Carbon dioxide (CO2) is a typical greenhouse gas, and its amount in the atmosphere has been on continuous rise because of the large-scale combustion of fossil fuels [1,2]. Meanwhile, CO2 is an ideal renewable and nontoxic carbon source in chemical synthesis [3,4]. CO2 capture and conversion strategy, which can not only reduce its adverse impact on the environment but also produce high value-added chemical products, has attracted great attentions and become a hot topic [5-7]. Benzimidazoles are widely used to synthesize many kinds of natural products and bioactive molecules, such as mebendazole [8], omeprazole [9], albendazole [10] and pantoprazole [11]. The most common synthetic route for benzimidazoles is direct synthesis from o-phenylenediamine with carbon sources, in which utilization of inexpensive and abundant CO2 as C1 source is very impressive. However, CO2 is a relatively stable molecule, so highly efficient catalysts are needed in this approach.
Frustrated Lewis pairs (FLPs) formed by the steric resistance of Lewis acids and bases have aroused significant attentions due to their highly efficient catalytic activities in non-metal-mediated catalysis [12-15]. Currently, FLPs are well investigated for small-molecule activation, such as H2 [16,17] and CO2 [18,19]. Chen et al. synthesized two copolymers installing bulky borane- and phosphine-containing blocks respectively and built them as the macromolecular FLP which can effectively bound CO2 and then serve as recyclable catalysts [19]. Usually, homogeneous FLP catalysts encounter separation difficulty from the reaction system thus resulting in poor catalyst recycle [20]. In order to solve this problem, FLPs combined with solid materials to form heterogeneous catalysts are explored [21,22]. Shyshkanov et al. reported a water-stable and recyclable MOF catalyst contained Lewis acidic sites (B centers) for the synthesis of benzimidazoles from o-phenylenediamine under CO2 atmosphere by the in situ formation of FLPs [23]. This proves that utilizing porous materials incorporating Lewis acidic centers would create FLPs during synthesis of benzimidazoles with CO2, which would act as promising heterogeneous catalysts.
Porous aromatic frameworks (PAFs) have been developed as a novel class of porous materials due to their robust skeletons and high surface areas [24]. Benefiting from the variety of synthesis and functionalization, it is possible to customize specific properties in PAF skeletons, which provides great opportunities for their applications in gas adsorption [25-27], separation [28,29], sensors [30-32], heterogeneous catalysis [33,34] and so on. Thomas group synthesized triphenylphosphine-based porous networks to mimic the basic sites employed in FLPs [35]. N-rich PAF-6 with layer structure was exfoliated into nanosheets to reveal its featured Lewis base sites and then served as heterogeneous FLP catalyst by interacting with the strong Lewis acid B(C6F5)3 [36]. Therefore, by virtue of the structural advantages of PAFs, introducing sterically encumbered B, P and N motifs would produce potential Lewis acid and base groups to achieve post-decorated FLPs, which are effective heterogeneous FLP catalysts for H2 and CO2 activation. Herein, a sterically hindered B motif (triphenylboron) was selected as building units and two B-based PAFs (PAF-165 and PAF-166) were synthesized via Friedel-Crafts alkylation. Both PAFs possess high surface areas (867 m2/g for PAF-165 and 754 m2/g for PAF-166) together with high thermal stabilities. Due to the presence of electron-deficient B centers, these PAFs exhibited highly enhanced CO2 adsorption abilities and selective adsorption over N2. At 273 K and 1 bar, the equimolar mixture selectivities of PAF-165 and PAF-166 estimated with the ideal adsorbed solution theory (IAST) are 106.2 and 196.5, respectively. Additionally, PAF-165 and PAF-166 showed excellent catalytic abilities in the synthesis of benzimidazole from o-phenylenediamine with CO2 source, in which triphenylboron units in PAFs acted as Lewis acid sites and form FLPs catalyst with substrates during the reaction.
