b Department of Hand and Foot, Clinical Medical College, Yangzhou University, Yangzhou 225001, China;
c School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China
Selenium is the element being discovered by Sweden chemist Jons Jacob Berzelius in 1818. It is named after Selene, the goddess of full moon in Greek mythology. Selenium-containing compounds/materials have been widely employed for their unique bio- and chemical activities [1–11]. It is an essential trace element for human beings and animals. It is one of the eight scattered elements in the world. In China, more than 70% of regions are selenium deficient [12]. Selenium deficiency may lead to various diseases, including the brain diseases. It will cause irreversible damage to human brain tissue. The human body can maintain the selenium content in advanced central brain tissue by mobilizing the selenium content in other tissues of the body [13]. Interestingly, when selenium is supplemented to selenium deficient animals, most of the element preferentially enters the brain tissues [14]. These phenomena indicate the significances of selenium for maintaining the brain functions of both human beings and animals. With the discovery of a series of selenoproteins and selenium-containing materials, the biological function of selenium and the effect and mechanism of selenium deficiency on the body have been studied more and more deeply. It is found that selenium can participate in many functions of the central nervous system, such as motor ability, physical coordination, brain memory and cognition, showing that the element can play an important role in brain signal transduction pathway (Fig. 1) [15]. Selenoproteins in the body are essential for maintaining normal brain function. The reduction of selenoprotein function will lead to brain cognitive dysfunction and nervous system coordination disorder. Appropriate selenium supplementation can alleviate the clinical symptoms of some neurodegenerative diseases [16]. Moreover, selenium has also been proved to be effective in antagonizing the toxicity of various heavy metals and the damage of environmental toxins to the nervous system [17,18]. Recently, the applications of selenium nanomaterials in nervous system afford additional opportunities for research in the field. Investigations on the relationship of selenium with health is booming. However, it should be noticed that excessive selenium intake may be harmful for health. The Institute of Medicine of the National Academy of Sciences stipulates that the maximum intake of selenium per adult per day should not exceed 400 µg [19]. This paper aims to review the recent progresses and give a perspective.
|
Download:
|
| Fig. 1. Relationship of selenium with the human nervous system diseases. | |
Selenoproteins are organoselenium compounds that widely exist in animals and plants. Brain tissue is one of the organs with the most abundant selenium content in human body. In the cases of lack of selenium in food, the selenium content and selenoprotein expression level of human liver, kidney, lung and other organs will be reduced, but the higher selenium content and more stable selenoprotein activity level can be maintained in the advanced central brain tissue, showing that the brain tissue has special advantages for selenium uptake. Transcription assays indicate that neurons, astrocytes and small glial cells in the brain can express a variety of selenoproteins. The areas with the highest content of selenoprotein are the hippocampus, the sniffing area and the cortical cerebellar cortex [20]. Selenium (majorly existed in selenocysteine form) is the active site of selenoprotein, which protects tissues from oxidative damage by increasing their activity to scavenge intracellular reactive oxygen species (ROS). Therefore, it has antioxidant function in the central nervous system. The reduction of selenoprotein expression in the brain will cause irreversible damage to neurons, leading to cognitive impairment, depression and anxiety.
Twenty-five selenoproteins have been found in human body (Table 1). Among them, selenoprotein P (SelP), selenoprotein M (SelM), selenoprotein H (SelH), selenoprotein K (SelK), selenoprotein R (SelR), selenoprotein W (SelW), glutathione peroxidase (GPx), thioredoxin reductase (TrxR) and deiodase (DIO) play the major function of antioxidant defense system in the brain [21,22]. The remaining selenoproteins include selenoproteins S (SelS), selenoprotein N (SelN), selenoprotein T (SelT), selenoprotein O (SelO), selenoprotein I (SelI), selenoprotein V (SelV), selenophosphate synthetase 2 (SPS2) and 15-kDa selenoprotein (Sep15).
SelP is not only the key molecule of selenium transport, storage and metabolism, but also the only selenium donor in brain tissue. It transfers selenium into brain tissue through the apolipoprotein E2 receptor antibody of cerebral vascular endothelial cells (ApoER2) to synthesize selenoprotein. In addition, SelP also has antioxidant, heavy metal detoxification and neurotrophic effects. Knocking out the gene that expresses SelP may lead to nerve damage and even death [23].
SelM is a kind of endoplasmic network selenoprotein. It is expressed in various tissues of mice, and the expression level of SelM is higher in brain [24]. Experiments on mouse neurons show that it can play a role in calcium regulation and reduce the level of ROS in the brain [25]. Over-expression of SelM can reduce the ROS level and apoptosis, while low expression of SelM will lead to apoptosis, in accordance with the results caused by the addition of hydrogen peroxide [25]. SelH, SelK and SelW also possess antioxidant effects, and among them, SelW shows higher expression level in brain [26]. SelW is a selenoprotein that is highly expressed in brain and muscle. It plays an important role in maintaining neural cell reduction homeostasis and synaptic plasticity.
