b Department of Chemical and Chemical Engineering, Hefei Normal University, Hefei 230601, China
Cellular mechanical force regulates numerous biological processes, such as cell proliferation, differentiation, apoptosis, polarization, adhesion, and migration [1-4]. Mechanical forces determine many aspects of biological processes from single cells to complex organisms [5]. In the process of tissue growth, various collective cell behaviors such as epiboly [6], intestinal epithelial space turnover [7] and apical constriction [8] exists. Due to the various morphologies of the tissues where the cells are located, and the cells must adapt to different external tissue environments to develop and grow. In a particular tissue, the cellular forces cause the tissue to undergo various transformations, such as bifurcation [9], rotation [7] and bending [10]. At the same time, in the special tissue morphology, the migration behavior of cellular force differs. Thus, studying the cell mechanics and behavior in various external environments is necessary. So, we tested the mechanical differences of collective cell migration in microchannels of different widths.
Cell mechanics and cell tissue environment determine the ability, path [11], and decision-making of cell migration. In these behaviors, cell-cell junctions act as a critical role in helping cells grow harmoniously in various fine structures. The cell-ECM (extracellular matrix) traction force research of collective migration is relatively mature [12], and the interaction between cellular adhesion and cellular force is also crucial for maintaining the integrity of special tissues. Cell-cell junction and cellular force are essential in maintaining the integrity of special tissues [13]. Cell-cell junctions can be divided into tight junctions, adhesive junctions (AJs), desmosomes [14] and gap junctions [2]. In cell-cell junctions, tight junctions and desmosomes are necessary to maintain the integrity and cohesion of epithelial cells, but AJs are essential to maintain coordinated cellular behavior [15]. AJs are the main cell-cell junctions that sense and respond to the tension at the cell-cell interface. Each structure in the AJs has an inseparable relationship with the intercellular force [15,16]. For example, the intercellular force is driven by the actomyosin contractility [2]. F-actin can indicate the cell orientation [17], and E-cadherin mediates cell-cell adhesion [18]. Biological processes driven by multiple cell interactions strongly depend on these cell-cell junctions [17,19].
The coordinated migration of epithelial cell sheets is regulated by mechanical force. We studied the mechanical properties of collective cell migration under different spatial constraints. Collective cell migration comes from the cell-cell cooperation. Thus, the study of intercellular forces elucidates collective cell migration. We designed a simple model that confined cells to microchannels of different widths to generalize some spatial cell constraints in vitro. We used a previously reported method [20,21] to measure the tension between living cells using a DNA spring probe with a fluorophore-quencher pair on molecular tension fluorescence microscopy (MTFM). At the same time, by using polydimethylsiloxane (PDMS) microchannels with different widths, changes in intercellular tensile force and energetic costs are observed in the narrow microchannels. In the narrow microchannel, we observe larger intercellular force and higher energetic costs, which is an important factor of intercellular mechanical transmission. The intercellular force during the cell migration needs to consume a mass of energy and take in enough glucose to meet energetic demand. The external constraints of the tissue are altered, and cell-cell connections and internal dynamics are altered to adjust to large-scale interactions over great distances across multiple cell bodies.
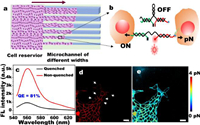
DNA-based force probe for microchannels of different widths: PDMS microchannels with different widths were fabricated, and the cells is strictly confined to microchannels of different widths for collective migration. As shown in Fig. 1a, under the action of intercellular force, the DNA spring probe (MTFM probe) changed from the quenched state of Cy3-BHQ2 to the open state, and the intercellular force was observed by the fluorescence signal (Fig. 1b). We also calculated the quenching efficiency was 81% (Fig. 1c). We used a worm-like chain (WLC) formula [22,23] to transform the fluorescence image on the cell membrane into piconewton (pN) image of intercellular force (Fig. S1 in Supporting information). The fluorescence (Fig. 1d) shows that the fluorescence intensity of cell-cell junction area is brighter than the other membrane areas, and the white arrow in Fig. 1d indicates the fluorescence intensity on non-cell-cell junction membranes that is slightly bright. This finding is consistent with that of Zhao [21], thereby indicating that the probe can well represent the intercellular tensile force (Figs. 1d and e). The feasibility of the probe (Fig. S2 in Supporting information) was verified by Y27632 pre-treatment, which proved that the MTFM probe can be used in real-time measurement between cells.