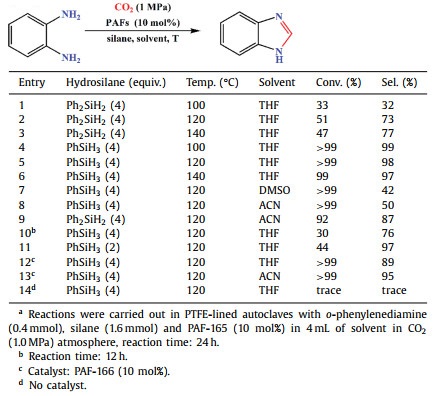
As illustrated in Scheme 1, PAF-165 and PAF-166 were synthesized via Friedel-Crafts alkylation reaction between triphenylboron and Br-substituted external cross-linkers (1, 4-bis(bromomethyl)benzene or 1, 3, 5-tris(bromomethyl)benzene). Both PAFs contain abundant Lewis acid active sites for the in situ formation of FLPs upon addition of o-phenylenediamine.
|
Download:
|
| Scheme 1. The synthetic routes of (a) PAF-165 and PAF-166 and (b) FLPs in situ formation with o-phenylenediamine. | |
Comparative FTIR spectra of PAF-165, PAF-166 and their corresponding monomers were collected to confirm the bonding features in their frameworks (Fig. S1 in Supporting information). The peaks at 1600 and 1460 cm−1 ascribed to benzene rings are retained after reaction. The absence of C-Br characteristic bands at 610 and 580 cm−1 along with the appearance of stretching vibrations at 2930 cm−1 for methylene group reveal that polymerization reaction completed successfully. To further illustrate the local structures of PAF-165 and PAF-166, solid-state 13C MAS NMR and 11B MAS NMR were performed [37]. As shown in Fig. S2a (Supporting information), the signals at 137 and 131 ppm are clearly observed, which are attributed to substituted and unsubstituted phenyl carbons in PAF skeletons, respectively. The signals at 32 and 15 ppm can be assigned to methylene linkers and methylene end groups, indicating that the Br-substituted external cross-linkers have participated the polymerization process. There is only one peak in 11B MAS NMR spectra of as-synthesized PAFs (Fig. S2b in Supporting information, 16.3 ppm for PAF-165 and 19.1 ppm for PAF-166), suggesting that boron centers are successfully introduced in PAFs skeletons without changes of connection ways. PXRD were then employed to explore the long-range order of resulted PAFs, and no obvious diffraction peak is observed (Fig. S3 in Supporting information), confirming disordered PAF structures which is common in the dynamic-driven irreversible reactions. SEM images (Figs. S4a and b in Supporting information) showed that both PAFs are well-defined spherical particles, while TEM images (Figs. S4c and d in Supporting information) indicated that these two PAFs possess worm-like porous textures. TGA curves (Fig. S5 in Supporting information) were collected to investigate PAFs' stabilities which show that both PAF-165 and PAF-166 are stable up to 350 ℃ in air condition. In addition, they cannot be dissolved in various solvents such as methanol, ethanol, dichloromethane, toluene, acetone, HCl solution (3 mol/L) and other common organic solvents. These results suggest that the resulted PAFs have high thermal stability together with high chemical stability. This is in favor of the recyclability of catalysts and plays an important role in heterogeneous catalytic systems [33].
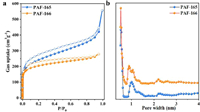
In order to investigate the porosity of PAF-165 and PAF-166, N2 adsorption-desorption measurements were carried out at 77 K. As shown in Fig. 1a, the adsorption capacities of PAFs increase sharply in the relative low-pressure region (P/P0 < 0.01) and hysteresis loops between the adsorption and desorption curves are observed, which suggest the presence of micropores and mesopores in their frameworks [27]. According to Brunauer-Emmett-Teller (BET) model, PAF-165 and PAF-166 present high surface areas of 867 and 754 m2/g, respectively. Meanwhile, their pore size distributions were calculated based on the nonlocal density functional theory (NLDFT), and provid two main pores around 0.5 and 1 nm as well as a few distributions at 2.2 nm (Fig. 1b), consistent with the results form sorption isotherms. The pore volumes of PAF-165 and PAF-166 were calculated to be 0.70 and 0.41 cm3/g, respectively. The high surface areas together with uniform porous textures of PAFs would provide enough space for the formation of FLPs and facilitate timely separation of reactants and products during catalytic process.
