b Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore
Piperazine, as an important heterocyclic organic base, has been used to produce derivatives with various structures and different activities due to its symmetry and two basic nitrogens [1-7]. Since 1951, piperazine has been developed for use in medicinal applications for gout and helminthiasis [8]. To date, the piperazine moiety has become the main scaffold for drug design as it can improve the pharmacokinetic profile of drug candidates and enhance the water solubility [9,10]. For example, furasosin [11] is a new antihypertensive drug in which the active ingredient prazosin is synthesized from piperazine, while aripiprazole [12,13] and quetiapine fumarate [14] which are used to treat schizophrenia, also contain piperazine substructures. Some reviews have summarized the evolving role of piperazine in drug design and discovery similar to Patel et al. [15]. Moreover, the hydrogen atom of the amino group in piperazine has been replaced by a long alkyl or alkoxy group for use as a wetting agent, emulsifier, or cleaning agent [16]. Considering these factors and the two special nitrogen atoms of piperazine, its agrochemical activity has also drawn much attention. Triforine [17] is the only marketed piperazine-containing agrochemical that protects fruits and horticultural and ornamental plants from plant diseases. As an important heterocycle, piperazine is usually used as a linker [18-20], such as with chloroacetyl chloride [21] and ethylamine [22], can act as a bridge connecting reactive groups, and has been used as a key means of introducing an active substructure into a molecule.
From 2000 to the present, there are no more than 100 articles on the application of piperazine in pesticides, and most of them focused on insecticidal and antifungal application. The medicinal use of piperazine is widely documented, Meanwell et al. [23,24] described almost exclusively in the field of drug design. However, there is currently no comprehensive overview of the applications of piperazine in new pesticide innovation. We used SciFinder and the web of European Patent Office (EPO) to search for relevant documents from 2000 to 2022 with "piperazine", "antifungal", "insecticidal", "herbicidal", "antiviral", "plant growth regulating activity", respectively. The structure–activity relationships (SARs) of each compound were analyzed to provide a reference for further applications of piperazine in pesticide development. This review provides rational guidelines for the design and synthesis of compounds containing piperazine systems, and these molecular scaffolds can be a rich source for future pesticide design.
2. Antifungal and antibacterial activityAs a linker, piperazine is often accompanied by an amide group when synthesizing antifungal and antibacterial agents. The amide has a broad spectrum of biological activities, the combination of amide and piperazine and then connecting other heterocyclic compounds can often achieve good results.
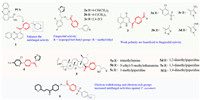
Phenazine-1-carboxylic acid (PCA) exhibits strong antifungal activity against phytopathogenic fungi. The linking of piperazine to PCA [25] followed by the addition to another heterocyclic group through a methylene linker yielded compound 1 (Fig. 1), this compound showed better EC50 values against most fungi than PCA. SAR analysis revealed that the introduction of a morpholinyl group could enhance antifungal activity, and compound 1 could be regarded as a potential fungicide candidate. Using a similar approach, Deng and coworkers [26] synthesized piperazine derivative to yield fungicides 2a-2c. The inhibitory effects of 2a-2c against Fusarium graminearum (200 µmol/L) were equal to that of triadimefon. Adding an isopropyl or a tert-butyl group on the benzene ring is much more favored for antifungal activity than adding methyl, ethyl, or other substituents. These data indicated that the larger the alkyl group was, the greater the steric hindrance and the stronger the antifungal activity. Fang and colleagues placed an amide on both ends of piperazine, and bioassays indicated that compounds 3a-3d displayed higher activity against Teleomorph than other compounds, possibly due to their weaker polarity [27]. Using piperazine as a bridge to connect trifluoromethyl pyridine and imidazole, creating a urea compound with significantly improved absorption and conductive properties and unexpectedly high antifungal activities. For instance, resulting compound 4 showed excellent antifungal activity against Alternaria solani, Fusarium oxysporum (F. oxysporum), Cercospora arachidicola Hori, Botryospharia dothidea, and Phytophthora capsici Leonian [28]. In addition, the active group was linked to natural ursolic acid via a piperazine moiety [29], and most of the compounds in this series (5a-5f) had good activities against Xanthomonas oryzae pv. Oryzae (EC50 of 0.37–7.97 µg/mL) and Xanthomonas axonopodis pv. Citri (EC50 of 1.03–10.9 µg/mL), and the active groups were all simple nitrogen-containing saturated aliphatic heterocycles or aliphatic secondary amines. The chalcone and benzene sulfonyl were also linked via the piperazine bridge [30] and gave the chalcone derivatives good antifungal activities. In particular, the electron-withdrawing and electron-rich groups on the benzene increased the antifungal activity, and the introduction of 2-pyridine (6) completely controlled Thanatephorus cucumeri and exhibited significant antifungal activity against anthracnose in vivo.
