Supramolecular chirality reflects asymmetric packing of molecules in aggregates, which offers a feasible way to produce materials for catalysis, sensing, display, information and chiroptical applications [1,2]. Precise manipulation of supramolecular chirality at hierarchical levels remains major challenges, and the development of novel protocols to rational design, prepare and control the properties of chiral supramolecular systems is still premature [3,4]. Compared to single component system, properties of multiple-component coassembly could be readily manipulated by varying the ratio and species [5]. The synergistic coassembly allows for the transfer, inversion, amplification of supramolecular chirality. To realize above behaviors, multiple noncovalent interactions between components have been built, such as hydrogen bonding [6–10], charge-transfer [11], π-π stacking [12], metal-ligand coordination [13], host-guest inclusion [14], halogen bonding [15] and arene-perfluorine interaction [16]. Among the above noncovalent forces, hydrogen bonding is an intensively explored interaction to manipulate and control the supramolecular chirality in multiple component systems. Pyridine, melamine, imidazole and cytimidine with sp2 hybridization N atoms are favourable acceptor to carboxylic acids [17]. Amine-acid salt bridge-type hydrogen bonds were also utilized to fabricate chiral coassemblies [18].
π-Conjugated amino acids and short peptides are a class of building units to give chiral nanoarchitectures with diversified size and dimensions [19]. The abundant hydrogen bonding sites including carbonyl and amide groups with alternative donor-acceptor arrays enable one-dimensional growth with the emergence of helical assembly and chiroptical responses. In this regard, the π-conjugated amino acids with specific topology might be potential hosts to include guests via hydrogen bonding to afford chiral coassemblies, which however have not been reported so far. We pictured that the C3-symmetrical π-conjugated amino acids would be favourable host candidates to accommodate small organic acids bearing both hydrogen bonding donor and acceptor. Thus, in this work, we designed and synthesized a series of C3-symmetrical π-conjugated amino acid derivatives protected by methoxy group to avoid unfavourable self-sorting (Scheme 1). These molecules show inclusion complexation towards organic carboxylic acids in organic solution and in the aggregated state. Experimental and computational results suggest multiple hydrogen bonds occurred between carboxylic acids and π-conjugated amino acid derivatives. Coassembly enhanced the chiral assembly with fine universality to organic acids with large substrate scope, whereby Cotton effects were boosted. The present systems are also potential chiroptical materials. Circularly polarized luminescence (CPL) tests showed relatively high dissymmetry g-factor at 10−2 order of magnitude [20], which can be tuned by coassemblies. This work provides a new manner using organic carboxylic acids to manipulate the supramolecular chirality and chiroptical properties of amino acid derivatives.
|
Download:
|
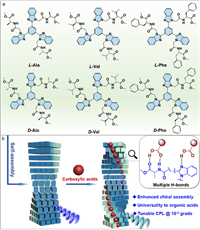
| Scheme 1. (a) Chemical structures of benzimidazole amino acid derivatives used in this work; (b) Schematic representation of carboxylic acids-tuned chiral self-assembly via complementary bonds and the properties. | |
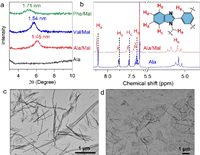
The building blocks including Ala, Val and Phe bearing typical amino acids residues were synthesized via four steps (details see Supporting information), of which structures were confirmed by the nuclear magnetic resonance (NMR) and mass spectra. Considering the hydrophobicity, a nanoprecipitation method was employed to trigger their self-assembly in aqueous media by dispersing stock solutions of dimethyl sulfoxide (DMSO, 100 mmol/L). The fraction of DMSO was less than 0.5 vol% for a 0.5 mmol/L self-assembly. Such as small ratio of DMSO has negligible effect on the self-assembly behavior. Colloidal dispersions were obtained for individual compounds, indicating the formation of nanostructures. We introduced a model malonic acid (Mal) to investigate the coassembly behaviors with the building units. Building units were premixed with Mal in DMSO followed by adding bulky water to initiate the coassembly. Phase behaviors were barely changed, with a still colloidal appearance. Thin-film X-ray diffraction (XRD) was employed to explore the occurrence of molecular level coassembly. The aggregates were centrifuged and air-dried before subjecting to XRD tests (Fig. 1a and Fig. S19 in Supporting information). The individual aggregates show amorphous phase by showing no apparent peaks in both small and wide angles, suggesting poor crystallinity in the self-assemblies. In the presence of Mal (1 molar equiv.), peaks at small angle region were observed with d-spacing values of 1.45, 1.54 and 1.71 nm for Ala/Mal, Val/Mal and Phe/Mal respectively. These peaks verify an enhanced long-range ordered coassembly brought by Mal. Despite the highly hydrophilic property of Mal in contrast to the amino acid derivatives, Mal could be incorporated into coassembly arrays via noncovalent forces. The supramolecular complexation was further probed by other techniques. In the 1H NMR spectra (Fig. 1b, Figs. S20 and S21 in Supporting information) carried out in CDCl3, the aromatic protons (Ha to He) display shifts to lower fields after the addition of 1 molar equiv. Mal. The enhanced deshielding effect suggests the host-guest complexation such as -COOH-imidazole hydrogen bonding occurred between the components even in the presence of strong competitive solvation (CDCl3). In the fourier transform infrared (FT-IR) spectra, the peak at 1662 cm−1 which is assigned as the amide-I band shows relative shrunk intensity (Fig. S22 in Supporting information). Hydrogen bonding rearrangement might be occurred between Ala and Mal. Under transmission electron microscopy (TEM) observation, nanorod architectures were generated from Ala with width and length at around 50 nm and several micrometres (Fig. 1c and Figs. S23–S28 in Supporting information). In the presence of Mal (1 molar equiv.), new structures with weaker contrast and smaller size emerged (Fig. 1d). Mal brings along specific hydrophilicity to the coassembly, resulting in the coassembled nanoarchitectures.
