b Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang 330063, China;
c School of Chemistry & Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China
Photocatalytic CO2 reduction to fuels is an ideal way to solve the energy crisis and greenhouse effect [1,2]. Many semiconductor photocatalysts had been developed for CO2 reduction [3,4]. Quantum dots (QDs) are excellent candidates for photocatalytic CO2 reduction due to their quantum confinement effect [5,6], large extinction coefficient [7,8], and easy modification of band structure [9]. And a lot of QDs photocatalysts had been developed, such as CdSe [10,11], CsPbBr1.5Cl1.5 [12], and Bi2WO6 [13-15]. However, the high surface energy and stabilizer of QDs cause poor stability and charge transfer [16,17]. Hence, the QDs photocatalysts need to be dispersed and stabilized.
The QDs-based heterojunction is an effective approach, which could disperse and stabilize QDs by the interaction between QDs and the support [18,19]. The CdSe QDs/g-C3N4 heterojunction showed improved photocatalytic stability [20,21]. And the good dispersion of CdSe QDs increased the photocatalytic activity [22,23]. However, most of the QDs-based heterojunction were linked by weak electrostatic interactions or/and van der Waals forces [24], which were not strong enough during the complex photocatalytic reactions [25]. In addition, the weak interactions between QDs and the support cause the high charge transfer barriers and easy recombination of photogenerated charges. Researchers have found that the chemical bonds linked heterojunction have better photocatalytic stability and activity. For example, the covalent-bond linked MOF/COF composites showed improved photogenerated charges transfer/migration and photocatalytic durability [26-29]. The semi-chemical interaction of Pt and g-C3N4 can improve the photocatalytic H2 evolution obviously, due to the fast charge transfer channels from the strong chemical bond [30]. Therefore, it is urgent to establish strong interactions between QDs and support for fast and efficient transfer of photogenerated charges.
Metal-organic frameworks (MOFs) are ideal supports for stabilizing QDs, own to the abundant functional groups and large surface area [31]. UiO-66(Zr) is a semiconductor [32-35], which can modified by organic ligands to increase the adsorption capacity of UiO-66 for CO2 and broaden the spectral adsorption range [31,36]. Herein, a new covalent-bonded QDs/MOFs heterojunction was successfully prepared by acylamino crosslinking. The CdSeTe QDs and NH2-UiO-66 were crossed by the acylamino (-CONH-), which was formed through amide condensation of –COOH of CdSeTe QDs and –NH2 of NH2-UiO-66, to form CdSeTe QDs/NH2-UiO-66 composites (NUC-X, X: the additive amount of CdSeTe QDs). The experimental results illustrated that the acylamino can act as a bridge for electron transmission and the NH2-UiO-66 can act as electron traps, which can effectively promote migration and suppress recombination of photogenerated electron-hole pairs. Compared with NH2-UiO-66, CdSeTe QDs, and the van der Waals heterostructure of NH2-UiO-66 and CdSeTe QDs, the obtained NUC-Xs had better photocatalytic activity and stability for photocatalytic CO2 reduction.
The NUC-X was prepared by a simple acid-assisted amide condensation reaction (Scheme 1). As shown in Fig. 1a, NUC-1200 exhibited similar diffraction peaks compared to NH2-UiO-66, suggesting that the structure of NH2-UiO-66 remained after the amide condensation with CdSeTe QDs. The FTIR spectra of the NUC-1200 and NH2-UiO-66 showed similar peaks in the region of 3342–3500 cm−1 (Fig. S1 in Supporting information), which can be attributed to the –NHx fraction [37]. And the weaker –NHx intensity of NUC-1200 than NH2-UiO-66 proved the reduction of –NH2, which was consumed by the amide condensation. As shown in Fig. 1b, the FTIR spectra of the obtained CdSeTe QDs showed a peak centered at 1582 cm−1 which is consistent with –COOH from the terminal of mercaptopropionic acid [38]. And NUC-1200 showed a distinct peak at 1685 cm−1, which can be assigned to the stretching band of acylamino [39]. These results indicated that the –NH2 of NH2-UiO-66 and the –COOH of CdSeTe QDs successfully formed the acylamino to obtain NUC-1200. In addition, the NUC-1200 showed similar FT-IR peaks as NH2-UiO-66, suggesting that the condensation had not changed the structure of NH2-UiO-66, which is consistent with the XRD results.