|
Download:
|
| Fig. 1. Amidepiperazine derivatives with antifungal (antibacterial) activity. | |
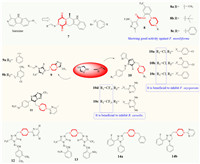
The advantages of diketopiperazine chiral enrichment can also mimic the advantages of preferential peptide conformations [31]. Wang and co-authors [32] synthesized a series of optically pure indole diketopiperazine acylhydrazones by combining the substructure of harmine and hydrazone into a fused aromatic ring. The antifungal activities of compound 7 (6S, 12aS) against Rhizoctonia cerealis (R. cerealis) and Sclerotinia sclerotiorum were comparable to that of chlorothalonil (Fig. 2), showing 100% inhibition of Phytophthora capsici at 50 µg/mL, which was higher than that of chlorothalonil (91% ± 1%). In addition, Wang also combined trifluorotoluene and piperazine and added some simple groups to the piperazine to yield compounds 8a-8c [33], and each has good activity against Fusarium moniliforme (F. moniliforme). Their inhibition rates are close to those of commercially available compounds, but all are not very prominent. The 1, 2, 4-triazole fused piperazine skeleton was introduced a triazole Schiff base via a methylene to yield compounds 9. This type of compounds (e.g. 9a and 9b) showed good antifungal activities against Cercospora arachidicola Hori, Physalospora piricola, Alternaria solan, and F. moniliforme, and were comparable to, or better than, the commercial triadimefon [34]. Moreover, similar work found that the compounds containing a 4-chlorophenyl group (10, R1 = -Cl) in the Schiff base showed favorable antifungal activities against F. oxysporum [35]. For example, compounds 10a-10c curbed F. oxysporum as well as chlorothalonil and carbendazim. Interestingly, a trifluoromethyl (R1 = -CF3) was favored for controlling R. cerealis. For example, both 10d and 10e displayed much higher antifungal activity than tridimefon. Furthermore, the -CF3 and -Cl groups in the Schiff base were more favored than the F-substituent. Zhang et al. [36] proposed a convenient method for connecting the novel 2-(trifluoromethyl)-6-arylimidazo[2,1-b][1,3,4]-thiadiazole chemical base on piperazine via a methyl group to give the novel structures (11) with good antifungal activity against Cercospora arachidicola (C. arachidicola). Wang and Zhang et al. [37,38] synthesized a series of new Mannich base and bis-Mannich base derivatives containing piperazine, and found that most of them had good antifungal activities. On the basis of the comparative molecular field analysis (CoMFA), it was found that compounds 12 and 13 had better antifungal activities against Pseudoperonospora cubensis, and the inhibition rates at 500 µg/mL were 96.9% and 84.9%, respectively. Compounds 14a and 14b had 65%–70% inhibition rates to C. arachidicola and Physalospora piricola at 50 µg/mL, showing good antifungal activities as well.