|
Download:
|
| Fig. 1. (a) N2 adsorption-desorption isotherms at 77 K and (b) the pore size distributions calculated from NLDFT for PAF-165 and PAF-166. | |
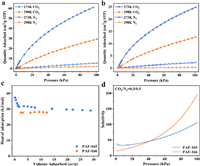
Given the highly porous frameworks and abundant B centers as Lewis acid sites in PAF-165 and PAF-166, CO2 adsorption measurements were conducted to explore PAFs' binding abilities to CO2 molecules. CO2 adsorption isotherms at 273 and 298 K were collected for each PAFs, while N2 adsorption under the same conditions were also carried out for comparison. As illustrated in Figs. 2a and b, both PAF-165 and PAF-166 exhibit obvious differences on CO2 and N2 adsorption. As the pressure increases, the adsorption capacities of CO2 raise correspondingly, whereas N2 adsorption capacities show negligible changes. This suggests that both PAFs possess greater affinity for CO2 than N2. At 273 K and 1 bar, PAF-165 possesses higher CO2 uptake (60.90 cm3/g) than PAF-166 (24.88 cm3/g), which is probably due to its higher surface area and lager pore volume (Table S1 in Supporting information). To further evaluate CO2 affinity with PAFs, the isosteric heats of adsorption (Qst) were calculated using the Clausius-Clapeyron equation [38]. At zero loading, Qst of CO2 are 37.4 and 27.8 kJ/mol for PAF-165 and PAF-166, respectively (Fig. 2c), higher than may reported porous materials such as PAF-18-OLi (29 kJ/mol) [27], PAF-26-COOK (32.6 kJ/mol) [29], Cu-BTC (30 kJ/mol) [39] and HCP-CN (26.9 kJ/mol) [40]. In addition, the well-developed ideal solution adsorbed theory (IAST) was employed to predict the CO2/N2 selectivity. Firstly, CO2 and N2 adsorption isotherms of PAF-165 and PAF-166 at 273 K were fitted by using the dual-site Langmuir Freundlich (DSLF) equation, which give well-fitted isotherms for both PAFs (Fig. S6 in Supporting information). The calculated selectivities of equimolar CO2 over N2 273 K are 33.8 for PAF-165 and 14.9 for PAF-166 at a pressure of 1.05 kPa (Fig. 2d), confirming their stronger CO2 capture abilities.
|
Download:
|
| Fig. 2. CO2 and N2 adsorption isotherms of (a) PAF-165 and (b) PAF-166 at 273 and 298 K, respectively. (c) Qst of CO2 and (d) IAST-predicted adsorption selectivity of equimolar CO2 and N2 at 273 K for PAF-165 and PAF-166. | |
As described above, PAF-165 and PAF-166 with high surface areas along with uniform pore size distributions were synthesized successfully. Benefiting from the presence of electron-deficient B centers, these two PAFs exhibit great selective adsorption abilities for CO2. The sterically hindered B building units could act as Lewis acid sites and take part in the in situ formation of FLPs with o-phenylenediamine, which will become efficient catalysts for CO2 activation and promote the synthesis of benzimidazoles. Compared to PAF-166, PAF-165 has higher surface area and larger pore volume, which is more favorable for the mass transfer process, so PAF-165 was firstly used to explore the optimal reaction conditions for the synthesis of benzimidazole from o-phenylenediamine, where CO2 was utilized as a C1 source. The type and amount of reducing agents, reaction temperatures, solvents and reaction times were fully investigated. The catalytic products were analyzed by GC–MS, in which diphenyl was used as internal standard and 2-methylbenzimidazole was determined to be the only by-product (Fig. S7 and Table S2 in Supporting information). First of all, two reducing agents, diphenylsilane (Ph2SiH2) and phenyl silane (PhSiH3) were chosen and the reactions were carried out in tetrahydrofuran (THF) at different temperatures including 100, 120 and 140 ℃ for 24 h. It shows that the conversion and selectivity in Ph2SiH2 reduced reactions (Table 1, entries 1–3) are at low levels, while PhSiH3 involved reactions obtain high conversions (> 99%) together with selectivities above 97% (Table 1, entries 4–6) at all temperatures. Then, another two solvents including dimethyl sulfoxide (DMSO) and acetonitrile (ACN) were tried. Although high conversions (> 99%) are achieved, the selectivities are only up to 50% (Table 1, entries 7 and 8). Ph2SiH2 was also tested in ACN system, and the conversion is higher than that in THF, but the selectivity is still not good enough (Table 1, entry 9). After that, the reaction time was reduced to 12 h (Table 1, entry 10) and dosage of PhSiH3 was cut down by 50% (Table 1, entry 11), respectively, whereas both conversions and selectivities decreased. Therefore, the optimal cyclization reaction proceeds in