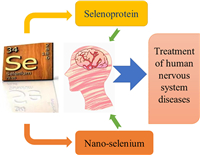
Selenoproteins GPx1 and GPx4 are selenium proteases widely present in brain tissue. With the decrease of their activities, the sensitivity of brain tissue to oxidative stress injury reduces correspondingly. It was also found that GPx4 can regulate the iron death process [27]. Activation of the Nrf2/ARE pathway enhances GPX1 activity [28]. It can reduce the production of pro-inflammatory factors and NO, and relieve the pro-inflammatory response of oxidative stress to the body. GPx1 can also resist acute oxidative stress, reduce NADPH oxidase (NOX)-mediated excess ROS, reduce endoplasmic reticulum stress, and maintain the stability of the internal environment [29]. In addition, GPx1 can mediate DNA methyltransferase 1 (DNMT1) expression, which plays an important role in alleviating DNA damage [30]. The relevant mechanisms are shown in Fig. 2.
|
Download:
|
| Fig. 2. The antioxidant mechanism of GPx1. | |
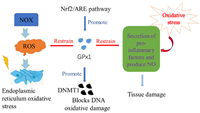
The reductive TrxR is beneficial for maintain the reduction state of Trx with NADPH and further restore a lot of organism disulfide. As shown in Fig. 3, the thioredoxin system can adjust cell redox balance. Thioredoxin reductase-1 (TrxR1) and thioredoxin reductase-2 (TrxR2) are important members of the TrxR family. In addition to their antioxidant functions, TrxR1 is also involved in DNA synthesis and cell signal transduction, which is related to the development of the nervous system [23].
|
Download:
|
| Fig. 3. Redox cycle and biological function of thioredoxin system. | |
SelR is a member of methionine sulfoxide reductase family. It can reduce the content of methionine sulfoxide generated by methionine residue oxidized by ROS [31]. The increase of methionine sulfoxide level is considered to be closely related to neurodegeneration. In addition, the degree of oxidation and aggregation of methionine at the 35th position in amyloid β-peptide (Aβ) is related to its cytotoxicity, indicating that SelR can significantly reduce cognitive impairment. In the control experiments on wild-type control mice, knockout of the gene of methionine sulfoxide reductase A can lead to the neurodegenerative diseases, increased phosphorylation of TAU protein (microtubule-associated protein), loss of integrity of astrocytes and increased Aβ precipitation [32]. Selenium deficiency may reduce the activity of SelR and increase the content of methionine sulfoxide, promoting the formation of Aβ small fiber oligomer to accelerate the development of cognitive impairment.
3. Nano-selenium for human nervous system disease treatmentNano-selenium is the Se(0) species appearing in gray, black and red colors. The ones in gray or black color are powders insoluble in water and are bio-inactive. The freshly prepared nano-selenium is in red color. It is unstable and easily inactivated [33]. The toxicity of nano-selenium may be relatively lower than that of the selenium compounds. In short-term and long-term toxicity experiments in mice, nano-selenium showed less oxidative damage, liver damage and less growth inhibition side effects than Na2SeO3 and selenomethionine [34]. Low dose nano-selenium also has the effects of anti-tumor and immune regulation. Nano-selenium is found to have a significant effect in cancer prevention and treatment, and is expected to be practically applied in the field [35,36]. Nano-selenium can play an antioxidant effect by inhibiting apoptosis. It can be used to up-regulate anti-apoptotic protein Bcl-2 and to reduce apoptotic protein Bax. The permeability of the mitochondrial membrane passage can be adjusted by nano-selenium to inhibit the promotion of apoptotic protein, such as cytochrome C, and this feature may be applied in the nervous system [37].
Early in 1997, bovine serum albumin (BSA) was employed as the stabilizer to prepare red nano-selenium [38]. It is bio-available and safe, and shows good antioxidant activity, which can significantly improve the abnormal oxidative stress state of cells [39]. The particle size of nano-selenium can affect its biological activity and cell uptake ability [40]. Therefore, it is important to choose the appropriate method to prepare the nano-selenium with appropriate size and morphology. Presently, nano-selenium can be synthesized via the chemical, physical and bio methods, and the chemical synthetic methods are practical for the low cost and high efficiency (Fig. 4). The aqueous solution of high valent selenium (such as H2SeO3 and its salts) can be reduced by vitamin C, glutathione, etc., to generate the red nano-selenium [33], which can be stabilized via the modification of the nano-selenium surfaces with polymers, carbohydrates, proteins, lipids and even medicines. The modification reagents usually possess a lot of hydroxyl or amino groups, and can lead to the stable electrostatic interaction with the nano-selenium surfaces to prevent the aggregation of the particles, ensuring the high dispersion and stability of nano-selenium. The release rate of nano-selenium under different conditions can be adjusted by changing the dosage and type of modification reagents, so as to improve its bioavailability and bioactivity in human body [40].