|
Download:
|
| Fig. 2. Piperazine derivatives with antifungal activity. | |
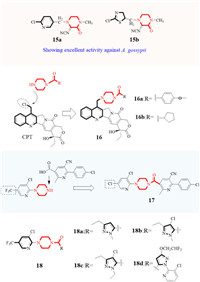
Piperazine itself has good anthelmintic properties, and is often used as medicine. For example, piperazine citrate effectively controls roundworms via muscle paralysis [39,40]. In the field of insecticides, piperazine is also used as an important linker for adding different functional groups. N-alkyl-substituted piperazine neonicotinoid derivatives were designed and synthesized by adding the pharmacophore of neonicotinoid pesticides. The resulting piperazine derivatives 15a and 15b (Fig. 3) showed excellent activity against Aphis gossypii, Besimm tabaci, Nilaparvata lugens, and Cicadella viridis [41]. Using natural (20S)-camptothecin (CPT) as the starting material, Li and coworkers added piperazine via a methylene linker at the 7-position of CPT, and adding diverse substituents to the piperazine yielded compound 16 [42]. These compounds showed excellent insecticidal activities against Tetranychus cinnabarinus (T. cinnabarinus) and Bursaphelenchu xylophilus, compounds 16a and 16b showed much lower LC50 values of 8.10 and 9.05 µg/mL, respectively, than that of CPT. The acaricidal activity could be enhance by replacement of trifluoromethyl with a chlorine atom, 17 exhibited an LC50 of 0.8977 µg/mL on T. cinnabarinus and proved to be more active than the spirodiclofen and pyridaben. In the field trials, 17 could effectively control Panonychus citri and Panonychus ulmi with long-lasting persistence and rapid action, which could be regarded as a potential candidate for acaricide [43]. As an important F-containing organic intermediate, trifluoromethyl pyridine is often used in pesticide design [44]. Wang and co-workers connected trifluoromethyl pyridine to piperazine and then added a substituted pyrazole at another end of piperazine to give insecticidal molecules 18 (Fig. 3) [45,46]. These compounds (18a-18d) showed more significant insecticidal activity on Plutella xylostella (P. xylostella) at 1 µg/mL than spirodiclofen.
|
Download:
|
| Fig. 3. Insecticidal activity of piperazine derivatives containing amide group. | |
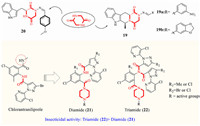
The diketopiperazine derivatives synthesized by Wang et al. showed good fungicidal activity, and some compounds (19a, 19b) (Fig. 4) also showed good insecticidal activity against P. xylostella and Culex pipiens pallens [31]. Wang [47] linked indole and hydrazone structures at the opposite ends of piperazine to provide compound 20. Which produced 100% mortality to mosquito larvae at 5 µg/mL and could be a novel skeleton for the optimization of insecticide. Li et al. replaced the N-methyl of chlorantraniliprole with a substituted piperazine, resulting in a series of phthalimides. These compounds showed excellent insecticidal activities against Mythimna separata (M. separata) and P. xylostella. In particularly, some of the compounds possessed LC50 values of 0.0022 to 0.0081 µg/mL toward the diamondback moth. Interestingly, the activities of triamide 22 were generally better than those of diamide 21. This type of piperazine-containing phthalimide compound could be used as a new insecticide lead structure for further study [48].
|
Download:
|
| Fig. 4. Piperazine derivatives containing amides with insecticidal activity. | |
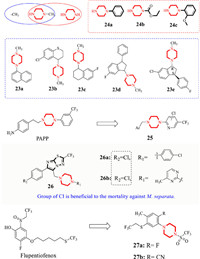
Starting from N-methylpiperazine, Steiner et al. added naphthalene (23a), thiochromane (23b), tetrahydronaphthalene (23c), and substituted indene (23d and 23e) to give novel skeletons with extensive acaricidal activity toward a variety of pests, including Bemisiais primarily, Dermatophagoides farinae, Dermatophagoides pteronyssinus and Tyrophagus putrescentiae, etc. (Fig. 5). Compound 23c displayed 1.5-3.2 times higher activity than DEET [49]. Interestingly, the N-methyl group could be removed, and some simple groups, including phenyl (24a and 24c) and ester (24b) could be added. The acaricidal activity against the above mites could be strengthened up to 4.7-5.7-fold compared with DEET [50]. 1-[(4-Aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP) is a 5-HT1A agonist that has been reported to display high affinity for the serotonin (5-HT) receptor in the parasitic nematode Haemonchus contortus. PAPP was modified by changing the trifluoromethyl benzene to trifluoromethyl pyridine, and the resulting trifluoromethyl pyridinyl piperazine derivatives (e.g., 25) displayed growth-inhibiting or larvicidal activities against M. separata. The introduction of electronegative groups (such as -Cl, -F and -CF3) and hydrophilic moieties, including heterocycles of -O/-N, increased the larvicidal activity of this type of compoud [51]. In 2018, Zhang et al. linked heterocycles to piperazine to obtain a new structure (found in 26a and 26b) with good insecticidal activities against oriental armyworm (M. separata) [36]. More recently, using flupentiofenox as the lead compound, Suzuki et al. disclosed a series of phenylpiperazine derivatives with a trifluoro sulfonyl group. The compounds containing fluorine atom (27a) and cyano (27b) at the 2-position of benzene (R) were particularly effective towards Tetranychus urticae (T. urticae). In particular, compound 27a displayed high efficacy (even at 3 µg/mL) to control T. urticae, Tetranychus kanzawai, and Panonychus citri [52].