|
Download:
|
| Fig. 1. (a) XRD pattern comparison of different samples; (b) 1H NMR spectra comparison of Ala and Ala/Mal in CDCl3 with 10 vol% CD3OD. (c, d) TEM images of Ala and Ala/Mal aqueous self-assembly respectively. Concentrations of all samples were fixed at 0.5 mmol/L. | |
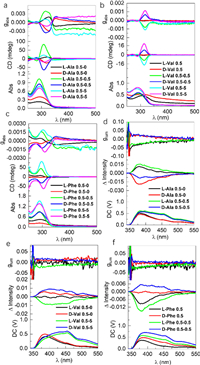
π-Conjugated amino acid derivatives have interesting chiroptical activities in their coassemblies. Then circularly dichroism (CD) and CPL spectra of individual and coassembled systems were collected (Fig. 2 and Fig. S29 in Supporting information). Exciton-type Cotton effects at the maxima absorbance of Ala were observed, which show positive and negative bands respectively for L- and D-enantiomer (Fig. 2a). Dissymmetry g-factor (gabs) was determined as ±0.002 at 325 nm. The Cotton effects of self-assembled Ala indicate the chirality transfer from amino acids to benzimidazole chromophores within chiral architectures. The introduction of Mal would enhance the CD signal intensities as well as the absorbance, which is consistent to the above conclusions with respect to the hydrophilicity brought by Mal. The chiral assemblies were expected to be enhanced based on the enlarged gabs values (±0.004 at 314 nm, 1 equiv. Mal). The enhancement of CD signal was also observed in the Val and Phe coassembled systems (Figs. 2b and c). Val individually shows negligible Cotton effects and gabs. The participation of Mal induces the emergence of Cotton effects with gabs up to ±0.001 at 316 nm (10 equiv. Mal). The maxima gabs of Phe was also boosted from ±0.0004 to ±0.0016 by coassembly. The intensity of Cotton effects in supramolecular self-assemblies are associated to the screw sense (degree) and distance between the chromophores. The boosted g-factors by Mal no doubtly improve the helical packing of π-conjugated amino acids. Photoluminescence (PL) spectra of different self-assemblies and coassemblies with Mal were collected in their solution state, as shown in Fig. S30 (Supporting information). Compared to the individual self-assemblies, the presence of 10 molar equiv. Mal would greatly shrink the PL intensity, which was observed in the three examples. A bathochromic shift occurred to Phe/Mal coassembly, in consistent with the CPL spectroscopy. The declined PL intensity suggests the participation of Mal into the coassemblies. We also evaluated their CPL properties to reveal their potential in chiroptical materials. Ala individually afforded PL and CPL peaks located at 380 nm (Fig. 3d). After adding Mal, shoulder peaks at around 450 nm emerged with a shift of CPL curves, which possibly caused by the enhanced aromatic interactions. The dissymmetry g-factors (glum) were determined as ±0.03 at 380 nm, which are among the highest values of organic self-assemblies [21]. Similarly, coassembly of Mal with Val or Phe induced the emergence of new PL and CPL bands at 450 nm with glum values at 10−2 grade (Figs. 2e and f). CPL spectra indicate that the present coassembly systems are potential chiroptical materials with tunable photophysical properties. CD and CPL spectroscopy reflect chirality at the ground and photoexcited state respectively. The changes of CD and CPL signal are relative independent to each other due to the structural relaxation and other factors. The coassembly between organic small acids and TBIB derivatives could change the chiral packing of chromophores which has significant influence on the Cotton effects, due to the CD signal is proportional to the screw sense between chromophores. However, this effect is not pronounced in the emergence of CPL spectra.