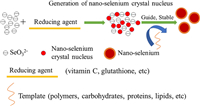
|
Download:
|
| Fig. 4. Diagram for the synthesis of nano-selenium. | |
In order to improve the stability of nano-selenium, people have paid much effort and tried to modify the nano-selenium with carbohydrates [41]. Polysaccharides are usually employed to modify the nano-selenium, and the related materials can be obtained via biosynthesis and chemical synthesis methods. In biosynthesis methods, bacteria, fungi, yeast, algae and plants are employed as the catalytic beds, while SeO32− is reduced by reducing enzymes to produce the nano-selenium [42]. It was found that the surface of the nano-selenium synthesized by the Bacillus paralicheniformis SR14 was covered with a stable nuclear shell structure [43]. In chemical synthesis methods, polysaccharides are usually employed to reduce the high valent selenium species into nano-selenium. They are also the stabilizers and dispersants for the system.
Brain nerve cells are vulnerable to oxidative damage caused by ROS because of their high oxygen consumption, high content of easily oxidized substrates, low content of antioxidant enzymes, and high ratio of membrane surface area to cytoplasmic volume [44]. Selenium is an important part of the brain's antioxidant defense system, and it can also improve the brain's oxidative stress via the regulation of Ca2+ channels, mitochondrial dysfunction, etc. [45]. Through multi-target action, nano-selenium can effectively reduce the oxidative stress state of the brain and delay the further pathological changes and development of nervous system diseases.
Alzheimer's disease (AD) is a complex progressive brain neurodegenerative disease, and its pathogenesis is mainly on the oxidative stress [45]. Chondroitin sulfate (CS) is a kind of glycosaminoglycan possessing the neuroprotective effect. It can be employed to stabilize nano-selenium to prepare the stable multi-target selenium chondroitin nanoparticles (Se@CS). By reducing ROS and malondialdehyde (MDA), the material can enhance the expression level of glutathione peroxidase (GPx) and reduce the damage caused by oxidative stress. On the other hand, it restrains the aggregation of amyloid β-protein and reduces the hyperphosphorylation of microtubule-associated protein tau to delay the development of AD. Presently, the complex pathogenesis, the difficult-to-overcome blood–brain barrier (BBB) and the development of the disease course which cannot be prevented led to huge challenges in the treatment of AD. Therefore, simple and easy-to-obtain selenium quantum dots (SeQDs) with a multitarget therapeutic effect were designed and prepared to overcome the issues. This new type of SeQDs are ultrasmall in size and can quickly break through the BBB. In vivo studies demonstrate that SeQDs can continuously accumulate in the brain after rapid passage of BBB and can quickly alleviate AD, significantly improve the memory impairment of AD mice, and improve their learning and memory ability [46].
Epilepsia is a kind of recurrent or unprovoked chronic neurodegenerative disease, during which, ROS and the inactivation of endogenous antioxidants will be produced in brain tissue. It was found that oral taking 0.5 mg/kg nano-selenium in advance could reduce oxidative stress by up regulating the expression of nuclear factor (Nrf2) and heme oxygenase 1 (HO-1). The inflammatory reaction and apoptosis cascade were also inhibited, while the duration of rigidity, muscle spasm and generalized seizures in mice were delayed after pentetrazol injection [47].
Nano-selenium was also successfully applied in the treatment of the common ischemic stroke. Polyethylene glycol-stabilized nano-selenium (OX26-PEG-Se NPs) was designed targeting to the monoclonal antibodies against cerebral capillary endothelial cells (OX26), and it was effective targeting ischemic stroke in vivo [44]. Selenium activates transcriptional factor TFAP2C and SP1 to enhance the other genes in the GPx4 protein and the transcription procedure (selenium group) to protect neurons. In the bleeding brain stroke model, the single dose of selenium enters the brain and it can promote the expression of antioxidant GPx4 protein, protect the neurons, and improve the behavior of iron death (Fig. 5) [48]. This provides infinite possibilities for nano-selenium in stroke treatment.