|
Download:
|
| Fig. 5. Derivatives containing piperazine fragments with insecticidal activity. | |
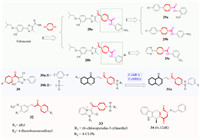
Piperazine derivatives often appear in medicinal structures. Many novel drugs for virus control have been reported in recent years, as this is a hot topic in the discovery of medicines [53,54], compounds containing the piperazine structure have also been found to control plant viruses. In 2013, Krishna Reddy doped piperazine with febuxostat to yield new urea and thiourea derivatives. The nitro (28a) and bromine (28b)-containing compounds (Fig. 6) showed good antiviral activity against tobacco mosaic virus (TMV) similar to of ningnanmycin. These types of compounds can also significantly increase the chlorophyll content in plants and have the potential to act as plant-inducing agents [55]. By changing the febuxostat scaffold to a simple substituted aromatic ring, Nagalakshmamma found that compounds 29a-29c could bond to the TMV-Hel enzyme to affect the self-assembly and replication processes of TMV, thus displaying good anti-TMV activity [56]. In 2014, Wu et al. placed the pyrazole amide and a substituted aromatic ring or sulfonyl moiety at opposite ends of piperazine to yield amide derivatives 30a and 30b, respectively. These molecules cured the disease caused by TMV as well as ningnanmycin [57]. Using the tomato chlorosis virus coat protein-oriented screening method, Jiang and co-authors discovered novel sulfonamide-containing chromone derivatives. In their work, sulfonyl and acyl groups were added to the N-position of piperazine. The resulting chromone derivatives possessed good activities toward tomato chlorosis virus (ToCV). In particularly, compounds 31a was screened via effective 3D Q-SAR models (CoMFA and CoMSIA). This compound was confirmed to show good affinity for the coat protein (CP) of ToCV, and the expression levels of the gene encoding CP could be inhibited by 67.2% [58]. Another representative work on unsaturated amide was the combination of natural ferulic acid, piperazine, and sulfonamide (32). These derivatives were found to exhibit various biological activities towards TMV and cucumber mosaic virus (CMV) [59,60]. In particular, the compound with R1 was an allyl and R2 was a 4-fluorobenzenesulfonyl group had good activities towards TMV and CMV [61].
|
Download:
|
| Fig. 6. Piperazine derivatives with antiviral activity. | |
More recently, Song's group placed an acyl group on the nitrogen atoms at both ends of piperazine for linkage with a imidazo[1, 2-a]pyridine mesoionic group to give a novel anti-viral slecketon (33) with good anti-potato virus Y (PVY) activity [62]. 3D Q-SAR study indicated the contribution of a steric field is confirmed to be much higher than that of the electrostatic fields, and both bulky groups and electron-withdrawing groups are beneficial for anti-PVY activity. For example, the introduction of bulky substituents at the R2 position and introduction of electron-donating groups at R1 could enhance the activities toward PVY. This is the first example of a mesoionic compound as a promising candidate for controlling plant viruses. The SAR study of diketopiperazine derivatives synthesized by Xie et al. [32] showed that spatial conformation is one of the most important factors that regulates antiviral activity, and the different spatial conformations of C6 and C12 had a great influence on the anti-TMV activity. Following the sequence C-12aR > C-12aS, trans > cis, it is worth noting that trans compound 34 (6S, 12aR) exhibited better anti-TMV activity, and the protective activity of 34 (EC50 = 394 µg/mL) was better than ribavirin (EC50 = 693 µg/mL).
