI:10.1038/nmat2986 |
| [100] |
C.S. Jin, J.F. Lovell, J. Chen, G. Zheng, ACS Nano 7 (2013) 2541-2550. DOI:10.1021/nn3058642 |
| [101] |
L. Li, Q. Yang, L. Shi, et al., J. Mater. Chem. B 7 (2019) 2247-2251. DOI:10.1039/c9tb00011a |
| [102] |
X.N. Yu, Y. Deng, G.C. Zhang, et al., ACS Appl. Mater. Interfaces 12 (2020) 17193-17206. DOI:10.1021/acsami.0c00375 |
| [103] |
X. Li, X.H. Peng, B.D. Zheng, et al., Chem. Sci. 9 (2018) 2098-2104. DOI:10.1039/C7SC05115H |
| [104] |
D. Gao, B. Zhang, Y. Liu, et al., Theranostics 9 (2019) 5315-5331. DOI:10.7150/thno.34418 |
| [105] |
D. Xi, M. Xiao, J. Cao, et al., Adv. Mater. 32 (2020) 1907855. DOI:10.1002/adma.201907855 |
| [106] |
L. Tang, F. Zhang, F. Yu, et al., Biomaterials 129 (2017) 28-36. DOI:10.1016/j.biomaterials.2017.03.009 |
| [107] |
N. Liu, P. O'Connor, V. Gujrati, et al., Adv. Healthc. Mater. 10 (2021) 2002115. DOI:10.1002/adhm.202002115 |
| [108] |
Y. Cao, D. Wei, L. Yang, et al., Adv. Healthc. Mater. 11 (2022) e2102526. DOI:10.1002/adhm.202102526 |
| [109] |
X. Wang, J. Lu, Y. Mao, et al., J. Control. Release 347 (2022) 14-26. DOI:10.1117/12.2627488 |
| [110] |
J. Yue, Q. Mei, P. Wang, et al., J. Nanobiotechnol. 20 (2022) 181. DOI:10.1186/s12951-022-01388-8 |
| [111] |
Q. Tian, Y. Li, S. Jiang, et al., Small 15 (2019) 1902926. DOI:10.1002/smll.201902926 |
| [112] |
S. Liu, Y. Zhou, C. Hu, et al., ACS Appl. Mater. Interfaces 12 (2020) 43456-43465. DOI:10.1021/acsami.0c12824 |
| [113] |
W. Sun, Y. Du, X. Liang, et al., Biomaterials 217 (2019) 119264. DOI:10.1016/j.biomaterials.2019.119264 |
| [114] |
Y. Tian, X. Wang, S. Zhao, et al., ACS Appl. Mater. Interfaces 11 (2019) 46626-46636. DOI:10.1021/acsami.9b18730 |
| [115] |
Z. Wang, B. Guo, E. Middha, et al., ACS Appl. Mater. Interfaces 11 (2019) 11167-11176. DOI:10.1021/acsami.8b22579 |
| [116] |
M. Li, S. Li, H. Zhou, et al., Nat. Commun. 11 (2020) 1126. DOI:10.1038/s41467-020-14963-0 |
| [117] |
Y. Yang, D. Hu, Y. Lu, et al., Acta Pharm. Sin. B 12 (2022) 2710-2730. DOI:10.1016/j.apsb.2021.08.021 |
| [118] |
D. Zhang, Y. Zheng, Z. Lin, et al., Small 15 (2019) e1902636. DOI:10.1002/smll.201902636 |
| [119] |
R.H. Deng, M.Z. Zou, D. Zheng, et al., ACS Nano 13 (2019) 8618-8629. DOI:10.1021/acsnano.9b02993 |
| [120] |
S. Qi, L. Lu, F. Zhou, et al., Theranostics 10 (2020) 1814-1832. DOI:10.7150/thno.38515 |
| [121] |
X. Li, G.L. Ferrel, M.C. Guerra, et al., Photochem. Photobiol. Sci. 10 (2011) 817-821. DOI:10.1039/c0pp00306a |
| [122] |
M.F. Naylor, W.R. Chen, T.K. Teague, et al., Br. J. Dermatol. 155 (2006) 1287-1292. DOI:10.1111/j.1365-2133.2006.07514.x |
| [123] |
M.F. Naylor, F. Zhou, B.V. Geister, et al., J. Biophotonics 10 (2017) 618-622. DOI:10.1002/jbio.201600271 |
| [124] |
D.J. Chen, X.S. Li, H. Zhao, et al., Oncol. Lett. 13 (2017) 1425-1431. DOI:10.3892/ol.2016.5530 |
| [125] |
D. Luo, K.A. Carter, D. Miranda, J.F. Lovell, Adv. Sci. (Weinh) 4 (2017) 1600106. DOI:10.1002/advs.201600106 |
 2023, Vol. 34
2023, Vol. 34 