|
Download:
|
| Fig. 1. Schematic diagram of MDCK cell migration under a confined microchannel and the method used to measure the intercellular force. (a) Cell migration under different spatial constraints. The purple arrow indicates the direction of cell migration. (b) The intercellular force spring probe was turned on under the action of intercellular force. The quenching effect of Cy3-BHQ2 was turned on, and the probe changed from OFF to ON. (c) After adding cDNA, the MTFM probe turned on, and the quenching efficiency of the DNA spring was obtained. (d) Fluorescent image of intercellular forces. The white arrow refers to the membrane areas of non-cell-cell junction. (e) pN force image was calculated from the typical fluorescence image. Scale bar in (d, e) was 25 µm. | |
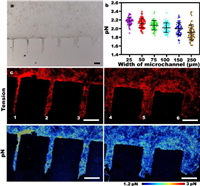
Effects of microchannels with different widths on intercellular forces in epithelial cells: The widths of the microchannel were approximately 25, 50, 75, 100, 150 and 250 µm (Fig. S3 in Supporting information). After the cells have migrated in the microchannel for about 24 h, we get the bright field (BF) image in Fig. 2a. The BF image shows that cells in narrow microchannels migrate farther than those in wide microchannels, which is consistent with the results of previous studies [24]. Therefore, to understand the differences of intercellular mechanics under spatial constraints, we used MTFM probe to observe the intercellular mechanics on different microchannels. Statistics on many groups of data were obtained, and a general phenomenon was found (Fig. 2b), with increasing microchannel width, the average value of the force on each microchannel and the average value of the non-zero point became increasingly smaller successively. This result is consistent with the cellular migration distance. As shown in Fig. 2c, we found that the fluorescence on 25 µm microchannel are brighter than 250 µm microchannel. Similarly, we can see that the force on the 75, 100, 150 and 250 µm microchannels are smaller in turn.
|
Download:
|
| Fig. 2. The distribution of intercellular forces on microchannels of different widths. (a) The bright field image of MDCK cell sheet after migrating on the channels of different widths for about 24 h. Scale bar is 200 µm. (b) The intercellular force count on all the microchannels. Each point represents the average value of the intercellular force on the microchannel except for 0. The results of at least 50 experiments were calculated. (c) Representative fluorescence and pN force maps representing intercellular forces on microchannels of different widths. All scale bars are 250 µm. The channels are labeled as 1, 2, 3, 4, 5 and 6 and correspond to widths of 25, 50, 75, 100, 150 and 250 µm, respectively. | |
On a narrow microchannel, because the width is small enough, and only a few cells can enter, cells spontaneously and roughly move in one orientation. On a wide microchannel, movement is similar to that in an unlimited space, which shows collective cell sheet migration. Cells spontaneously migrate to the blank area, because enough space for migration is available.
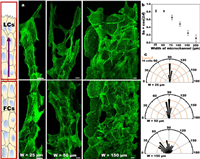
Epithelial cytoskeleton and cell orientation in microchannels with different widths: AJs in the cell-cell junction structure mediate tissue integrity [15,16]. Intercellular force is directly related to AJs in the process of cell migration. Thus, the mechanical properties of AJs under microchannel of different widths need to be analyzed (Fig. S4 in Supporting information). AJs are usually composed of E-cadherin, which couples cell-cell junction with the cytoskeleton of F-actin (Fig. S5 in Supporting information). The orientation of F-actin filaments represents the cell orientation [17,25], and we speculate that cell orientation might differ in various confined microchannels. Fig. 3a shows that in the 25 µm microchannels, the orientation of F-actin filaments is mostly toward the direction of cell movement or the axis of the channel, which is relatively orderly. However, in the 150 µm microchannels, cell orientation is random. The F-actin filaments have various orientations in the wide microchannels, but they are strongly aligned with the long axis in the narrow channels. F-actin filaments are oriented parallel to the orbital in narrow channels, but they have no preferred orientation in wide microchannels. The distribution of F-actin is uniform, and stress fibers are visible in any channel. Narrow microchannels can promote cell polarization, which in turn can improve the migration ability [2]. Meanwhile, physical constraints promote cytoskeleton reorganization. To better quantify these, we used the method proposed by Benoit Ladoux et al. [25], the nematic actin order parameter defined as Sa = cos(2θ) (Fig. 3b). In 25 µm and 50 µm channels, most of the actin filaments are parallel to the axis of microchannel, and the value of Sa in the 25 µm microchannel is close to 0.8. By contrast, in the 250 µm microchannels, the random the orbital in narrow channels, but they have no orientation of actin filaments can be seen, and the value of Sa in the 250 µm microchannel is close to 0. We wanted to understand cell body orientation, and our method involved the use of the angle between the long axis of cell body and the microchannel axis (Fig. 3c). Achieving a more efficient cell migration involves a great relationship with the intercellular communication. The orientation of each cell depends on its neighboring cells. Cell-cell junction and actin cytoskeleton are chemically and mechanically coupled to each other; they interact, thereby changing the cell front-back polarization [26]. At the same time, we also observed the orientation field (Figs. S6 and S7 in Supporting information), which showed that there were topological defects in the wide channel, but the orientation field was ordered in the narrow channel.