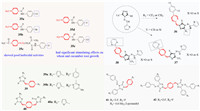
5. Herbicidal and plant growth regulating activityCompounds containing a piperazine ring are less often used as herbicides or plant growth regulators. Stoilkova and colleagues [63] found that compounds containing N-methyl-piperazine (35a and 35b) showed good herbicidal activities. Compound 35c (Fig. 7) containing a -F exhibited the highest activity against Triticum aestivum at 100 µmol/L. In contrast, compounds containing 4-fluoro (35d) or 4‑chloro (35e) had significant stimulatory effects on wheat and cucumber root growth depending on the halogen and increased in the order -Cl < -F < -Br. Wang et al. [64] introduced a triazole Mannich base with a furan/thiophene structure into N-methyl piperazine, and added a substituted aromatic ring to give 36–38. These compounds showed herbicidal activities against dicotyledon rape and regulated the growth of rape root. This type of compound can be regarded as a potential inhibitor of ketol-acid reductor isomerase (KARI). Interestingly, trifluoromethyl was much more favorable than the other groups, and the addition of pyridine and pyrimidine positively influenced their activity toward rape. Zhang et al. [65] synthesized a series of new Mannich base and bis-Mannich base derivatives containing piperazine (compounds 39 and 40), When R1 was furan-2-yl instead of pyridine-3-yl, it had more obvious herbicidal activity against dicotyledonous rape (Brassica campestris). Especially, compounds 39a, 39b and 40a all showed strong rice KARI inhibitory activities, which were comparable to the known effective in vitro KARI inhibitor N‑hydroxy-N-isopropyloxamate (IpOHA). The density functional theory (DFT) calculation and molecular docking results showed that the oxadiazole, furan and piperazine ring of high activity inhibitors (39a and 40a) may make an important contribution to the activities of KARI. Differently, Mannich base derivatives (such as 41 and 42) synthesized by Wang et al. [66] had obvious herbicidal activities against Echinochloa crusgalli, and strong in vitro inhibitory activities against KARI, but showed very weak herbicidal activities against Brassica campestris. The reason may be related to the structural differences between 1, 3, 4-oxadiazole ring and 1, 2, 4-triazole ring, and the substituents on the ring. In addition, for compounds 41 and 42, when R1 was 2-F and R2 was F, the corresponding compounds had good herbicide activities. This will provide useful information for the design and discovery of new pesticides for KARI.
|
Download:
|
| Fig. 7. Piperazine derivatives with herbicidal and plant growth regulating activity. | |
Recent studies have demonstrated that piperazine is an important skeleton in the discovery of novel agrochemicals. It's often used as a "bridge" for the inclusion of different groups, including amide or sulfonyl substructures, or directly connected to heterocycles, alkyl groups, etc. In general, the activities of piperazine-containing compounds tend to be better when electron-withdrawing groups (-F, -Cl, etc.) are present in the corresponding substructures, rather than electron-donating groups (-CH3, etc.). Many compounds containing piperazine have shown promise as novel pesticides. For example, piperazine compounds employing trifluoromethyl pyridine, chalcone, and sulfonyl moieties have shown good insecticidal activity.
However, as a "bridge", the chemical reaction type to connect with piperazine is also very limited (most of the reaction are nucleophilic substitutions), and the probability for obtaining active compounds is not very high. Piperazine derivatives are widely used in drug discovery, and many drug candidates have been discovered. Their SARs and mechanisms of action in medicine have been widely reported. Consequently, we think that some of the experiences in medical properties activity can provide a reference for the discovery of pesticides. For example, a piperazine has been connected to quinolone [67], fluoroquinolone [68], oxazolidinone, etc. [69] which could enhance antibacterial activity, and such a substructure could also be add to agrochemical piperazine molecules. In addition, the piperazine fused helical heterocyclic systems, cubic rings and dicyclopentane derivatives to expand biological activities, these applications can also be introduced to agrochemicals discovery. We believe that the modes of action, potential targets and physicochemical properties of piperazine will inform the rational design and optimization of active compounds with this structure.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe financial supports from National Natural Science Foundation of China (NSFC, Nos. 32072445, 21762012), the Program of Introducing Talents to Chinese Universities (No. D20023), the S & T Planning Project of Guizhou Province (No. [2017]5788), and Graduate Research Fund in Guizhou Province (No. YJSKYJJ[2021]038).
