A fluoranthene is composed of a naphthalene and a benzene which are connected by a five-membered ring. Therefore, the fluoranthene is one of the smallest non-alternant polycyclic aromatic hydrocarbons [1-3] and its derivatives always exhibit unique optoelectronic properties [4-6], such as intense photoluminescence (PL) resistance to oxygen quenching, small Stokes shifts, and long PL lifetime. In the past decades, fluoranthenes have attracted increasing interest due to their outstanding applications in organic light-emitting diodes (OLEDs) [7], organic field-effect transistors (OFET) [8,9], perovskite solar cells [10,11], and fluorescent sensors [12]. Until now, a number of synthetic methodologies have been developed for the preparation of fluoranthene and its derivatives [13-15]. To endow the fluoranthene additional optoelectronic properties, substituents are required to extend the π-conjugation [16,17]. However, it is still unfavorable to functionalize the fluoranthene framework at different positions, most likely due to the relatively low selectivity and reactivity. In view of the intriguing photophysical properties of fluoranthene-containing organic semiconductors, it is still desirable to develop simple methods for the synthesis of fluoranthene derivatives and the extension of the π-conjugation through different positions.
The Diels-Alder (D-A) reaction is one of the most powerful synthetic approaches for the construction of cyclic compounds [18,19]. Numerous dienes and alkynes have been developed and utilized to produce six-membered aromatic rings via [4 + 2] D-A reactions [20-22]. Among the various dienes, thiophenes have been successfully converted to benzene rings via intermolecular [4 + 2] annulations, followed by sulfur extrusion reactions [23-25]. However, the intramolecular [4 + 2] annulation between the thiophene and alkyne has been rarely reported. In this paper, a catalyst-free intramolecular [4 + 2] annulation between the thiophene and the alkyne is utilized to construct fluoranthenes (Scheme 1). Various functional groups can be precisely introduced into the fluoranthenes at different positions by simply tuning the substituents on the thiophenes and alkynes. Therefore, this protocol provides a simple pathway to extend the conjugation of the fluoranthene and construct large polycyclic aromatic hydrocarbons.
|
Download:
|
| Scheme 1. Synthesis of fluoranthene derivatives via intramolecular [4 + 2] annulation reactions between thiophenes and alkynes. | |
The straightforward synthetic approach to the fluoranthene started from 1,8-dibromonaphthalene (1), as shown in Scheme 1. To prepare the asymmetric precursor 2 with thienyl and alkynyl substituents, palladium-catalyzed Sonogashira [26] and Suzuki [27] coupling reactions were alternately conducted. It should be noted that the sequence of the two-step coupling reactions can be random and the overall yields of the two steps are over 60% in both routes with reversed sequences. Subsequently, via catalyst-free intramolecular [4 + 2] annulation between thiophene and alkyne, fluoranthene derivative 3 could be produced.
The annulation condition was initially investigated using 1-(3-methylthien-2-yl)−8-(1-octynyl)naphthalene (2a) as the model starting material. Effects of solvent, temperature, and additive on the isolated yields have been systematically investigated. Firstly, the reactions were carried out by stirring the solutions of 2a (1.0 mmol) in different solvents (7 mL) at the temperatures around their boiling points (Table S1 in Supporting information, entries 1–6). In the solvents with low boiling points, such as tetrahydrofuran and acetonitrile, no starting material was consumed. With the solvent boiling point increasing, the yields enhanced to 10%, 45% and 54%, in toluene, N,N-dimethylformamide, and o-xylene, respectively. When the reaction was carried out at 180 ℃ using 1-methyl-2-pyrrolidinone (NMP) as the solvent, the yield dramatically improved to 89%. To figure out whether the reaction was temperature-dependent or solvent-dependent, the annulation reactions were further conducted in NMP at different temperatures (Table S1, entries 6–9). Similar to the results in lower boiling point solvent, the yields gradually decreased to 65%, 10% and 0%, at 160, 120 and 80 ℃, respectively. Interestingly, the solvent was not necessary for this annulation reaction. When 2a was heated at 180 ℃ without any solvent, 3a could also be produced but in low yield of 21% (Table S1, entry 10). Since NMP was regarded as a weak base, some basic additives were introduced to verify the possible contribution of a base in this annulation reaction (Table S1, entries 11–18). The results indicated that the introduction of basic additives, such as K2CO3, triethylamine (Et3N), 4-dimethylaminopyridine (DMAP), and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) did not assist the annulation reactions. Therefore, the temperature is crucial for this annulation reaction and further investigation was carried out by stirring the corresponding NMP solutions at 180 ℃. It should be noted that no obvious intermolecular reaction could be detected. After the reaction, only two spots, i.e., the desired product and the starting material, could be found on the thin-layer chromatography plate. The relatively lower yields could be attributed to the incompletely consumed starting material.