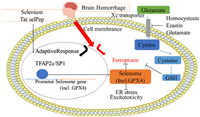
|
Download:
|
| Fig. 5. Selenium-driven transcriptional adaptation procedures to prevent iron death and treatment of stroke. | |
Lead widely exists in air, dust, soil, water and industrial products [49]. Since the lead absorption rate in gastrointestinal tract of children is much higher than that of the adults, it may lead to even more serious health problems for kids. Therefore, lead poisoning for children is a major public health problem in many countries [50,51]. In recent years, the researches on lead neurotoxicity have attracted much attention. Among the effects of lead on human tissues and systems, the nervous system is the most seriously affected [52]. Long term exposure to lead can cause irreversible damage to learning and memory functions [53]. The blood lead concentration exceeding 70 µg/L will damage the human central nervous system, resulting in the decline of memory and learning ability [54]. Lead is a strong neurotoxin, and due to the incomplete development of blood brain barrier in children, its neurotoxicity is more prominent than that for adults [55]. Neurological symptoms and complications of lead poisoning include the acute cerebral disease, peripheral neuropathy, hearing loss and neurological behavior defects, such as hyperactivity, developmental delay [56]. Indeed, animal experiments have confirmed that the inhibitory effect of lead poisoning on neuron continues from the development period to adulthood [57]. It could also lead to the obvious increasing of neuronal cell synaptic gap, the decreasing of postsynaptic density, active zone length and interface curvature and the changes in the expression of neural cell adhesion molecule (NCAM) protein in hippocampal neurons leading to the neuronal apoptosis [58–60].
Lead poisoning is closely related to the balance of antioxidant system in the body. The oxidative stress indexes such as superoxide dismutase (SOD), GPx, glutathione S-transferase (GST), catalase (CAT), total antioxidant capacity (TAOC), nitric oxide synthase (NOS) activities, and malondialdehyde (MDA) nitric oxide (NO), hydrogen peroxide (H2O2) and glutathione (GSH) contents are commonly used to evaluate lead-induced oxidative stress. By regulating mitochondrial dynamics and inhibiting the increase of apoptosis related genes, selenium can counteract the oxidative stress and apoptosis of cells caused by lead [61]. Low level lead exposure may inhibit the expression of neurocyte adhesion molecules (NCAM) and change the activity of sialyltransferase, resulting in the damage of formation and consolidation of learning and memory. For this process, selenium can reverse the reducing of NCAM caused by lead and alleviate the neurotoxicity of the metal [60]. Therefore, selenium can reduce the content of lipid peroxides produced by lead on the body, alleviate the lipid peroxidation reaction caused by heavy metals, and excrete it in a non-toxic form through bile, so as to accelerate the excretion of lead, reduce the accumulation of lead, and alleviate the oxidative stress caused by the toxicity of the heavy metals [62].
Investigations on 324 workers exposing to lead confirmed the effect of selenium on relieving lead poisoning [63]. The patients were divided into two groups, i.e., the low selenium intake group and the high selenium intake group. It was found that the contents of 8-hydroxydeoxyguanosine (8-OHdG) and lipopolysaccharide (LPS) and the activity of SOD in the serum samples of the patients in high selenium intake group were obviously higher than those of the patients in low selenium intake group. Contrarily, the activities of GSH, GPx and catalase in the serum samples of the patients in high selenium intake group are significantly higher than those of the patients in low selenium intake group. Spearman correlation shows that the selenium level is positively correlated with GPx activity and GSH levels [63].
Selenium has been widely reported in animals to alleviate the toxicity of heavy metals through selenoprotein. Investigations on the effects of selenium on selenoprotein expression in chicken cartilage induced by lead indicated that selenium could alleviate the decrease of GPx1, GPx2, GPx4, Txnrd2, Txnrd3, Dio1, Dio2, SelT, SelK, SelS, SelM, SelU, SelI, SelO, Sepn1, Sepx1, SelW and Sep15 in chicken meniscus tissue caused by lead. It could also alleviate the decrease of GPx2, GPx3, GPx4, Txnrd1, Txnrd2, Dio2, Dio3, SelT, SelK, SelH, SelM, SelI, SelO, Selpb, Sepn1, Sepx1, SelW, Sep15 and SPS2 in chicken bone sword tissue caused by lead. These results demonstrate that selenium antagonizes the changes of selenoprotein expression caused by lead toxicity [64].
5. Conclusions and perspectivesIn summary, selenium is an important trace element for brain development and nervous system function maintenance. Selenoproteins are the main forms of selenium in human brain, and they play important physiological roles. Nano-selenium can be employed for the treatment of a series of nervous system diseases, such as AD, epilepsia and ischemic stroke. The heavy metal elimination features of selenium can be applied in the treatment of lead poisoning of nervous system, which is caused by the lead pollution and is especially harmful to children for causing mental retardation. Thus, investigations on the relationship of selenium with human nervous system are of both practical and theoretical values.