| [1] |
D.I. Bugaenko, Chem. Heterocycl. Compd. 53 (2017) 1277-1279. DOI:10.1007/s10593-018-2205-2 |
| [2] |
V.R. Solomon, C. Hu, H. Lee, Bioorg. Med. Chem. 18 (2010) 1563-1572. DOI:10.1016/j.bmc.2010.01.001 |
| [3] |
M. Kimura, T. Masuda, K. Yamada, et al., Bioorg. Med. Chem. Lett. 14 (2004) 4287-4290. DOI:10.1016/j.bmcl.2004.05.091 |
| [4] |
A. Garg, A. Kumar, V. Singh, et al., J. Cardiovasc. Dis. Res. 12 (2021) 686-695. |
| [5] |
B.N. Acharya, R. Ghorpade, K.P. Singh, D. Kumar, S. Nayak, Monatsh. Chem. 152 (2021) 357-366. DOI:10.1007/s00706-021-02752-4 |
| [6] |
S. Elliott, Drug Test. Anal. 3 (2011) 430-438. DOI:10.1002/dta.307 |
| [7] |
M. Borzsonyi, A. Pinter, G. Torok, A. Surjan, L. Nadasdi, IARC Sci. Publ. 19 (1978) 477-484. |
| [8] |
G. Mouriquand, E. Roman, J. Coisnard, J., Med. Lyon 32 (1951) 189-195. |
| [9] |
A.K. Rathi, R. Syed, H.S. Shin, R.V. Patel, Expert Opin. Ther. Pat. 26 (2016) 777-797. DOI:10.1080/13543776.2016.1189902 |
| [10] |
E. Lacivita, M. Leopoldo, P. De Giorgio, F. Berardi, R. Perrone, Bioorg. Med. Chem. 17 (2009) 1339-1344. DOI:10.1016/j.bmc.2008.12.015 |
| [11] |
S.J. Matthews, Conn. Med. 42 (1978) 509-510. |
| [12] |
X.J. Liu, T.T. Wang, Y.L. Zhong, X. Wang, J., Pharm. Sci. 30 (2013) 253-255. DOI:10.1007/s11712-013-9319-0 |
| [13] |
A. Les, K. Badowska-Roslonek, M. Laszcz, et al., Acta Pol. Pharm. 67 (2010) 151-157. |
| [14] |
N.S. Gunasekara, C.M. Spencer, CNS Drugs 9 (1998) 325-340. DOI:10.2165/00023210-199809040-00007 |
| [15] |
R.V. Patel, S.W. Park, Mini-Rev. Med. Chem. 13 (2013) 1579-1601. DOI:10.2174/13895575113139990073 |
| [16] |
Q.S. Zhang, H.M. Zhang, B.N. Guo, Fine Chem. 23 (2006) 435-438. |
| [17] |
J.B. Bourke, T.R. Nelsen, D. Eichler, J., Agric. Food Chem. 25 (1977) 36-39. DOI:10.1021/jf60209a023 |
| [18] |
K.E. Gettys, Z. Ye, M. Dai, Synthesis (Mass) 49 (2017) 2589-2604. DOI:10.1055/s-0036-1589491 |
| [19] |
S.S. Sajadikhah, M. Nassiri, Chem. Heterocycl. Compd. 57 (2021) 905-907. DOI:10.1007/s10593-021-02998-0 |
| [20] |
R.H. Zhang, H.Y. Guo, H. Deng, J. Li, Z.S. Quan, J., Enzyme Inhib. Med. Chem. 36 (2021) 1165-1197. DOI:10.1080/14756366.2021.1931861 |
| [21] |
P.P. Chen, Z.Y. Zhou, Y.F. Zhou, et al., CN Patent 114262273, 2022.
|
| [22] |
X.W. Wang, H. Han, W.Z. Tang, Q.J. Guo, CN Patent 114315593, 2022.