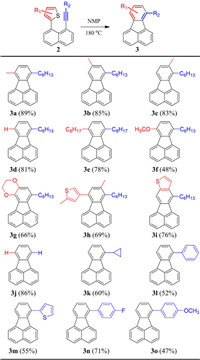
With the optimized reaction condition, the scope of the substituents R1 and R2 on the thienyl and the alkynyl groups, respectively, were evaluated (Scheme 2). Firstly, the substituted positions of R1 on the thiophene ring were studied. When the methyl group was substituted at 3-, 4- and 5-positions of the thiophene ring, products 3a, 3b and 3c with methyl groups substituted at different positions were obtained in high yields over 80%, indicating that the substitution on the 3-, 4- and 5-position of the thiophene group had a negligible effect on the annulation reaction. Moreover, when no substituent was on the thiophene ring or a longer alkyl group was substituted at 3-position, 3d and 3e were produced in yields around 80%. Furthermore, electron-donating units, such as 3-methoxylthiophene and 3,4-ethylenedioxythiophene, served as dienophiles and provided 3f and 3g, respectively. Additionally, other decorated thiophene derivatives, such as 5,5′-dimethyl-3,3′-bithiophene and thieno[3,2-b]thiophene, were also suitable for this [4 + 2] annulation reaction and corresponding products 3h and 3i were afforded in high yields. Subsequently, the universality of the alkynes was explored. When both R1 and R2 were hydrogen atoms, fluoranthene 3j without any substituent was obtained in 86% yield. It is interesting to note that when R1 was 3-hexylthiophene and R2 was a hydrogen atom, compound 3d could also be produced. In addition to the linear alkyl group, a cyclopropyl substituent with a secondary carbon was introduced on the fluoranthene skeleton (3k). Moreover, when the alkyl groups were replaced by aryl groups, phenyl-substituted 3l and thienyl-substituted 3m were afforded in moderate yields. Furthermore, electron-withdrawing fluoride and electron-donating methoxyl groups were further attached at the para-position of the phenyl groups. Compounds 3n and 3o were obtained in yields of 71% and 47%, respectively, which indicates that an electron-deficient substituent is more favorable for this annulation reaction in comparison to an electron-rich group.
|
Download:
|
| Scheme 2. Scope of the substituents on the thiophene and the alkyne units. | |
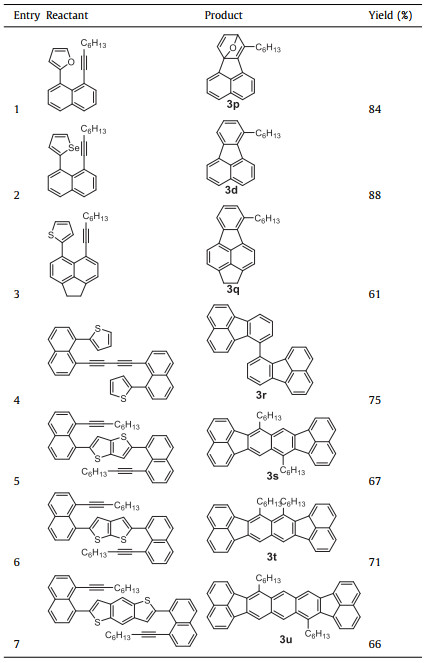
In addition to the two substituents R1 and R2 on the thiophene and the alkyne units, the scope of the 1-ethynyl-8-thienylnaphthalene skeleton was also investigated. As shown in Table 1, when furan and selenophene derivatives were used as the reactants (Table 1, entries 1 and 2), the [4 + 2] D-A reactions could occur. However, the oxygen could not be extruded and an oxygen bridged product 3p was obtained. Meanwhile, selenophene-based precursor could be converted to corresponding fluoranthene 3d, similar to thiophene-based reactant. In view of cost and synthetic feasibility, thiophene derivatives were much more convenient for this annulation reaction in comparison to selenophene analogues. Moreover, when 1,2-dihydroacenaphthalene was used instead of naphthalene, fluoranthene 3q was provided in 61% yield. Furthermore, when the precursors were dimerized, single bond linked fluoranthene dimer 3r was afforded in 75% yield. Interestingly, when thieno[3,2-b]thiophene and thieno[2,3-b]thiophene were used as building blocks for this annulation reaction, isomeric polycyclic aromatic hydrocarbons 3s and 3t were obtained in similar yields of 67% and 71%, respectively. Upon further introduction of a benzene ring, conjugation-extended product 3u was produced in 66% yield. All these examples demonstrate the universality of this intramolecular [4 + 2] annulation between thiophenes and alkynes towards fluoranthene derivatives.