THF system under the reduced effect of PhSiH3 (4 equiv.) for 24 h. PAF-166 was utilized to verify its catalytic activity using the above condition, and conversion greater than 99% is obtained (Table 1, entry 12). ACN system was also tried with PAF-166, which accomplishes high conversion (> 99%, Table 1, entry 13). Under the same optimized reaction condition, conversions of > 99% are obtained in both PAF-165 and PAF-166 catalyzed systems (Table 1, entries 5 and 12), whereas selectivity is higher in the reaction catalyzed by PAF-165. This might be attributed to the higher surface area and larger pore volume of PAF-165, which can provide more effective contact areas for the formation of FLPs with o-phenylenediamine and is also beneficial for the separation of reactants and products. Furthermore, the cyclization of o-phenylenediamine was conducted without the addition of PAF catalysts (Table 1, entry 14), and only trace benzimidazole was detected by GC–MS analysis, proving the critical role of PAF catalysts in this reaction. Their catalytic performances are comparable with many other catalysts, such as SION-105, Rh@PS, Au/TiO2 (Table S3 in Supporting information).
|
|
Table 1 Optimization of reaction conditions for the synthesis of benzimidazoles from o-phenylenediamine.a |
Considering economic cost and environmental friendliness, the recyclability of PAF catalysts was also investigated. PAF-165 and PAF-166 with distinguished thermal and chemical stability can be easily separated from the reaction system via filtration or centrifugation. After being washed with dichloromethane and methanol thoroughly and then dried under vacuum for 6 h, these PAFs were used in the next cycle. After five successful cycles, the conversions of o-phenylenediamine cyclization under the optimal reaction conditions reach up to 99% for PAF-165 and 92% for PAF-166 (Fig. S8 in Supporting information). The structural features of recycled PAFs were studied with FTIR (Fig. S9 in Supporting information), SEM (Fig. S10 in Supporting information) and TGA (Fig. S11 in Supporting information), respectively. All the results are in good agreements with the fresh PAFs catalysts, indicating their excellent recyclability.
Furthermore, the reaction mechanism of PAFs catalyzed o-phenylenediamine cyclization via in situ formation of FLPs with sterically hindered B units in PAFs and o-phenylenediamine substrates were speculated based on the previous reported methods [19,23]. As illustrated in Fig. 3, B center in PAFs and N atom of o-phenylenediamine react with CO2 molecule to form CO2 adduct in step Ⅰ. Then, Si atom in the reducing agent PhSiH3 connects with one O atom in CO2 adduct (step Ⅱ), and subsequently PhH2SiOH releases from the reaction to give out N-(2-aminophenyl)formamide (step Ⅲ), which produces the final product 1H-1, 3-benzodiazole by dehydration reaction (step Ⅳ).
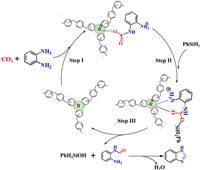
|
Download:
|
| Fig. 3. Proposed reaction mechanism for the cyclization of o-phenylenediamine catalyzed by PAF-165. | |
In summary, by selecting triphenylboron as building units, two novel B-based PAFs, PAF-165 and PAF-166, are successfully synthesized via Friedel-Crafts alkylation reaction. Profiting from the highly porous frameworks, uniform pore size distributions and the presence of electron-deficient B centers, PAF-165 and PAF-166 exhibit great CO2 adsorption capacity as well as highly selective CO2 adsorption abilities over N2. Because B centers are sterically hindered in resultant PAFs, they act as Lewis acid sites and combine with Lewis basis substrates (o-phenylenediamine) to in situ form FLPs, which present effectively catalytic performance in the synthesis of benzimidazole from o-phenylenediamine, where CO2 is utilized as a C1 source. Meanwhile, the reaction mechanism of o-phenylenediamine cyclization catalyzed by B-based PAFs is speculated to better understand the relationship of PAFs structures and catalytic activity. This work offers a promising strategy for developing FLPs-involved porous materials as heterogeneous catalysts for the synthesis of benzimidazoles, which also is an effective way for CO2 capture and conversion.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful for the financial support by the Fundamental Research Funds for the Central Universities (No. 2412019FZ008), the National Natural Science Foundation of China (Nos. 22131004 and U21A20330) and the "111" Project (No. B18012).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.05.073.