However, there are still some challenges in the field. For example, the application scope of nano-selenium is not large enough, and it is majorly limit in the academic research, other than the practical applications. The investigation on the combined application of nano-selenium and antioxidants is not deep enough. The safeties of selenium materials need to be further clarified during the clinical practices. Up to present, a series of effective selenium-containing drugs have been developed for treating neurocognitive dysfunction, but cognitive decline is multifaceted and the fact that the toxicity of high doses of selenium may impede the development of the related medicines.
Indeed, the toxicity of selenium containing drugs depends not only on the dose of selenium, but also on the form of selenium. The toxicity of inorganic selenium is significantly higher than that of organoselenium species [65]. Fortunately, there have been many methods for the synthesis of organoselenium compounds owing to the rapid development of selenium chemistry during the last decade [66–68]. Besides the chemical synthesis methods, selenium-enriched yeast can transform toxic inorganic selenium into the absorbable organoselenium species for pharmaceutical applications [69]. Organoselenium compounds/materials can be used as the nutrient enhancers [70,71] and they are ideal selenium forms for the development of medicines [72,73]. The selenium content in plants and animals is greatly affected by the conditions. The randomness of the process of selenium replacing sulfur makes the content of selenium protein in plants and animals uncertain, which also affects its development in pharmaceutical industry.
Development of the selenium-containing medicines for the treatment of human nervous system diseases is unfolding and has attracted much attention. For example, Ebselen is a synthetic organoselenium drug molecule originally designed for GPx mimic, and the clinical trials of it were also being held. It is being investigated as a possible treatment for reperfusion injury and stroke, hearing loss and tinnitus, bipolar disorder and Huntington's disease [74–79]. Moreover, continuous efforts are been made for new forms of organic selenium, inorganic selenium and their responsive polymeric selenium and low-molecular weight selenium species, which provide the foundation for the future research of selenium and nervous system [80–90]. This field is of both challenges and opportunities and deserves further investigations.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank Jiangsu Provincial Six Talent Peaks Project (No. XCL-090) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for support. We thank the support of the fund of the joint-laboratory of Shanghai Dingya Pharmaceutical Chemical Technology Co., Ltd. with Yangzhou University (2022-2027).
| [1] |
W. Zhou, X. Xiao, Y. Liu, et al., Chin. J. Org. Chem. 42 (2022) 1849-1855. DOI:10.6023/cjoc202201023 |
| [2] |
Z. Zhu, S. Sun, X. Jing, Chem. Pap. 76 (2022) 401-408. DOI:10.1007/s11696-021-01875-6 |
| [3] |
Z. Zhu, S. Sun, S. Tang, S. Chu, X. Zhang, Mol. Catal. 515 (2021) 111923. DOI:10.1016/j.mcat.2021.111923 |
| [4] |
X. Xiao, C. Guang, J. Xu, Green Chem. 23 (2021) 4647-4655. DOI:10.1039/d1gc00961c |
| [5] |
F. Liu, J. Zhan, Y. Sun, et al., Chin. J. Org. Chem. 41 (2021) 2099-2104. DOI:10.6023/cjoc202011012 |
| [6] |
J. Huang, R. Qian, S. Wang, et al., Chin. J. Org. Chem. 41 (2021) 1639-1645. DOI:10.6023/cjoc202008032 |
| [7] |
H. Cao, R. Qian, L. Yu, Catal. Sci. Technol. 10 (2020) 3113-3121. DOI:10.1039/d0cy00400f |
| [8] |
H. Cao, Y. Yang, X. Chen, et al., Chin. Chem. Lett. 31 (2020) 1887-1889. DOI:10.1016/j.cclet.2020.01.027 |
| [9] |
X. Mao, P. Li, T. Li, et al., Chin. Chem. Lett. 31 (2020) 3276-3278. DOI:10.1016/j.cclet.2020.06.033 |
| [10] |
Y. Ge, J. Kong, C. Yang, et al., Chin. J. Org. Chem. 40 (2020) 1760-1765. DOI:10.6023/cjoc201912022 |
| [11] |
M. Liu, Y. Li, L. Yu, Q. Xu, X. Jiang, Sci. China Chem. 61 (2018) 294-299. DOI:10.1007/s11426-017-9158-y |
| [12] |
Z. Peng, J. Huang, Overview of Research on Selenium Resources in Enshi, the Selenium Capital of the World, 1st Ed, Tsinghua University Press, Beijing, 2012.