|
Download:
|
| Fig. 2. Chiroptical activities. (a–c) Absorption, CD and gabs factor spectra comparison of Ala, Val and Phe self-assemblies with different molar ratios of Mal, respectively; (d–f) CPL spectra of Ala, Val and Phe self-assemblies and coassemblies with different concentrations and ratios of Mal, respectively. (λex = 300 nm). The ratios stand for the milli molar per liter (mmol/L). Absolute configuration (L- or D-) was marked to compare the enantiomeric effect. | |
|
Download:
|
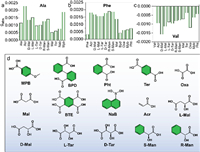
| Fig. 3. (a–c) gabs values of Ala, Phe and Val coassemblies (L-configuration) with organic acids (excess, 10 equiv.) at 310 nm, 310 nm and 300 nm respectively, blank samples were also added for comparison; (d) The chemical structures of expanded carboxylic acids. | |
Then we envisioned that, other than Mal, other types of aromatic carboxylic acids may also be potential species for enhanced chiroptical activities. Acidification of the self-assemblies by hydrochloric acid did not arouse apparent chiroptical changes, which supports the crucial role of carboxylic acids. As shown in Fig. 3, a series of organic carboxylic acids were employed, and their gabs values at the corresponding absorbance were summarized. The coassembly effects are different for the three amino acid derivatives. For Ala, only specific acids such as acrylic acid and p-methoxy phenylboronic acid could enhance the g-factors, while Phe and Val with negligible Cotton effects, were generally enhanced after coassembly. No apparent selectivity was found (Fig. 3a). Nevertheless, the enhancement of Cotton effects shows universality for Phe and Val (Figs. 3b and c, Fig. S31 in Supporting information). All the employed organic acids are capable of enhancing gabs up to 10 folds. This situation is more pronounced for Val due to the negligible Cotton effects individually. Although different small organic acids possess different geometry and structures, they show slight difference with respect to the Cotton effect enhancement. It means the key factor of organic acids is the COOH group that provides sufficient hydrogen bonds, while the other structural factors could hardly contribute to the molecular chiral self-assemblies. It is worth noting that although chiral natural acids were used, no apparent bias effects were observed, and the chirality of acids hardly transfer to chromophores via noncovalent forces. It implies the inherent chirality is the major factor to control over the overall chirality and chiroptical activities. It is however understandable. The chiral centres of amino acids are conjugated covalently to the imidazole core, while the organic chiral acids attached to the building units via hydrogen bonds are weak in nature. Therefore, it is expectedly that chiral acids could hardly influence the chirality and chiroptical behaviors.
In order to give insights into the self-assembly mechanism, full-atom molecular dynamic simulation (MD) was carried out (Fig. 4 and Fig. S32 in Supporting information). The π-conjugated amino acid derivatives were dispersed in a simulation box (10 nm × 10 nm × 10 nm) full of water molecules, which underwent 50 ns equilibrium. Ala formed aggregates with extended one-dimensional (1D) growth (Fig. 4a), which is in good agreement with the fibrous structures under TEM observation. The highlighted 1D molecular extension is primarily driven by aromatic interactions in addition to the hydrogen bonds between amides. The aromatic interactions comprise typical π-π stacking as well as the CH··· π close contacts between benzimidazole planes. By introducing Mal (3 equiv.), 1D aggregates were not interfered in the presence of hydrophilic organic acids (Fig. 4b). Many Mal molecules have not been incorporated into aggregates due to the competitive solvation effects in water. Mal molecules inside the Ala assembly are evenly distributed. Expectedly, the supramolecular complexation between Ala and Mal is driven primarily by complementary hydrogen bonds. Typically, carboxylic acids form duplex hydrogen bonds (distance < 2.0 Å) with amide groups, which stabilize the complexes. The MD simulation between Val and Mal also verifies the similar binding interactions between amides and carboxylic acids (Fig. S32 in Supporting information). The MD results are in accordance with the NMR and FTIR results as well, confirming the crucial rule of hydrogen bonds in the complexation.