|
|
Table 1 Scope of the skeletons for the annulation reactions. |
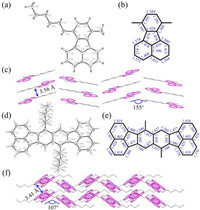
To gain insight into the molecular conformation, yellow single crystals of 3a and 3s suitable for X-ray diffraction were obtained by slow evaporation from mixed solutions of chloroform and methanol at room temperature. As shown in Fig. 1, the bond lengths of the C-C bonds in the benzene rings range from 1.360 Å to 1.439 Å, which are typical C-C bond lengths for delocalized aromatic systems. However, the bonds lengths of the two unshared C-C bonds in the five-membered rings (C5-C9 and C6-C7 in 3a, and C3-C4 and C10-C11 in 3s) are in the region of 1.476–1.480 Å, suggesting their single bond characteristics. Moreover, the fluoranthene skeletons in both molecules of 3a and 3s are planar. Therefore, relatively small interplane distances of 3.56 and 3.41 Å can be found for 3a and 3s which display herringbone packing structures with herringbone angles of 155° and 107°, respectively. Moreover, the shortest C-H···π distances are measured to be 2.85 and 3.01 Å for 3a and 3s (Fig. S3 in Supporting information), respectively, suggesting the weak intermolecular interactions among the planar fluoranthene molecules.
|
Download:
|
| Fig. 1. (a, d) ORTEP diagram with ellipsoid contour probability level of 50%, (b, e) bond lengths of the core motifs, and (c, f) molecular packings of 3a and 3s. | |
The mechanism for this intramolecular [4 + 2] annulation reaction between thiophenes and alkynes is proposed and shown in Fig. S4 (Supporting information). First, the 1,3-diene subunit in the thiophene ring reacts with the alkyne unit and produces the intermediate compound IM via the transition state TS, which is similar to the traditional [4 + 2] Diels-Alder reaction. Subsequently, the sulfur atom decomposes to produce the product via S-extrusion reaction due to the large ring tension. The feasibility of the [4 + 2] D-A reaction was further verified by density functional theory (DFT) calculations. The reaction pathway and the relative Gibbs free energies for the starting material SM, the transition state TS, and the S-bridged intermediate IM at 453.15 K were calculated using Gaussian 16 program [28] with the B3LYP functional and the 6–31G(d) basis set. As shown in Fig. S5 (Supporting information), although the [4 + 2] D-A reaction is endothermic only at 2.8 kcal/mol, a large energy barrier of 37.3 kcal/mol can be found for the first [4 + 2] D-A reaction, which explains the high temperature condition in this annulation reaction.
The photophysical properties of the as-prepared fluoranthene derivatives were further investigated by measuring their UV–vis absorption and photoluminescence (PL) spectra in toluene solutions (ca. 5 × 10−6 mol/L). As shown in Fig. S6 (Supporting information), 3j displays two absorption bands in the region of 250–300 and 300–400 nm, assigning to the S0 → S2 and S0 → S1 electron transitions, respectively. Meanwhile, 3j exhibits the maximum PL wavelength at 442 nm. Under the same condition, compounds 3r, 3s and 3u with different conjugation skeletons demonstrate absorption maxima at 362, 454 and 478 nm, and PL maxima at 461, 484, and 499 nm, which is obviously due to the extended effective conjugation length. Fig. S7 (Supporting information) shows the photographs of the dilute solutions of 3j, 3r, 3s and 3u under the irradiation of a UV light. Strong blue or blue-green emissions can be observed, suggesting their intense luminescent characteristics.
In summary, a catalyst-free intramolecular [4 + 2] annulation between thiophenes and alkynes has been established to construct fluoranthene derivatives. Various functional groups can be introduced to the different positions of fluoranthenes by simply tuning the substituents on the 1-ethynyl-8-thienylnaphthalene skeleton. Consequently, the conjugation of the fluoranthene can be facilely extended through different directions. Overall, this work provides not only a synthetic methodology towards fluoranthenes with substituent functionalized at different positions, but also an effective pathway to construct large polycyclic aromatic hydrocarbons containing fluoranthene moieties.