|
| [13] |
R.F. Burk, K.E. Hill, Biochim. Biophys. Acta 1790 (2009) 1441-1447. DOI:10.1016/j.bbagen.2009.03.026 |
| [14] |
G.A. Trapp, J. Millam, J. Neurochem. 24 (1975) 593-595. DOI:10.1111/j.1471-4159.1975.tb07682.x |
| [15] |
N.D. Solovyev, J. Inorg. Biochem. 153 (2015) 1-12. DOI:10.1016/j.jinorgbio.2015.09.003 |
| [16] |
R. Pillai, J.H. Uyehara-lock, F.P. Bellinger, IUBMB Life 66 (2014) 229-239. DOI:10.1002/iub.1262 |
| [17] |
L.L. Liu, C.M. Li, Z.W. Zhang, et al., Biol. Trace Elem. Res. 158 (2014) 176-185. DOI:10.1007/s12011-014-9919-5 |
| [18] |
P.D. Whanger, Nutr. Neurosci. 4 (2001) 81-97. DOI:10.1080/1028415X.2001.11747353 |
| [19] |
R. Boyd, Nat. Chem. 3 (2011) 570. DOI:10.1038/nchem.1076 |
| [20] |
Y. Zhang, Y. Zhou, U. Schweizer, et al., J. Biol. Chem. 283 (2008) 2427-2438. DOI:10.1074/jbc.M707951200 |
| [21] |
J.C. Peeler, E. Weerapana, Acc. Chem. Res. 52 (2019) 2832-2840. DOI:10.1021/acs.accounts.9b00379 |
| [22] |
M.W. Pitts, C.N. Byrns, A.N. Ogawa-Wong, et al., Biol. Trace Elem. Res. 161 (2014) 231-245. DOI:10.1007/s12011-014-0060-2 |
| [23] |
A. Dominiak, A. Wilkaniec, P. Wroczyński, et al., Curr. Neuropharmacol. 14 (2016) 282-299. DOI:10.2174/1570159X14666151223100011 |
| [24] |
S.V. Novoselov, M. Rao, N.V. Onoshko, et al., EMBO J. 21 (2002) 3681-3693. DOI:10.1093/emboj/cdf372 |
| [25] |
M.A. Reeves, F.P. Bellinger, M.J. Berry, Antioxid. Redox Signal. 12 (2010) 809-818. DOI:10.1089/ars.2009.2883 |
| [26] |
N. Morozova, E.P. Forry, E. Shahid, et al., Genes Cells 8 (2003) 963-971. DOI:10.1046/j.1365-2443.2003.00687.x |
| [27] |
C. Moreau, J.A. Duce, O. Rascol, et al., Mov. Disord. 33 (2018) 568-574. DOI:10.1002/mds.27275 |
| [28] |
S. Chu, Z. Niu, Q. Guo, et al., Eur. J. Pharmacol. 882 (2020) 173258-173298. DOI:10.1016/j.ejphar.2020.173258 |
| [29] |
Z. Wang, J. Yang, J. Qi, et al., Microvasc. Res. 131 (2020) 104012. DOI:10.1016/j.mvr.2020.104012 |
| [30] |
F. Gan, Y. Zhou, Z. Hu, et al., Int. J. Biol. Macromol. 146 (2020) 18-24. DOI:10.1016/j.ijbiomac.2019.11.221 |
| [31] |
H.Y. Kim, V.N. Gladyshev, Biochem. J. 407 (2007) 321-329. DOI:10.1042/BJ20070929 |
| [32] |
R. Pal, D.B. Oien, F.Y. Ersen, et al., Exp. Brain Res. 180 (2007) 765-774. DOI:10.1007/s00221-007-0903-6 |
| [33] |
C. Mellinas, A. Jiménez, M.D.C. Garrigós, Molecules 24 (2019) 4048-4067. DOI:10.3390/molecules24224048 |
| [34] |
A. Bhattacharjee, A. Basu, S. Bhattacharya, Nucleus 62 (2019) 259-268. DOI:10.1007/s13237-019-00303-1 |
| [35] |
J. Zhang, H. Wang, X. Yan, et al., Life Sci. 76 (2005) 1099-1109. DOI:10.1016/j.lfs.2004.08.015 |
| [36] |
H. Wang, J. Zhang, H. Yu, Free Radic. Biol. Med. 42 (2007) 1524-1533. DOI:10.1016/j.freeradbiomed.2007.02.013 |
| [37] |
K.M. Sadek, M.A. Lebda, T.K. Abouzed, et al., Metab. Brain Dis. 32 (2017) 1659-1673. DOI:10.1007/s11011-017-0053-x |
| [38] |
J.S. Zhang, X.Y. GAO, L.D. Zhang, et al., BioFactors 15 (2001) 27-38. DOI:10.1002/biof.5520150103 |