| [1] |
D.H. Nam, P. De Luna, A. Rosas-Hernandez, et al., Nat. Mater. 19 (2020) 266-276. DOI:10.1038/s41563-020-0610-2 |
| [2] |
P. Gao, L. Zhang, S. Li, Z. Zhou, Y. Sun, ACS Cent. Sci. 6 (2020) 1657-1670. DOI:10.1021/acscentsci.0c00976 |
| [3] |
H. Gu, L. Zhong, G. Shi, et al., J. Am. Chem. Soc. 143 (2021) 8679-8688. DOI:10.1021/jacs.1c02326 |
| [4] |
S. Mukhopadhyay, R. Shimoni, I. Liberman, et al., Angew. Chem. Int. Ed. 60 (2021) 13423-13429. DOI:10.1002/anie.202102320 |
| [5] |
Z. Yang, B. Yu, H. Zhang, et al., ACS Catal. 6 (2016) 1268-1273. DOI:10.1021/acscatal.5b02583 |
| [6] |
J. Liu, C. Chen, K. Zhang, L. Zhang, Chin. Chem. Lett. 32 (2021) 649-659. DOI:10.1016/j.cclet.2020.07.040 |
| [7] |
L.H. Han, J.Y. Li, Q.W. Song, et al., Chin. Chem. Lett. 31 (2020) 341-344. DOI:10.1016/j.cclet.2019.06.030 |
| [8] |
Y.L. Li, D. Thomas, A. Deutzmann, et al., Sci. Rep. 9 (2019) 16775-16783. DOI:10.1038/s41598-019-53290-3 |
| [9] |
M. Gilard, B. Arnaud, J.C. Cornily, et al., J. Am. Coll. Cardiol. 51 (2008) 256-260. DOI:10.1016/j.jacc.2007.06.064 |
| [10] |
L. Prchal, R. Podlipna, J. Lamka, et al., Environ. Sci. Pollut. Res. Int. 23 (2016) 13015-13022. DOI:10.1007/s11356-016-6472-0 |
| [11] |
K. Takeuchi, A. Konaka, M. Nishijima, S. Kato, T. Yasuhiro, J. Gastroenterol. Hepatol. 14 (1999) 251-257. |
| [12] |
D.W. Stephan, Science 354 (2016) aaf7229. DOI:10.1126/science.aaf7229 |
| [13] |
D.J. Scott, T.R. Simmons, E.J. Lawrence, et al., ACS Catal. 5 (2015) 5540-5544. DOI:10.1021/acscatal.5b01417 |
| [14] |
J. Lam, K.M. Szkop, E. Mosaferi, D.W. Stephan, Chem. Soc. Rev. 48 (2019) 3592-3612. DOI:10.1039/C8CS00277K |
| [15] |
D.J. Scott, M.J. Fuchter, A.E. Ashley, Angew. Chem. Int. Ed. 53 (2014) 10218-10222. DOI:10.1002/anie.201405531 |
| [16] |
A. Willms, H. Schumacher, T. Tabassum, et al., Chem. Commun. 10 (2018) 1835-1843. |
| [17] |
S. Mummadi, A. Brar, G. Wang, et al., Chemistry 24 (2018) 16526-16531. DOI:10.1002/chem.201804370 |
| [18] |
S. Zhang, Z.M. Xia, Y. Zou, et al., J. Am. Chem. Soc. 141 (2019) 11353-11357. DOI:10.1021/jacs.9b03217 |
| [19] |
L. Chen, R.J. Liu, Q. Yan, Angew. Chem. Int. Ed. 57 (2018) 9336-9340. DOI:10.1002/anie.201804034 |
| [20] |
F.W. Ding, Y.L. Zhang, R. Zhao, et al., Chem. Commun. 53 (2017) 9262-9264. DOI:10.1039/C7CC04709F |
| [21] |