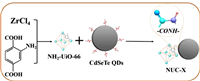
|
Download:
|
| Scheme 1. Schematic illustration of the preparation of NUC-X. | |
|
Download:
|
| Fig. 1. (a) XRD spectra for NH2-UiO-66, CdSeTe QDs and NUC-1200. (b) FTIR spectra for NH2-UiO-66, CdSeTe QDs and NUC-1200. XPS spectra of (c) C 1s in NH2-UiO-66 and NUC-1200. (d) N 1s in NH2-UiO-66 and NUC-1200. | |
The surface chemical composition and electronic states of NH2-UiO-66 and NUC-1200 were studied by XPS. Both NH2-UiO-66 and NUC-1200 have the characteristic peaks corresponding to C, N, O, and Zr elements, while the presence of three other elements of Cd, Se, and Te in the obtained NUC-1200 (Fig. S2 in Supporting information). This also indicated that CdSeTe QDs and NH2-UiO-66 were successfully combined. The high-resolution C 1s spectrum of NH2-UiO-66 has three main peaks located at 284.8, 286.3, and 288.7 eV (Fig. 1c), corresponding to C=C, C–N (C–NH2), and C=O, respectively [40]. Compared with NH2-UiO-66, the C–N (286.1 eV) in NUC-1200 had a stronger intensity and smaller bonding energy, indicating the formation of acylamino. The N 1s spectrum of NH2-UiO-66 can be deconvolved into two peaks located at 399.2 and 400.5 eV (Fig. 1d), which correspond to the tertiary nitrogen (N–Cx) and –NH2 groups, respectively [41]. For the NUC-1200, a new peak centered at 401.1 eV is attributed to the C–N from covalent amide linkage and the bonding energies of N 1s were bigger than that of NH2-UiO-66, which can be assigned to the decrease of electron density around N atoms due to the successful formation of the acylamino [42]. These results proved the strong interaction between NH2-UiO-66 and CdSeTe QDs and the successful construction of acylamino crosslinking. The increased intensity of the –OH peak in the O 1s spectrum of NUC-1200 indicated the successful introduction of CdSeTe QDs (Fig. S3 in Supporting information). The Zr 3d spectra of NH2-UiO-66 and NUC-1200 both have two peaks at 185.2 and 182.8 eV, which can be attributed to Zr 3d5/2 and Zr 3d3/2 (Fig. S4 in Supporting information), respectively [43].
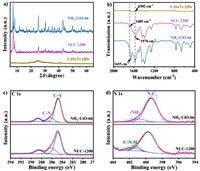
The morphology of the as-prepared samples was investigated by transmission electron microscopy (TEM). As shown in Fig. S5 (Supporting information), the synthesized CdSeTe QDs were uniform particles with a size of 5.2 nm. For NH2-UiO-66, a typical crystal stacking structure with a smooth surface was observed (Fig. 2a). The TEM images of NUC-1200 indicate that CdSeTe QDs were uniformly distributed on the surface of NH2-UiO-66 (Figs. 2b and c). Although the NH2-UiO-66 in NUC-1200 was almost indistinguishable, the particle morphology of CdSeTe QDs changed slightly after binding with NH2-UiO-66, suggesting that NH2-UiO-66 combined with a certain amount of CdSeTe QDs through covalent bonding. The spatial distribution of different elements in NUC-1200 was investigated by elemental mapping analysis [44]. These results indicated that the CdSeTe QDs were uniformly distributed on the surface of NH2-UiO-66 (Figs. 2b and d).
|
Download:
|
| Fig. 2. TEM image of the (a) NH2-UiO-66, (b, c) NUC-1200, the black dots are CdSeTe QDs; and (d) TEM with elemental mapping images of NUC-1200. | |
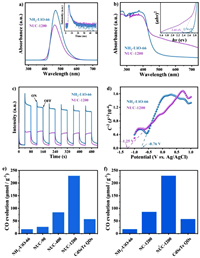
The photo-electrochemical properties of the prepared samples were also investigated. As shown in Fig. 3a, the NUC-1200 had a lower PL intensity than that of NH2-UiO-66 indicating that the acylamino in NUC-1200 can transfer the photogenerated charges fast and inhibit recombination [45,46]. As can be seen in Fig. 3b, the NUC-1200 had a stronger UV–vis adsorption intensity than NH2-UiO-66 in the region of 450–750 nm, indicating the enhanced light absorption capacity. Furthermore, the smaller band gap of NUC-1200 (2.41 eV) than that of NH2-UiO-66 (2.74 eV) making it an extended light absorption range (inset in Fig. 3b). As shown in Fig. 3c, the transient photocurrent intensity of NUC-1200 was much higher than NH2-UiO-66, which can be assigned to the faster transfer and separation of photogenerated carriers between NH2-UiO-66 and CdSeTe QDs [47]. The flat band potential energy of NH2-UiO-66 and NUC-1200 were −0.76 and −1.26 V vs. Ag/AgCl respectively (Fig. 3d). Since the flat band potential of n-type semiconductors is equal to the Fermi level [48], the Fermi levels of NH2-UiO-66 and NUC-1200 were −0.15 and −0.65 V vs. RHE, respectively. Based on this, the CB of NH2-UiO-66 and NUC-1200 can be calculated to be −0.35 and −0.85 V, respectively [37]. The matched band potential between NH2-UiO-66 and CdSeTe QDs allows photogenerated carriers to be effectively separated.