| [39] |
K. Bai, B. Hong, J. He, et al., Nutrients 12 (2020) 857-872. DOI:10.3390/nu12030857 |
| [40] |
X. Zhai, C. Zhang, G. Zhao, et al., J. Nanobiotechnol. 15 (2017) 4. DOI:10.14445/23942568/ijaes-v4i5p102 |
| [41] |
Y. Yang, X. Fan, H. Cao, et al., Catal. Sci. Technol. 8 (2018) 5017-5023. DOI:10.1039/c8cy01413b |
| [42] |
D. Mandal, M.E. Bolander, D. Mukhopadhyay, et al., Appl. Microbiol. Biotechnol. 69 (2006) 485-492. DOI:10.1007/s00253-005-0179-3 |
| [43] |
Y.Z. Cheng, X. Xiao, X.X. Li, et al., Carbohydr. Polym. 178 (2017) 18-26. DOI:10.1016/j.carbpol.2017.08.124 |
| [44] |
H. Amani, R. Habibey, F. Shokri, et al., Sci. Rep. 9 (2019) 6044-6058. DOI:10.1038/s41598-019-42633-9 |
| [45] |
F. Gao, J. Zhao, P. Liu, et al., Int. J. Biol. Macromol. 142 (2020) 265-276. DOI:10.1016/j.ijbiomac.2019.09.098 |
| [46] |
X. Guo, Q. Lei, Y. Liu, et al., ACS Appl. Mater. Interfaces 13 (2021) 30261-30273. DOI:10.1021/acsami.1c00690 |
| [47] |
X. Yuan, Z. Fu, P. Ji, et al., Int. J. Nanomed. 15 (2020) 6339-6353. DOI:10.2147/ijn.s259134 |
| [48] |
I Alim, J.T. Caulfield, Y. Chen, et al., Cell 177 (2019) 1262-1279. DOI:10.1016/j.cell.2019.03.032 |
| [49] |
Y.K. Yen, C.Y. Lai, Nanomaterials (Basel) 10 (2020) 2454. DOI:10.3390/nano10122454 |
| [50] |
K.L. Hon, C.K. Fung, A.K. Leung, Hong Kong Med. J. 23 (2017) 616-621. |
| [51] |
E. Erdenebayar, K.D. Santos, A. Edwards, et al., BMC Public Health 19 (2019) 163. DOI:10.1186/s12889-019-6486-x |
| [52] |
D.C. Bellinger, Pediatrics 113 (2004) 1016-1022. DOI:10.1542/peds.113.s3.1016 |
| [53] |
I. Al-Saleh, M. Nester, A. Mashhour, et al., J. Environ. Pathol. Toxicol. 28 (2009) 283-302. |
| [54] |
C. Dominguéz, E. Solé, A. Fortuny, Mol. Cell. Biochem. 237 (2002) 47-53. DOI:10.1023/A:1016547519763 |
| [55] |
J. Keosaian, T. Venkatesh, S. D'Amico, et al., Glob. Adv. Health Med. 8 (2019) 1-6. |
| [56] |
H. Okatch, M. Cherney, B. Mokshefsky, et al., Int. J. Env. Res. Public Health 16 (2019) 2281. DOI:10.3390/ijerph16132281 |
| [57] |
B. Wang, G. Feng, C. Tang, et al., Neurol. Sci. 34 (2013) 1181-1188. DOI:10.1007/s10072-012-1215-6 |
| [58] |
M. Wang, H.J. Fu, Y.M. Xiao, et al., Brain Res. 1530 (2013) 76-81. DOI:10.1016/j.brainres.2013.07.028 |
| [59] |
C. Venero, A.I. Herrero, K. Touyarot, et al., Eur. J. Neurosci. 23 (2006) 1585-1595. DOI:10.1111/j.1460-9568.2006.04663.x |
| [60] |
Z.Q. Deng, H.J. Fu, Y.M. Xiao, et al., Environ. Toxicol. Phar. 39 (2015) 221-228. DOI:10.1016/j.etap.2014.11.010 |
| [61] |
X. Jin, Z. Xu, X. Zhao, et al., Chemosphere 180 (2017) 259-266. DOI:10.1016/j.chemosphere.2017.03.130 |
| [62] |
H. Gurer, N. Ercal, Free Radic. Biol. Med. 29 (2000) 927-945. DOI:10.1016/S0891-5849(00)00413-5 |
| [63] |
N. Pawlas, M. Dobrakowski, A. Kasperczyk, et al., Biol. Trace Elem. Res. 170 (2016) 1-8. DOI:10.1007/s12011-015-0435-z |
| [64] |
H. Gao, C.P. Liu, S.Q. Song, et al., Biol. Trace Elem. Res. 172 (2016) 234-241. DOI:10.1007/s12011-015-0579-x |