|
| [23] |
N.A. Meanwell, O. Loiseleur, J., Agric. Food Chem. 70 (2022) 10942-10971. DOI:10.1021/acs.jafc.2c00726 |
| [24] |
N.A. Meanwell, O. Loiseleur, J., Agric. Food Chem. 70 (2022) 10972-11004. DOI:10.1021/acs.jafc.2c00729 |
| [25] |
F. Han, R. Yan, M. Zhang, et al., Nat. Prod. Res. 34 (2020) 1282-1287. DOI:10.1080/14786419.2018.1556656 |
| [26] |
S.C. Deng, X.J. Li, X. Zhu, et al., Chin. J. Pestic. Sci. 23 (2021) 77-86. DOI:10.1117/12.2605808 |
| [27] |
H.B. Fang, Z.F. Chen, X.W. Hua, et al., Med. Chem. Res. 31 (2022) 485-496. DOI:10.1007/s00044-022-02852-8 |
| [28] |
M.H. Wang, H.B. Zhu, Z. Peng, L.Z. Xu, CN Patent 108675991, 2018.
|
| [29] |
S. Yang, P.Y. Wang, M. Xiang, et al., CN Patent 110776548, 2020.
|
| [30] |
Q. Zhou, X.M. Tang, S. Chen, et al., J. Agric. Food Chem. 70 (2022) 1029-1036. DOI:10.1021/acs.jafc.1c05933 |
| [31] |
B.E.D.M. El-Gendy, M.E. Rateb, Bioorg. Med. Chem. Lett. 25 (2015) 3125-3128. DOI:10.1016/j.bmcl.2015.06.010 |
| [32] |
J.L. Xie, W.T. Xu, H.J. Song, et al., J. Agric. Food Chem. 68 (2020) 5555-5571. DOI:10.1021/acs.jafc.0c00875 |
| [33] |
Q.M. Wang, J.M. Guo, Y.X. Liu, et al., CN Patent 113024538, 2021.
|
| [34] |
B.L. Wang, Z.M. Li, Y. Zhang, CN Patent 106749262, 2017.
|
| [35] |
Y. Zhang, Y.Z. Zhan, Y. Ma, et al., Chin. Chem. Lett. 29 (2018) 441-446. DOI:10.1016/j.cclet.2017.08.035 |
| [36] |
Y. Zhang, Z. Li, H. Song, B. Wang, Chin. J. Chem. 36 (2018) 635-638. DOI:10.1002/cjoc.201800110 |
| [37] |
B.L. Wang, Y.X. Shi, Y. Ma, et al., J. Agric. Food Chem. 58 (2010) 5515-5522. DOI:10.1021/jf100300a |
| [38] |
L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Chin. Chem. Lett. 27 (2016) 163-167. DOI:10.1016/j.cclet.2015.09.015 |
| [39] |
D.D. Brown, S.Z. Siddiqui, M.D. Kaji, S.G. Forrester, Vet. Parasitol. 185 (2012) 201-209. DOI:10.1016/j.vetpar.2011.10.006 |
| [40] |
M.T. Hoekenga, Am., J. Trop. Med. Hyg. 4 (1955) 1088-1090. DOI:10.4269/ajtmh.1955.4.1088 |
| [41] |
J.G. Samaritoni, D.A. Demeter, J.M. Gifford, et al., J. Agric. Food Chem. 51 (2003) 3035-3042. DOI:10.1021/jf021185r |
| [42] |
Z.B. Li, Z. Chen, A.L. Chen, et al., Chin. J. Pestic. Sci. 17 (2015) 136-142. |
| [43] |
Y. Xie, Y. Xu, C.L. Liu, et al., Pest Manag. Sci. 73 (2017) 945-952. DOI:10.1002/ps.4369 |
| [44] |
Z.Z. Zheng, A.L. Dai, Z.C. Jin, Y.R. Chi, J. Wu, J., Agric. Food Chem. 70 (2022) 11019-11030. DOI:10.1021/acs.jafc.1c08383 |
| [45] |
M.H. Wang, Z. Peng, H.Q. Cui, J.Q. Cui, L.Z. Xu, CN Patent 108822082, 2018.
|
| [46] |
M.H. Wang, L.Z. Xu, Z. Peng, H.Q. Cui, J.X. Sun, CN Patent 109336882, 2019.
|
| [47] |
Q.M. Wang, H.J. Song, L.L. Li, et al., CN Patent 110759896, 2020.