A. Willms, H. Schumacher, T. Tabassum, et al., ChemCatChem 10 (2018) 1835-1843. DOI:10.1002/cctc.201701783 |
| [22] |
Z. Niu, W. Zhang, P.C. Lan, B. Aguila, S. Ma, Angew. Chem. Int. Ed. 58 (2019) 7420-7424. DOI:10.1002/anie.201903763 |
| [23] |
S. Shyshkanov, T.N. Nguyen, F.M. Ebrahim, K.C. Stylianou, P.J. Dyson, Angew. Chem. Int. Ed. 58 (2019) 1-6. DOI:10.1002/anie.201813481 |
| [24] |
Y. Tian, G. Zhu, Chem. Rev. 120 (2020) 8934-8986. DOI:10.1021/acs.chemrev.9b00687 |
| [25] |
T. Ben, H. Ren, S.Q. Ma, et al., Angew. Chem. Int. Ed. 48 (2009) 9457-9460. DOI:10.1002/anie.200904637 |
| [26] |
Z.J. Yan, H. Ren, H.P. Ma, et al., Microporous Mesoporous Mater. 173 (2013) 92-98. DOI:10.1016/j.micromeso.2013.02.006 |
| [27] |
H. Ma, H. Ren, X. Zou, et al., J. Mater. Chem. A 1 (2013) 752-758. DOI:10.1039/C2TA00616B |
| [28] |
L. Jiang, Y. Tian, T. Sun, et al., J. Am. Chem. Soc. 140 (2018) 15724-15730. DOI:10.1021/jacs.8b08174 |
| [29] |
H. Ma, H. Ren, X. Zou, et al., Polym. Chem. 5 (2014) 144-152. DOI:10.1039/C3PY00647F |
| [30] |
H. Ma, B. Li, L. Zhang, D. Han, G. Zhu, J. Mater. Chem. A 3 (2015) 19346-19352. DOI:10.1039/C5TA05447H |
| [31] |
T. Ma, X. Zhao, Y. Matsuo, et al., J. Mater. Chem. C 7 (2019) 2327-2332. DOI:10.1039/C8TC06288A |
| [32] |
A.T. Vilian, P. Puthiaraj, C.H. Kwak, et al., ACS Appl. Mater. Interfaces 8 (2016) 12740-12747. DOI:10.1021/acsami.6b03942 |
| [33] |
Y.T. Yang, T.N. Wang, X.F. Jing, G.S. Zhu, J. Mater. Chem. A 7 (2019) 10004-10009. DOI:10.1039/C8TA12099D |
| [34] |
L.P. Jing, J.S. Sun, F. Sun, P. Chen, G. Zhu, Chem. Sci. 9 (2018) 3523-3530. DOI:10.1039/C8SC00510A |
| [35] |
M. Trunk, J.F. Teichert, A. Thomas, J. Am. Chem. Soc. 139 (2017) 3615-3618. DOI:10.1021/jacs.6b13147 |
| [36] |
Q. Meng, Y. Huang, D. Deng, et al., Adv. Sci. 7 (2020) 2000067. DOI:10.1002/advs.202000067 |
| [37] |
Y. Yang, Y. Yang, T. Wang, et al., Microporous Mesoporous Mater. 306 (2020) 110393-110340. DOI:10.1016/j.micromeso.2020.110393 |
| [38] |
H. He, F. Sun, B. Aguila, et al., J. Mater. Chem. A 4 (2016) 15240-15246. DOI:10.1039/C6TA05098K |
| [39] |
Z. Liang, M. Marshall, A.L. Chaffee, Energy Procedia 1 (2009) 1265-1271. DOI:10.1016/j.egypro.2009.01.166 |
| [40] |
Y. Cheng, S. Razzaque, Z. Zhan, B. Tan, Chem. Eng. J. 426 (2021) 130731. DOI:10.1016/j.cej.2021.130731 |
 2023, Vol. 34
2023, Vol. 34