| [65] |
M.H.M. Sari, L.M. Ferreira, V.C. Prado, et al., Eur. J. Pharm. Biopharm. 178 (2022) 69-81. DOI:10.1016/j.ejpb.2022.07.018 |
| [66] |
M. Wang, Q. Fan, X. Jiang, Org. Lett. 18 (2016) 5756-5759. DOI:10.1021/acs.orglett.6b03078 |
| [67] |
L. Shao, Y. Li, J. Lu, et al., Org. Chem. Front. 6 (2019) 2999-3041. DOI:10.1039/c9qo00620f |
| [68] |
Y. Liu, H. Ling, C. Chen, et al., Synlett 30 (2019) 1698-1702. DOI:10.1055/s-0037-1612083 |
| [69] |
T. Wang, X. Lou, G. Zhang, et al., Bioengineered 10 (2019) 335-344. DOI:10.1080/21655979.2019.1644853 |
| [70] |
L. Xian, Q. Li, T. Li, L. Yu, Chin. Chem. Lett. 34 (2023) 107878. DOI:10.1016/j.cclet.2022.107878 |
| [71] |
X. Xiao, Z. Shao, L. Yu, Chin. Chem. Lett. 32 (2021) 2933-2938. DOI:10.1016/j.cclet.2021.03.047 |
| [72] |
H. Cao, R. Ma, S. Chu, et al., Chin. Chem. Lett. 32 (2021) 2761-2764. DOI:10.1016/j.cclet.2021.03.029 |
| [73] |
M. Liu, X. Zhang, S. Chu, et al., Chin. Chem. Lett. 33 (2022) 205-208. DOI:10.1016/j.cclet.2021.05.061 |
| [74] |
M. Parnham, H. Sies, Expert Opin. Investig. Drug. 9 (2000) 607-619. DOI:10.1517/13543784.9.3.607 |
| [75] |
T. Yamaguchi, K. Sano, K. Takakura, et al., Stroke 29 (1998) 12-17. DOI:10.1161/01.STR.29.1.12 |
| [76] |
J. Kil, C. Pierce, H. Tran, et al., Hear. Res. 226 (2007) 44-51. DOI:10.1016/j.heares.2006.08.006 |
| [77] |
J. Kil, E.E. Harruff, R.J. Longenecker, Hear. Res. 413 (2022) 108209. DOI:10.1016/j.heares.2021.108209 |
| [78] |
N. Singh, A.C. Halliday, J.M. Thomas, et al., Nat. Commun. 4 (2013) 1332. DOI:10.1038/ncomms2320 |
| [79] |
R.P. Mason, M. Casu, N. Butler, et al., Nat. Genet. 45 (2013) 1249-1254. DOI:10.1038/ng.2732 |
| [80] |
G. Mugesh, W.W.D. Mont, H. Seis, Chem. Rev. 101 (2001) 2125-2179. DOI:10.1021/cr000426w |
| [81] |
X. Huang, X.M. Liu, Q. Luo, et al., Chem. Soc. Rev. 40 (2011) 1171-1184. DOI:10.1039/C0CS00046A |
| [82] |
H. Xu, W. Cao, X. Zhang, Acc. Chem. Res. 46 (2013) 1647-1658. DOI:10.1021/ar4000339 |
| [83] |
J. Shang, S. Li, T. Pan, et al., Chem. Commun. 56 (2020) 15052-15055. DOI:10.1039/d0cc05750a |
| [84] |
Q. Xu, Z. Cui, J. Yao, et al., Chin. Chem. Lett. 32 (2021) 4024-4028. DOI:10.1016/j.cclet.2021.05.058 |
| [85] |
Y. Huang, Y. Fu, M. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 4406-4414. DOI:10.1002/anie.201910615 |
| [86] |
P. Li, Z. Qi, L. Yu, H. Zhou, Catal. Sci. Technol. 12 (2022) 2241-2247. DOI:10.1039/d1cy02274a |
| [87] |
P. Li, K. Cao, X. Jing, Y. Liu, L. Yu, New J. Chem. 45 (2021) 17241-17246. DOI:10.1039/d1nj03311e |
| [88] |
Q. Wang, P. Li, T. Li, et al., Ind. Eng. Chem. Res. 60 (2021) 8659-8663. DOI:10.1021/acs.iecr.1c01437 |
| [89] |
H. Cao, P. Li, X. Jing, H. Zhou, Chin. J. Org. Chem. 42 (2022) 3890-3895. DOI:10.6023/cjoc202205005 |
| [90] |
Z. Zhu, W. Wang, L. Zeng, F. Zhang, J. Liu, Catal. Commun. 142 (2020) 106031. DOI:10.1016/j.catcom.2020.106031 |
 2023, Vol. 34
2023, Vol. 34