|
Download:
|
| Fig. 3. Cytoskeleton and cell orientation of epithelial cell sheets in microchannels of different widths. (a) Fluorescence images of F-actin filaments stained with Phalloidin-Alexa488 on microchannels of different widths. The diagram on the left describes the definition of LCs and FCs on the microchannel. LCs: The front area of the leading cell. FCs: Follower cells. The purple arrow represents the direction of cell migration. Scale bar: 10 µm. (b) Sa = cos(2θ), θ is derived from the angle of the F-actin filaments relative to the long axis of the microchannel (according to five independent experiments). (c) Polar graph plotting the histogram of the distribution of the cell orientation on the microchannel; 90 degrees indicates orientation along the axis of the microchannel, and 0 and 180 degrees indicate that the cell orientation is perpendicular to the long axis direction. | |
At the same time, in order to study contractility difference results in intercellular force variations. We tested the distribution of phosphorylated myosin light chain (pMLC) represented by the cytoskeletal actomyosin contractility. Figs. S8-S10 (Supporting information) show that most of the F-actin and pMLC were colocalized, indicating that actomyosin contractility is the most important contribution to intercellular force [27].
The influence of energy on confined microchannel of epithelial cell sheets: Cells need energy to migrate and usually produce ATP to meet their energetic needs [28,29]. The intercellular pull is produced by the movement of ATP-dependent actomyosin on the actin filaments of the cytoskeleton. We used PercevalHR [29,30], a ratiometric intracellular ATP/ADP fluorescent biosensor to measure cellular energetic costs.
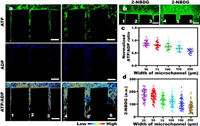
As shown in Fig. 4a, the energetic costs on the 25 µm and 50 µm microchannels is significantly greater than the energetic costs on the 150 µm and 250 µm microchannels. The results presented here are similar to the results of pMLC (Fig. S8 in Supporting information) and intercellular force, which prove from the side that the cellular mechanical properties are directly related to energy. Glucose is an energetic source used for maintaining cell activity and homeostasis [30,31]. Glucose uptake depends on the utilization of intracellular glucose. Enhanced glucose uptake protects cells during energy absorption [32]. When cells were incubated with the fluorescent glucose analog 2-NBDG, glucose uptake was measured. It can be seen from Fig. 4b that the fluorescence intensity of 2-NBDG is higher in the narrow channel.
|
Download:
|
| Fig. 4. The ATP/ADP ratio and glucose uptake of cell migration in microchannels of different widths. (a) ATP/ADP ratio of cells on different width microchannels. From left to right were ATP, ADP fluorescence image, and ATP/ADP ratio maps. All scale bars, 250 µm. (b) Fluorescence image of glucose uptake of 2-NBDG in microchannels of different widths. All scale bars, 250 µm. (c) The scatter plot is derived from the average value of the ATP/ADP of the images of 30 different microchannel combinations. ATP/ADP is normalized, and the value on the 25 µm microchannel is used as a standard for normalization. (d) The scatter plot is calculated from the average value of 2-NBDG fluorescence values of at least 50 different microchannel combinations. Every scatter point is the average value on each microchannel, and the average value does not include zero value. | |
Therefore, we separately counted the ATP/ADP and 2-NBDG fluorescence intensity of many groups. It is observed that in Figs. 4c and d, the phenomenon of 2-NBDG is consistent with the phenomenon of ATP/ADP. