b Department of Chemistry, Engineering Center of Catalysis and Synthesis for Chiral Molecules, Fudan University, Shanghai 200433, China;
c Shanghai Engineering Research Center of Industrial Asymmetric Catalysis of Chiral Drugs, Fudan University, Shanghai 200433, China
Carbon dioxide (CO2) is an attractive C1 source that gains growing interest in producing high value-added fine chemicals in recent years [1–9]. One of the most promising approaches of chemical fixation of CO2 is the conversion of CO2 into carboxylic acids which has been extensively studied in organic synthesis [10–13].
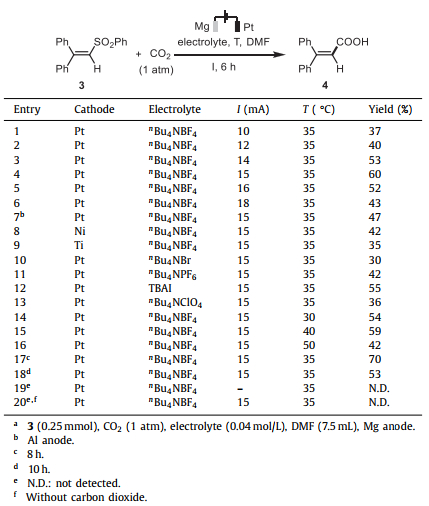
α,β-Unsaturated carboxylic acids and their derivatives are important skeletons in bioactive molecules and they are also versatile building blocks involved in many useful transformations [14–16]. The preparation of α,β-unsaturated carboxylic acids from CO2 with alkynes [17], alkenes [18–24], or allenes [25] has been well-documented. Nevertheless, control of the stereoselectivity needs to be specially noticed in the preparation of vinyl derivatives. Getting a mixture of cis and trans vinyl products leads to wasting a part of the material if the product with an unwanted configuration is discarded. On the other hand, it is difficult to isolate the target product from the isomers. Thus, stereoselective synthesis of α,β-unsaturated carboxylic acids with retention of the original configuration from parent alkenes is highly desirable. BuLi has been used to convert vinyl halides into the corresponding vinyl lithium reagents, which could then attack carbon dioxide to give α,β-unsaturated carboxylic acids in keeping the original alkene geometry [26]. However, the substrate scope is limited by the poor tolerance with electrophilic groups in the presence of BuLi reagent.
In addition, transition-metal-catalyzed carboxylation of vinyl derivatives, such as haloalkenes [27–33], vinyl boronic acid esters [34–38], vinyl sodium sulfinates [39] and so on [40–42], has been developed as a robust method to construct α,β-unsaturated carboxylic acids. The original configuration of the parent precursor is usually preserved. Transition-metal-free organic synthesis is environmentally benign and sustainable that gained more and more attention in 21 century [43]. Nevertheless, stereoselective carboxylation of vinyl derivatives under transition-metal-free and mild conditions to produce α,β-unsaturated carboxylic acids is elusive. König and coworkers have reported a photocatalytic C—H carboxylation of styrenes to afford E-cinnamic acids (Scheme 1b) [44]. Additionally, synthetic electrochemistry plays an important role for organic transformations [45–50]. An electrochemical reduction of β-bromostyrenes was reported by Kuang and co-workers (Scheme 1c) [51]. Notably, either the E-bromostyrene or the Z-bromostyrene affords a mixture of cinnamic acids. This is attributed to the low energy barrier to inversion for α-hydrogen vinyl radicals (for the ethenyl radical is 3.3 kcal/mol) [52] that leads to a mixture of cis- and trans-products. The authors found that the stereospecific electrochemical carboxylation of β-bromostyrenes was only achieved when the nickel salt is added as a catalyst. The electrochemical defluorinative carboxylation of gem-difluoroalkenes under transition metal-free conditions has been developed recently, providing the α-fluoroacrylic acids with the Z/E selectivities from 1/2 to 20/1 (Scheme 1d) [53]. Thus, stereoselective electrochemical carboxylation of vinyl derivatives remains challenging under transition-metal-free conditions. Given that the easily available organosulfones are versatile building blocks in organic synthesis, they may also serve as promising reaction partners in reductive functionalization [54–58]. Herein, we report the electrochemically stereoselective carboxylation of α,β-unsaturated sulfones to afford a series of α,β-unsaturated carboxylic acids.
|
Download:
|
| Scheme 1. Carboxylation of olefinic compounds with CO2. | |
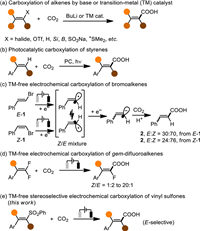
In a preliminary investigation, (2-(phenylsulfonyl)ethene-1,1-diyl)dibenzene (3) in a DMF solution containing nBu4NBF4 was initially tried to react with CO2 at atmospheric pressure under electrolysis with a Mg anode and a Pt cathode in an undivided cell. To our delight, when a constant current of 10 mA was applied between the two electrodes at 35 ℃, the expected vinyl carboxylic acid 4 was obtained after 6 h albeit in a poor yield (Table 1, entry 1). The following investigations disclosed that the yield was heavily influenced by the current intensity. The yield was gradually improved accompanying the increase of the current in the range from 10 mA to 15 mA (entries 1–4), whereas it soon dropped if the current of more than 15 mA was applied (entries 5 and 6). Either the replacement of the anode by an Al plate or the change of the cathode to a Ni plate or a Ti plate led to a decreased yield (entries 7–9). Besides nBu4NBF4, some other electrolytes were also tested in the reactions, but no improvement was made (entries 10–13). In addition, the optimal temperature was around 35 ℃. Both the elevation and the reduction of temperatures offered relatively negative results (entries 14–16). Gratifyingly, the yield was improved to 70% when the reaction time was properly extended to 8 h (entries 17 and 18). No desired products were obtained when no current was applied or the reaction was conducted open to the air (entries 19 and 20). It demonstrated that both the electrolysis and concentrated carbon dioxide were critical to this carboxylation.
|
|
Table 1 Optimization of desulfonylative carboxylation of the vinyl sulfone 3. |
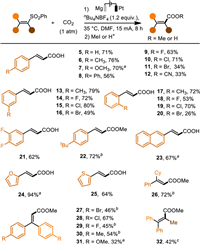
Then a series of vinyl sulfones were investigated under the optimal conditions (Scheme 2). (2-(Phenylsulfonyl)vinyl)benzene bearing electron-donating groups (EDGs) on the phenyl ring reacted smoothly with CO2 to afford the cinnamic acid derivatives (5–7, 22) in good yields. Electron-withdrawing groups (EWGs) were also tolerated on the phenyl ring, giving the desired products (8–12) in relatively low yields compared with EDGs. Additionally, the substituents on the meta-position of the phenyl ring (13–16) of (2-(phenylsulfonyl)vinyl)benzenes gave better results than those on ortho- (17–20) and para-positions (5–12, 22). Noteworthy, the aryl halides, which were prone to electrochemical dehalogenative carboxylations [59,60], were compatible with this methodology affording the expected products (9–11, 14–16, 18–21) in moderate to good yields. Vinyl sulfones containing other (hetero)arenes, like naphthalene (23), furan (24), thiophene (25) furnished the desulfonylative carboxylation products in moderate to excellent yields. In particular, all these products were obtained in trans-configuration. An array of trisubstituted vinyl sulfones also worked well in the desulfonylative carboxylation, giving the trisubstituted vinyl carboxylates (26–31) in moderate to good yields. Satisfyingly, the tetrasubstituted vinyl sulfone (32) was also suitable in the protocol by increasing the constant current to 30 mA. In addition, the aliphatic vinyl sulfones and (E)−1,2-diphenylethenyl sulfone were also tested but found not suitable for the present protocol (Scheme S2 in Supporting information).
|
Download:
|
| Scheme 2. Scope of desulfonylative carboxylation of vinyl sulfones with CO2. Reaction conditions: in an undivided cell with a Mg anode and a Pt cathode, sulfone (0.3 mmol, 0.04 mol/L), nBu4NBF4 (0.05 mol/L) in DMF at 35 ℃. a 13 mA, 10 h. b 10 mA, 8 h. c 30 mA, 6 h. | |
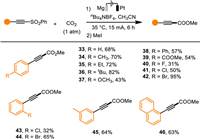
Our attention was next turned to explore the substrate scope of various alkynyl sulfones (Scheme 3). Delightfully, when acetonitrile was used as the solvent instead of DMF, the electrochemically desulfonylative carboxylation of phenylethynyl sulfone proceeded smoothly, delivering the methyl 3-phenylpropiolate (33) in acceptable yield. The alkyl (34–36, 45), methoxyl (37), phenyl (38), carboxylate (39) and halides (40–44) on the phenyl ring of the arylethynyl sulfone were well tolerated in the transformations. To our surprise, the substrates with a bromo substituent (42, 44) showed much better efficiency than the ones with either a fluoro (40) or a chloro substituent (41, 43). In addition, the 1-naphthalenylethynyl sulfone also yielded the desired propiolate (46) in a moderate yield. Nevertheless, the alkylethynyl sulfones were not suitable under the present conditions (Scheme S3 in Supporting information). Apart from vinyl and alkynyl sulfones, aryl sulfones were also tested in our methodology. To our delight, the tosyl group was readily removed under the electroreductive conditions to produce benzoic acid, 1-naphthoic acid, and 2-thiophenecarboxylic acid in moderate to good yields (Scheme S1 in Supporting information).
|
Download:
|
| Scheme 3. Scope of desulfonylative carboxylation of alkynyl sulfones with CO2. Reaction conditions: in an undivided cell with a Mg anode and a Pt cathode, sulfone (0.3 mmol, 0.04 mol/L), nBu4NBF4 (0.05 mol/L) in MeCN at 35 ℃. | |
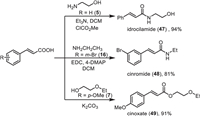
Given that cinnamic acid derivatives are important bioactive compounds, the aforementioned cinnamic acids, obtained from the electrochemically desulfonylative carboxylation of vinyl sulfones, could be further converted into active pharmaceutical ingredients (APIs) (Scheme 4). For instance, the idrocilamide (47), a marketed drug for relaxation of skeletal muscle or treatment of muscular pain, could be obtained in 94% yield via the coupling of 5 and the 2-aminoethan-1-ol. Another marketed antiepileptic drug, cinromide (48), could be prepared from the condensation of 16 with the ethyl amine in 81% yield. Besides, the cinoxate (49), which was used as an active ingredient in sunscreen, also could be readily obtained in an excellent yield.
|
Download:
|
| Scheme 4. Synthesis of APIs from the cinnamic acids 5, 16 and 7. | |
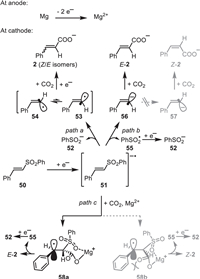
The possible mechanisms are proposed in Scheme 5. Considering that the reduction potential of vinyl sulfone 50 had a lower reduction potential (Ep = −1.75 V vs. SCE, in DMF) than that of CO2 (E = −2.21 V vs. SCE, in DMF) [61], the reduction of vinyl sulfone 50 is prior to CO2 on the cathode, giving a radical anion 51. There are three possible routes involved in the following process. In path a, the C-S bond dissociation of 51 produces the benzenesulfinic acid anion (52) and a bent α-hydrogen vinyl radical 53 in trans-configuration, which may invert feasibly to bring the cis-vinyl radical 54. Thereby the further reduction affords cinnamic acid 2 in a mixture of Z/E isomers, which should be analogical to the electrochemical debrominative carboxylation of the vinyl bromide. Nevertheless, the formation of the sulfonyl radical 55 and the vinyl anion 56 (path b) from the fragmentation of 51 also cannot be excluded. The bent vinyl anion has a high energy barrier to inversion (57) [62,63]. Therefore, only E-2 is obtained in this case. In addition, the radical anion 51 may also react with CO2, giving the intermediate 58a or 58b (path c). The oxygen atoms of the sulfonyl and carboxylic acid groups in 58a or 58b were coordinated by Mg2+ [64]. Due to the steric hindrance effect, the less hindered 58a was more favorable than the other. Thus, E-2 was selectively obtained by liberation of the sulfonyl radical 55. Furthermore, taking into account the fact that all the cinnamic acid derivatives obtained in the present protocol were highly in trans-configuration, paths b or c are more likely involved than path a.
|
Download:
|
| Scheme 5. Discussion of the proposed mechanism. | |
In summary, we have reported a stereoselective electrochemical decarboxylation of vinyl and alkynyl sulfones under transition-metal-free conditions. Highly E-selective cinnamic acids and their derivatives are obtained in the protocol, distinct from the results of electrochemical debrominative carboxylations. Thereby, the vinyl radical pathway may be excluded in our case. The resulted products, cinnamic acids, could be further utilized to synthesize high value-added APIs, such as the idrocilamide, cinromide, and cinoxate, which arouses potential interest in terms of fixation and utilization of CO2. In addition, alkynylsulfones are also suitable in the present protocol, affording a range of arylpropiolates in moderate to good yields. Other synthetic useful electrochemical fixation of CO2 is currently explored in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (No. 21901041) and the Fuzhou University (No. 0041/511095) is gratefully acknowledged.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.107956.
| [1] |
T. Sakakura, J.C. Choi, H. Yasuda, Chem.Rev. 107 (2007) 2365-2387. DOI:10.1021/cr068357u |
| [2] |
J.R. Cabrero-Antonino, R. Adam, M. Beller, Angew. Chem. Int. Ed. 58 (2019) 12820-12838. DOI:10.1002/anie.201810121 |
| [3] |
S. Dabral, T. Schaub, Adv. Synth. Catal. 361 (2019) 223-246. DOI:10.1002/adsc.201801215 |
| [4] |
J. Davies, J.R. Lyonnet, D.P. Zimin, R. Martin, Chem 7 (2021) 2927-2942. DOI:10.1016/j.chempr.2021.10.016 |
| [5] |
A. Modak, P. Bhanja, S. Dutta, B. Chowdhury, A. Bhaumik, Green Chem. 22 (2020) 4002-4033. DOI:10.1039/D0GC01092H |
| [6] |
D.G. Yu, L.N. He, Green Chem. 23 (2021) 3499-3501. DOI:10.1039/D1GC90036F |
| [7] |
C. Huang, J. Liu, H.H. Huang, X. Xu, Z. Ke, Chin. Chem. Lett. 33 (2022) 262-265. DOI:10.1016/j.cclet.2021.06.046 |
| [8] |
J. Liu, C. Chen, K. Zhang, L. Zhang, Chin. Chem. Lett. 32 (2021) 649-659. DOI:10.1016/j.cclet.2020.07.040 |
| [9] |
Y. Liu, H. Yang, X. Fan, B. Shan, T.J. Meyer, Chin. Chem. Lett. 33 (2022) 4691-4694. DOI:10.1016/j.cclet.2021.12.063 |
| [10] |
X.F. Wu, F. Zheng, Top. Curr. Chem. 375 (2016) 4. DOI:10.1007/s41061-016-0091-6 |
| [11] |
Z. Yang, Y. Yu, L. Lai, et al., Green Synth. Catal. 2 (2021) 19-26. DOI:10.1016/j.gresc.2021.01.009 |
| [12] |
C.S. Yeung, Angew. Chem. Int. Ed. 58 (2019) 5492-5502. DOI:10.1002/anie.201806285 |
| [13] |
G. Yuan, C. Qi, W. Wu, H. Jiang, Curr. Opin. Green Sustain. Chem. 3 (2017) 22-27. DOI:10.1016/j.cogsc.2016.11.006 |
| [14] |
D.M. Kitcatt, S. Nicolle, A.L. Lee, Chem.Soc. Rev. 51 (2022) 1415-1453. DOI:10.1039/D1CS01168E |
| [15] |
S.A. Zalivatskaya, N.D. Zakusilo, V.A. Vasilyev, Mini-Rev. Org. Chem. 18 (2021) 992-1011. DOI:10.2174/1570193X17999201228153003 |
| [16] |
S.F. Zhu, Q.L. Zhou, Acc. Chem. Res. 50 (2017) 988-1001. DOI:10.1021/acs.accounts.7b00007 |
| [17] |
L. Feng, X. Li, B. Liu, E. Vessally, J. CO2 Util. 40 (2020) 101220. DOI:10.1016/j.jcou.2020.101220 |
| [18] |
N. Huguet, I. Jevtovikj, A. Gordillo, et al., Chem. Eur. J. 20 (2014) 16858-16862. DOI:10.1002/chem.201405528 |
| [19] |
I. Jevtovikj, S. Manzini, M. Hanauer, F. Rominger, T. Schaub, Dalton Trans. 44 (2015) 11083-11094. DOI:10.1039/C5DT01040C |
| [20] |
S. Manzini, A. Cadu, A.C. Schmidt, et al., ChemCatChem 9 (2017) 2269-2274. DOI:10.1002/cctc.201601150 |
| [21] |
S.C. Stieber, N. Huguet, T. Kageyama, et al., Chem. Commun. 51 (2015) 10907-10909. DOI:10.1039/C5CC01932J |
| [22] |
S. Tanaka, K. Watanabe, Y. Tanaka, T. Hattori, Org. Lett. 18 (2016) 2576-2579. DOI:10.1021/acs.orglett.6b00918 |
| [23] |
Z. Zhang, T. Ju, M. Miao, et al., Org. Lett. 19 (2017) 396-399. DOI:10.1021/acs.orglett.6b03601 |
| [24] |
Z. Zhang, L.L. Liao, S.S. Yan, et al., Angew. Chem. Int. Ed. 55 (2016) 7068-7072. DOI:10.1002/anie.201602095 |
| [25] |
T. Ju, Y.Q. Zhou, K.G. Cao, et al., Nat. Catal. 4 (2021) 304-311. DOI:10.1038/s41929-021-00594-1 |
| [26] |
G. Cahiez, D. Bernard, J.F. Normant, Synthesis 1976 (1976) 245-248. DOI:10.1055/s-1976-25384 |
| [27] |
S.K. Bhunia, P. Das, S. Nandi, R. Jana, Org. Lett. 21 (2019) 4632-4637. DOI:10.1021/acs.orglett.9b01532 |
| [28] |
T. Mita, K. Suga, K. Sato, Y. Sato, Org. Lett. 17 (2015) 5276-5279. DOI:10.1021/acs.orglett.5b02645 |
| [29] |
K. Paridala, S.M. Lu, M.M. Wang, C. Li, Chem.Commun. 54 (2018) 11574-11577. DOI:10.1039/C8CC06820H |
| [30] |
K. Shimomaki, T. Nakajima, J. Caner, N. Toriumi, N. Iwasawa, Org. Lett. 21 (2019) 4486-4489. DOI:10.1021/acs.orglett.9b01340 |
| [31] |
Y. Wang, X. Jiang, B. Wang, Chem.Commun. 56 (2020) 14416-14419. DOI:10.1039/D0CC06451C |
| [32] |
S.L. Xie, X.Y. Cui, X.T. Gao, et al., Org. Chem. Front. 6 (2019) 3678-3682. DOI:10.1039/C9QO00923J |
| [33] |
S.S. Yan, D.S. Wu, J.H. Ye, et al., ACS Catal. 9 (2019) 6987-6992. DOI:10.1021/acscatal.9b02351 |
| [34] |
J. Hong, O.S. Nayal, F. Mo, Eur. J. Org. Chem. 2020 (2020) 2813-2818. DOI:10.1002/ejoc.202000288 |
| [35] |
Y. Makida, E. Marelli, A.M. Slawin, S.P. Nolan, Chem.Commun. 50 (2014) 8010-8013. DOI:10.1039/c4cc03650f |
| [36] |
T. Ohishi, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 47 (2008) 5792-5795. DOI:10.1002/anie.200801857 |
| [37] |
P.J. Riss, S. Lu, S. Telu, F.I. Aigbirhio, V.W. Pike, Angew. Chem. Int. Ed. 51 (2012) 2698-2702. DOI:10.1002/anie.201107263 |
| [38] |
K. Ukai, M. Aoki, J. Takaya, N. Iwasawa, J. Am. Chem. Soc. 128 (2006) 8706-8707. DOI:10.1021/ja061232m |
| [39] |
S. Sun, J.T. Yu, Y. Jiang, J. Cheng, Adv. Synth. Catal. 357 (2015) 2022-2026. DOI:10.1002/adsc.201500101 |
| [40] |
T.V.Q. Nguyen, W.J. Yoo, S. Kobayashi, Asian J. Org. Chem. 7 (2018) 116-118. DOI:10.1002/ajoc.201700519 |
| [41] |
T. Yanagi, R.J. Somerville, K. Nogi, R. Martin, H. Yorimitsu, ACS Catal. 10 (2020) 2117-2123. DOI:10.1021/acscatal.9b05141 |
| [42] |
W.J. Yoo, J. Kondo, S. Kobayashi, Chem.Lett. 48 (2019) 1248-1250. DOI:10.1246/cl.190577 |
| [43] |
Y. Kim, C.J. Li, Green Synth. Catal. 1 (2020) 1-11. DOI:10.1016/j.gresc.2020.06.002 |
| [44] |
M. Schmalzbauer, T.D. Svejstrup, F. Fricke, et al., Chem 6 (2020) 2658-2672. DOI:10.1016/j.chempr.2020.08.022 |
| [45] |
J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Chin. J. Catal. 42 (2021) 1445-1450. DOI:10.1016/S1872-2067(20)63750-0 |
| [46] |
J. Jiang, Z. Wang, W.M. He, Chin. Chem. Lett. 32 (2021) 1591-1592. DOI:10.1016/j.cclet.2021.02.067 |
| [47] |
X. Wang, S. Wu, Y. Zhong, et al., Chin. Chem. Lett. (2022). |
| [48] |
Y. Wu, J.Y. Chen, J. Ning, et al., Green Chem. 23 (2021) 3950-3954. DOI:10.1039/D1GC00562F |
| [49] |
Z.L. Wu, J.Y. Chen, X.Z. Tian, et al., Chin. Chem. Lett. 33 (2022) 1501-1504. DOI:10.1016/j.cclet.2021.08.071 |
| [50] |
J. Zhong, Y. Yu, D. Zhang, K. Ye, Chin. Chem. Lett. 32 (2021) 963-972. DOI:10.1016/j.cclet.2020.08.011 |
| [51] |
C. Kuang, Q. Yang, H. Senboku, M. Tokuda, Chem.Lett. 34 (2005) 528-529. DOI:10.1246/cl.2005.528 |
| [52] |
C. Galli, A. Guarnieri, H. Koch, P. Mencarelli, Z. Rappoport, J. Org. Chem. 62 (1997) 4072-4077. DOI:10.1021/jo962373h |
| [53] |
S.L. Xie, X.T. Gao, H.H. Wu, F. Zhou, J. Zhou, Org. Lett. 22 (2020) 8424-8429. DOI:10.1021/acs.orglett.0c03051 |
| [54] |
J.S. Zhong, Z.X. Yang, C.L. Ding, et al., J. Org. Chem. 86 (2021) 16162-16170. DOI:10.1021/acs.joc.1c01261 |
| [55] |
H. Senboku, Y. Minemura, Y. Suzuki, H. Matsuno, M. Takakuwa, J. Org. Chem. 86 (2021) 16077-16083. DOI:10.1021/acs.joc.1c01516 |
| [56] |
M. Nambo, Y. Tahara, J.C. Yim, D. Yokogawa, C.M. Crudden, Chem.Sci. 12 (2021) 4866-4871. DOI:10.1039/D1SC00133G |
| [57] |
J. Xuan, Z.J. Feng, J.R. Chen, L.Q. Lu, W.J. Xiao, Chem.Eur. J. 20 (2014) 3045-3049. DOI:10.1002/chem.201304898 |
| [58] |
X. Zhou, C. Ni, L. Deng, J. Hu, Chem.Commun. 57 (2021) 8750-8753. DOI:10.1039/D1CC03258E |
| [59] |
H. Senboku, A. Katayama, Curr. Opin. Green Sustain. Chem. 3 (2017) 50-54. DOI:10.1016/j.cogsc.2016.10.003 |
| [60] |
H. Tateno, K. Nakabayashi, T. Kashiwagi, H. Senboku, M. Atobe, Electrochim. Acta 161 (2015) 212-218. DOI:10.1016/j.electacta.2015.01.072 |
| [61] |
E. Lamy, L. Nadjo, J.M. Saveant, J. Electroanal. Chem. 78 (1977) 403-407. DOI:10.1016/S0022-0728(77)80143-5 |
| [62] |
P. Caramella, K.N. Houk, Tetrahedron Lett. 22 (1981) 819-822. DOI:10.1016/0040-4039(81)80005-6 |
| [63] |
W. Ghattas, C.R. Hess, G. Iacazio, et al., J. Org. Chem. 71 (2006) 8618-8621. DOI:10.1021/jo061022s |
| [64] |
I. Nishiguchi, T. Matsumoto, T. Kuwahara, M. Kyoda, H. Maekawa, Chem.Lett. 31 (2002) 478-479. DOI:10.1246/cl.2002.478 |
 2023, Vol. 34
2023, Vol. 34