|
Download:
|
| Fig. 3. (a) Steady-state photoluminescence spectra and time-resolved photoluminescence decays spectra of NH2-UiO-66 and NUC-1200. (b) UV–vis diffuse reflectance spectra of NH2-UiO-66 and NUC-1200. (c) Transient photocurrent responses spectra of NH2-UiO-66 and NUC-1200. (d) Mott-Schottky spectra of NH2-UiO-66 and NUC-1200. Photocatalytic performance (e) comparison of NH2-UiO-66, CdSeTe QDs and NUC-X composites, (f) comparison of NH2-UiO-66, CdSeTe QDs, NC-1200 and NUC-1200 composites. | |
The photocatalytic CO2 reduction measurements of the as-prepared catalysts had been performed. The catalysts were dispersed in acetonitrile aqueous solution (acetonitrile: water = 4:1), while triethanolamine (TEOA) was the electron donor and tris(2,2′-bipyridine)ruthenium(Ⅱ) chloride hexahydrate (C30H24Cl2N6Ru-6H2O) as the photosensitizer. In Fig. 3e, the NH2-UiO-66 exhibited the poorest photocatalytic CO evolution rate, while the NUC-Xs showed a better CO evolution rate, indicating that CdSeTe QDs played an important role. Among NUC-Xs, the optimized NUC-1200 achieved a CO evolution rate of 228.68 µmol/g, which was 13 and 4 times higher than NH2-UiO-66 and CdSeTe QDs, respectively. The comparison of the photocatalytic performances were also made between this work and some related works (Table S1 in Supporting information), which proved the superior performance of NUC-1200. To further explore the effect of acylamino on photocatalytic activities, NC-1200 was constructed by van der Waals interaction between NH2-UiO-66 and CdSeTe QDs. As shown in Fig. 3f, NC-1200 had a much lower CO evolution rate (85.7 µmol/g) compared with NUC-1200 (228.68 µmol/g). Therefore, this enhanced photocatalytic performance of NUC-1200 can mainly be attributed to the formation of acylamino. In addition, NUC-1200 showed good stability after 7 cycles owing to the strong interaction (Fig. S6 in Supporting information).
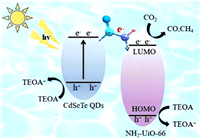
Based on the above results, the photocatalytic enhanced mechanism of NUC-1200 was proposed (Scheme 2). Firstly, NH2-UiO-66 was linked with CdSeTe QDs by covalent bonding, thus allowing the rapid electron transfer between MOFs and QDs via acylamino. Then, the matched band structure between NH2-UiO-66 and CdSeTe QDs facilitated the transfer of photogenerated electrons from the CdSeTe QDs to the CB of NH2-UiO-66 via amide-bonded bridges. Finally, the covalent bonding can significantly improve the stability of heterojunction photocatalysts.
|
Download:
|
| Scheme 2. Photocatalytic mechanism of the charge transfer for CO2RR over the NUC-1200 under light irradiation. | |
In conclusion, we had developed a novel covalent bonding heterojunction photocatalysts by a simple amide condensation. The characterization results confirmed the presence of acylamino in the synthesized NUC-Xs. Experimental results showed that the photogenerated electrons generated on CdSeTe QDs can be transferred to NH2-UiO-66 and used for photocatalytic CO2RR. At the same time, the acylamino served as an electron transfer bridge between NH2-UiO-66 and CdSeTe QDs for the transfer and separation of photogenerated carriers. As a result, the obtained NUC-1200 had a better photocatalytic CO2RR performance. This work provides a new perspective for the design and development of novel heterojunction photocatalysts.
Declaration of competing interestThe authors declare that they have no known competing fifinancial interests or personal relationships that could have appeared to inflfluence the work reported in this paper.