|
Download:
|
| Fig. 4. MD results. (a) Morphology of Ala in a simulation box after 50 ns equilibrium, and the represented molecular packing and aromatic interactions; (b) morphology of Ala/Mal coassembly (1:3 by molar), and the distribution of Mal in Ala assembly as well as the detailed noncovalent interactions. | |
In summary, we unveil the small organic carboxylic acids as the key components to modulate the supramolecular chirality and chiroptical properties of π-conjugated amino acids. The introduction of carboxylic acids boosted the dissymmetry factors of the aqueous self-assemblies. The present systems show strong CPL activities with glum at 10−2 grade. Complementary hydrogen bonds facilitated the coassembly induced by the experimental and simulation results. This work establishes an alternative protocol using commercially available organic acids to control and prepare advanced chiroptical materials.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (Nos. 21901145, 22171165). We also acknowledge the financial support from Youth Cross-Scientific Innovation Group of Shandong University (No. 2020QNQT003).
| [1] |
M. Liu, L. Zhang, T. Wang, Chem. Rev. 115 (2015) 7304-7397. DOI:10.1021/cr500671p |
| [2] |
E. Yashima, N. Ousaka, D. Taura, et al., Chem. Rev. 116 (2016) 13752-13990. DOI:10.1021/acs.chemrev.6b00354 |
| [3] |
L. Zhang, L. Qin, X. Wang, et al., Adv. Mater. 26 (2014) 6959. DOI:10.1002/adma.201305422 |
| [4] |
Z. Wang, A. Hao, P. Xing, Chin. Chem. Lett. 32 (2021) 1390-1396. DOI:10.1016/j.cclet.2020.10.032 |
| [5] |
D. Wang, H. Liu, W. Wang, Chin. Chem. Lett. 33 (2022) 1488-1492. DOI:10.1016/j.cclet.2021.08.040 |
| [6] |
P. Xing, Y. Zhao, Acc. Chem. Res. 51 (2018) 2324-2334. DOI:10.1021/acs.accounts.8b00312 |
| [7] |
A. Das, S. Ghosh, Angew. Chem. Int. Ed. 53 (2014) 1092-1097. DOI:10.1002/anie.201308396 |
| [8] |
P. Xing, P. Li, H. Chen, et al., ACS Nano 11 (2017) 4206-4216. DOI:10.1021/acsnano.7b01161 |
| [9] |
G.F. Liu, L.Y. Zhu, W. Ji, Angew. Chem. Int. Ed. 55 (2016) 2411-2415. DOI:10.1002/anie.201510140 |
| [10] |
S. Dhiman, A. Jain, M. Kumar, J. Am. Chem. Soc. 139 (2017) 16568-16575. DOI:10.1021/jacs.7b07469 |
| [11] |
Z. Yu, A. Erbas, F. Tantakitti, J. Am. Chem. Soc. 139 (2017) 7823-7830. DOI:10.1021/jacs.7b02058 |
| [12] |
Z. Wang, Y. Li, A. Hao, et al., Angew. Chem. Int. Ed. 60 (2021) 3138-3184. DOI:10.1002/anie.202011907 |
| [13] |
P. Xing, Z. Zhao, A. Hao, Chem. Commun. 53 (2016) 1246-1249. DOI:10.1039/C5CC08858E |
| [14] |
L. Zhu, X. Li, S. Wu, J. Am. Chem. Soc. 135 (2013) 9174-9180. DOI:10.1021/ja403722t |
| [15] |
L. Ji, Q. He, D. Niu, et al., Chem. Commun. 55 (2019) 11747-11750. DOI:10.1039/C9CC06305F |
| [16] |
S. An, A. Hao, P. Xing, ACS Nano 15 (2021) 15306-15315. DOI:10.1021/acsnano.1c06178 |
| [17] |
J. Zhao, B. Wang, A. Hao, Nanoscale 14 (2021) 1779-1786. DOI:10.1039/D1NR06254A |
| [18] |
P. Xing, Y. Li, S. Xue, J. Am. Chem. Soc. 141 (2019) 9946-9954. DOI:10.1021/jacs.9b03502 |
| [19] |
Y. Wang, W. Qi, R. Huang, J. Am. Chem. Soc. 137 (2015) 7869-7880. DOI:10.1021/jacs.5b03925 |
| [20] |
S. Fleming, R.V. Ulijn, Chem. Soc. Rev. 43 (2014) 8150-8177. DOI:10.1039/C4CS00247D |
| [21] |
Y. Sang, J. Han, T. Zhao, et al., Adv. Mater. (2019) 1900110. DOI:10.1002/adma.201900110 |
 2023, Vol. 34
2023, Vol. 34 