|
| [48] |
H. Li, H. Liu, Y. Zhang, et al., Chin. Chem. Lett. 32 (2021) 2893-2898. DOI:10.1016/j.cclet.2021.02.002 |
| [49] |
G. Steiner, T. Schmidt, M. Kordes, et al., WO Patent 2004080170, 2004.
|
| [50] |
C.H. Lee, H.W. Kim, H.S. Lee, Pest Manag. Sci. 65 (2009) 704-710. DOI:10.1002/ps.1728 |
| [51] |
M.Y. Cai, Z. Li, F. Fan, et al., J. Agric. Food Chem. 58 (2010) 2624-2629. DOI:10.1021/jf902640u |
| [52] |
J. Suzuki, A. Ootaka, S. Onoue, M. Onoue, J., Pestic. Sci. 46 (2021) 189-197. DOI:10.1584/jpestics.d21-007 |
| [53] |
A. Jain, J. Chaudhary, H. Khaira, B. Chopra, A. Dhingra, Drug Res. 71 (2021) 62-72. DOI:10.1055/a-1323-2813 |
| [54] |
R.R. Kumar, B. Sahu, S. Pathania, et al., ChemMedChem 16 (2021) 1878-1901. DOI:10.1002/cmdc.202100045 |
| [55] |
R.C.K. Reddy, S. Rasheed, D.S. Rao, et al., Sci. World J. 2013 (2013) 682603. |
| [56] |
V. Nagalakshmamma, M. Venkataswamy, C. Pasala, et al., Bioorg. Chem. 102 (2020) 104084. DOI:10.1016/j.bioorg.2020.104084 |
| [57] |
Q. Wu, Z.C. Wang, T. Yang, Int., J. Mol. Sci. 30 (2014) 273-279. DOI:10.1179/1433075X13Y.0000000134 |
| [58] |
D.H. Jiang, J.X. Chen, N.N. Zan, et al., J. Agric. Food Chem. 69 (2021) 12126-12134. DOI:10.1021/acs.jafc.1c02467 |
| [59] |
D.Y. Hu, Q.Q. Wan, S. Yang, et al., J. Agric. Food Chem. 56 (2008) 998-1001. DOI:10.1021/jf072394k |
| [60] |
D.G. Zhou, D.D. Xie, F.C. He, B.A. Song, D.Y. Hu, Bioorg. Med. Chem. Lett. 28 (2018) 2091-2097. DOI:10.1016/j.bmcl.2018.04.042 |
| [61] |
X.H. Gan, T. Yuan, D. Liu, B.A. Song, D.Y. Hu, CN Patent 113461639, 2021.
|
| [62] |
C.Y. Li, R.J. Song, S.Q. He, et al., J. Agric. Food Chem. 70 (2022) 7375-7386. DOI:10.1021/acs.jafc.2c01813 |
| [63] |
G.M. Stoilkova, P. Yonova, K. Ananieva, Plant Growth Regul. 72 (2014) 303-312. DOI:10.1007/s10725-013-9861-0 |
| [64] |
Y. Zhang, X.H. Liu, Y.Z. Zhan, et al., Bioorg. Med. Chem. Lett. 26 (2016) 4661-4665. DOI:10.1016/j.bmcl.2016.08.059 |
| [65] |
B.L. Wang, L.Y. Zhang, X.H. Liu, et al., Bioorg. Med. Chem. Lett. 27 (2017) 5457-5462. DOI:10.1016/j.bmcl.2017.10.065 |
| [66] |
B.L. Wang, Y.X. Shi, Y.Z. Zhan, et al., Chin. J. Chem. 33 (2015) 1124-1134. DOI:10.1002/cjoc.201500436 |
| [67] |
A. Foroumadi, F. Soltani, M.H. Moshafi, R. Ashraf-Askari, Farmaco 58 (2003) 1023-1028. DOI:10.1016/S0014-827X(03)00191-5 |
| [68] |
A. Foroumadi, S. Ghodsi, S. Emami, et al., Bioorg. Med. Chem. Lett. 16 (2006) 3499-3503. DOI:10.1016/j.bmcl.2006.03.103 |
| [69] |
B.B. Lohray, V.B. Lohray, B.K. Srivastava, et al., Bioorg. Med. Chem. Lett. 16 (2006) 1557-1561. DOI:10.1016/j.bmcl.2005.12.025 |
 2023, Vol. 34
2023, Vol. 34 