AcknowledgmentsWe gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 52100186 and 52170082), the Natural Science Foundation of Jiangxi Province (No. 20212ACB203008).
| [1] |
X.B. Li, C.H. Tung, L.Z. Wu, Angew. Chem. Int. Ed. 58 (2019) 10804-10811. DOI:10.1002/anie.201901267 |
| [2] |
S.Q. Gong, X. Teng, Y.L. Niu, et al., Appl. Catal. B: Environ. 298 (2021) 120521. DOI:10.1016/j.apcatb.2021.120521 |
| [3] |
X.D. Li, Y.F. Sun, J.Q. Xu, et al., Nat. Energy 4 (2019) 690-699. DOI:10.1038/s41560-019-0431-1 |
| [4] |
J.L. White, M.F. Baruch, J.E. Pander Ⅲ, et al., Chem. Rev. 115 (2015) 12888-12935. DOI:10.1021/acs.chemrev.5b00370 |
| [5] |
Y.E. Panfil, M. Oded, U. Banin, Angew. Chem. Int. Ed. 57 (2018) 4274-4295. DOI:10.1002/anie.201708510 |
| [6] |
L. Shi, X.H. Ren, Z.X. Zhang, et al., J. Catal. 401 (2021) 271-278. DOI:10.1016/j.jcat.2021.08.006 |
| [7] |
D.Y. Wang, Y.Y. Yin, C.W. Feng, et al., Catalysts 11 (2021) 275. DOI:10.3390/catal11020275 |
| [8] |
S.C. Zhu, S. Li, B. Tang, et al., J. Catal. 404 (2021) 56-66. DOI:10.1016/j.jcat.2021.09.001 |
| [9] |
J.D. Wang, H.R. Ning, J. Wang, et al., ACS Appl. Nano Mater. 5 (2022) 5617-5624. DOI:10.1021/acsanm.2c00573 |
| [10] |
H.J. Peng, P.Q. Zheng, H.Y. Chao, RSC Adv. 10 (2020) 551-555. DOI:10.1039/C9RA08801F |
| [11] |
Z.J. Zhao, Z.L. Liu, Z.X. Zhu, Chin. Chem. Lett. 32 (2021) 2474-2478. DOI:10.1016/j.cclet.2021.01.004 |
| [12] |
S.H. Guo, J. Zhou, X. Zhao, J. Catal. 369 (2019) 201-208. DOI:10.1016/j.jcat.2018.11.004 |
| [13] |
W.L. Dai, W.W. Xiong, J.J. Yu, et al., ACS Appl. Mater. Interfaces 12 (2020) 25861-25874. DOI:10.1021/acsami.0c04730 |
| [14] |
J.W. Wang, W.K. Zhang, X.B. Zhang, et al., J. Alloy. Compd. 826 (2020) 154217. DOI:10.1016/j.jallcom.2020.154217 |
| [15] |
Y.S. Zeng, Q. Yin, Z. Liu, et al., Chem. Eng. J. 435 (2022) 134918. DOI:10.1016/j.cej.2022.134918 |
| [16] |
C.S. Li, X.S. Zou, W.H. Lin, et al., ACS Appl. Nano Mater. 4 (2021) 6280-6289. DOI:10.1021/acsanm.1c01096 |
| [17] |
Y. Xiao, Z.Y. Peng, W.L. Zhang, et al., Appl. Surf. Sci. 494 (2019) 519-531. DOI:10.1016/j.apsusc.2019.07.175 |
| [18] |
P. Wang, X.B. Zhou, Y. Shao, et al., J. Colloid Interface Sci. 601 (2021) 186-195. DOI:10.1016/j.jcis.2021.05.132 |
| [19] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [20] |
T.Y. Qiu, L. Wang, B.Y. Zhou, et al., ACS Appl. Nano Mater. 5 (2022) 702-709. DOI:10.1021/acsanm.1c03471 |
| [21] |
F. Raziq, A. Hayat, M. Humayun, et al., Appl. Catal. B: Environ. 270 (2020) 118867. DOI:10.1016/j.apcatb.2020.118867 |
| [22] |
J.S. Han, F.X. Dai, Y. Liu, et al., Appl. Surf. Sci. 467 (2019) 1033-1039. |
| [23] |
S. Raheman AR, H.M. Wilson, B.M. Momin, et al., Renew. Energ. 158 (2020) 431-443. DOI:10.1016/j.renene.2020.05.140 |
| [24] |
R.T. Guo, X.Y. Liu, H. Qin, et al., Appl. Surf. Sci. 500 (2020) 144059. DOI:10.1016/j.apsusc.2019.144059 |
| [25] |
V. Georgakilas, J.N. Tiwari, K.C. Kemp, et al., Chem. Rev. 116 (2016) 5464-5519. DOI:10.1021/acs.chemrev.5b00620 |
| [26] |
F. Li, D.K. Wang, Q.J. Xing, et al., Appl. Catal. B: Environ. 243 (2019) 621-628. DOI:10.1016/j.apcatb.2018.10.043 |
| [27] |
D.R. Sun, S. Jang, S.J. Yim, et al., Adv. Funct. Mater. 28 (2018) 1707110. DOI:10.1002/adfm.201707110 |
| [28] |
D.R. Sun, D.P. Kim, ACS Appl. Mater. Interfaces 12 (2020) 20589-20595. DOI:10.1021/acsami.0c04537 |
| [29] |
Y.W. Peng, M.T. Zhao, B. Chen, et al., Adv. Mater. 30 (2018) 1705454. DOI:10.1002/adma.201705454 |
| [30] |
Z.A. Zhong, L.S. Chen, L.S. Zhang, et al., Chin. Chem. Lett. 33 (2022) 3061-3064. DOI:10.1016/j.cclet.2021.09.057 |
| [31] |
L.F. Hong, R.T. Guo, Z.W. Zhang, et al., J. CO2 Util. 51 (2021) 101650. DOI:10.1016/j.jcou.2021.101650 |
| [32] |
X.L. Chen, Y. Cai, R. Liang, et al., Appl. Catal. B: Environ. 267 (2020) 118687. DOI:10.1016/j.apcatb.2020.118687 |
| [33] |
D.R. Sun, Y.H. Fu, W.J. Liu, et al., Chem. Eur. J. 19 (2013) 14279-14285. DOI:10.1002/chem.201301728 |
| [34] |
D.R. Sun, W.J. Liu, M. Qiu, et al., Chem. Commun. 51 (2015) 2056-2059. DOI:10.1039/C4CC09407G |
| [35] |
F.Y. Yu, X. Jing, Y. Wang, et al., Angew. Chem. Int. Ed. 60 (2021) 24849. DOI:10.1002/anie.202108892 |
| [36] |
H. Zhao, X. Yang, R. Xu, et al., J. Mater. Chem. A 6 (2018) 20152-20160. DOI:10.1039/C8TA05970E |
| [37] |
X.H. Jiang, Q.J. Xing, X.B. Luo, et al., Appl. Catal. B: Environ. 228 (2018) 29-38. DOI:10.1016/j.apcatb.2018.01.062 |
| [38] |
Z.X. Pan, K. Zhao, J. Wang, et al., ACS Nano 7 (2013) 5215-5222. DOI:10.1021/nn400947e |
| [39] |
A. Hayat, T.A.M. Taha, A.M. Alenad, et al., Energy Technol 9 (2021) 2100091. DOI:10.1002/ente.202100091 |
| [40] |
J.W. Ren, S. Lv, S.Q. Wang, et al., Sep. Purif. Technol. 274 (2021) 118973. DOI:10.1016/j.seppur.2021.118973 |
| [41] |
Z.K. Huang, H.J. Yu, L. Wang, et al., J. Hazard. Mater. 436 (2022) 129052. DOI:10.1016/j.jhazmat.2022.129052 |
| [42] |
B. Ma, G. Chen, C. Fave, et al., J. Am. Chem. Soc. 142 (2020) 6188-6195. DOI:10.1021/jacs.9b13930 |
| [43] |
J.W. Ren, Y. Meng, X. Zhang, et al., Sep. Purif. Technol. 296 (2022) 121423. DOI:10.1016/j.seppur.2022.121423 |
| [44] |
L.S. Zhang, X.H. Jiang, Z.A. Zhong, et al., Angew. Chem. Int. Ed. 60 (2021) 1-6. DOI:10.1002/anie.202015604 |
| [45] |
Z.L. Tang, C.J. Wang, W.J. He, et al., Chin. Chem. Lett. 33 (2022) 939-942. DOI:10.1016/j.cclet.2021.07.020 |
| [46] |
H.N. Che, P.F. Wang, J. Chen, et al., Appl. Catal. B: Environ. 316 (2022) 121611. DOI:10.1016/j.apcatb.2022.121611 |
| [47] |
X.H. Jiang, L.S. Zhang, H.Y. Liu, et al., Angew. Chem. Int. Ed. 59 (2020) 23112. DOI:10.1002/anie.202011495 |
| [48] |
D. Amarajothi, S.P. Andrea, M.A. Abdullah, et al., ChemCatChem 11 (2019) 899. DOI:10.1002/cctc.201801452 |
 2023, Vol. 34
2023, Vol. 34 