Declaration of competing interestThe authors declare no competing financial interest
AcknowledgmentsThe authors thank Dr. Lingling Li at the Instrumental Analysis Center of SJTU for single crystal analysis. This work was financially supported by National Key Research and Development Program of China (No. 2018YFA0209401), National Natural Science Foundation of China (Nos. 22171053, 21733003), and Natural Science Foundation of Shanghai (No. 21ZR1409600).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.06.065.
| [1] |
A. Konishi, M. Yasuda, Chem. Lett. 50 (2021) 195-212. DOI:10.1246/cl.200650 |
| [2] |
H. Takano, N. Shiozawa, Y. Imai, K.S. Kanyiva, T. Shibata, J. Am. Chem. Soc. 142 (2020) 4714-4722. DOI:10.1021/jacs.9b12205 |
| [3] |
T. Wu, M. Chen, Y. Lee, M. Kuo, Y. Wu, Angew. Chem. Int. Ed. 52 (2013) 1289-1293. DOI:10.1002/anie.201208200 |
| [4] |
I.B. Berlman, H.O. Wirth, O.J. Steingraber, et al., J. Am. Chem. Soc. 90 (1968) 566-569. DOI:10.1021/ja01005a003 |
| [5] |
A. Slodek, A. Maroń, M. Pająk, Chem. Eur. J. 24 (2018) 9622-9631. DOI:10.1002/chem.201801123 |
| [6] |
X. Li, M. Li, W. Yao, H. Lu, Y. Zhao, C. Chen, RSC Adv. 5 (2015) 18609-18614. DOI:10.1039/C4RA17112H |
| [7] |
S. Kumar, D. Kumar, Y. Patil, S. Patil, J. Mater. Chem. C. 4 (2016) 193-200. DOI:10.1039/C5TC02926K |
| [8] |
Q. Yan, Y. Zhou, B. Ni, et al., J. Org. Chem. 73 (2008) 5328-5339. DOI:10.1021/jo800606b |
| [9] |
X. Sun, M. Liao, X. Yu, et al., Chem. Sci. 13 (2022) 996-1002. DOI:10.1039/d1sc06807e |
| [10] |
X. Sun, Q. Xue, Z. Zhu, et al., Chem. Sci. 9 (2018) 2698-2704. DOI:10.1039/C7SC05484J |
| [11] |
X. Yu, Z. Li, X. Sun, et al., Nano Energy 82 (2021) 105701. DOI:10.1016/j.nanoen.2020.105701 |
| [12] |
N. Venkatramaiah, S. Kumar, S. Patil, Chem. Commun. 48 (2012) 5007-5009. DOI:10.1039/c2cc31606d |
| [13] |
M. Yamaguchi, M. Higuchi, K. Tazawa, K. Manabe, J. Org. Chem. 81 (2016) 3967-3974. DOI:10.1021/acs.joc.6b00553 |
| [14] |
N. Ogawa, Y. Yamaoka, K.I. Yamada, K. Takasu, Org. Lett. 19 (2017) 3327-3330. DOI:10.1021/acs.orglett.7b01538 |
| [15] |
J. Karunakaran, A.K. Mohanakrishnan, Eur. J. Org. Chem. 2017 (2017) 6747-6762. DOI:10.1002/ejoc.201701137 |
| [16] |
L.M. Geary, T. Chen, T.P. Montgomery, M.J. Krische, J. Am. Chem. Soc. 136 (2014) 5920-5922. DOI:10.1021/ja502659t |
| [17] |
T. Wu, H. Hsin, M. Kuo, C. Li, Y. Wu, J. Am. Chem. Soc. 133 (2011) 16319-16321. DOI:10.1021/ja2067725 |
| [18] |
J. Li, T. Liu, Y. Chen, Acc. Chem. Res. 45 (2012) 1491-1500. DOI:10.1021/ar3000822 |
| [19] |
K.C. Nicolaou, S.A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 41 (2002) 1668-1698. DOI:10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z |
| [20] |
M.E. Welker, Molecules 25 (2020) 30740-30763. |
| [21] |
D. Tejedor, A. Diaz-Diaz, R. Diana-Rivero, S. Delgado-Hernández, F. García-Tellado, Org. Lett. 20 (2018) 7987-7990. DOI:10.1021/acs.orglett.8b03558 |
| [22] |
M. Xu, X. You, Y. Zhang, et al., J. Am. Chem. Soc. 143 (2021) 8993-9001. DOI:10.1021/jacs.1c04759 |
| [23] |
D.B. Clapp, J. Am. Chem. Soc. 61 (1939) 2733-2735. DOI:10.1021/ja01265a051 |
| [24] |
C. Song, X. Dong, Z. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 12206-12210. DOI:10.1002/anie.201905971 |
| [25] |
W.G. Kanhounnon, F. Richard, G.A. Kpotin, et al., Top. Catal. 64 (2020) 288-296. |
| [26] |
R. Chinchilla, C. Najera, Chem. Soc. Rev. 40 (2011) 5084-5121. DOI:10.1039/c1cs15071e |
| [27] |
A. Suzuki, Angew. Chem. Int. Ed. 50 (2011) 6722-6737. DOI:10.1002/anie.201101379 |
| [28] |
M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian, Inc., Wallingford CT, 2019.
|
 2023, Vol. 34
2023, Vol. 34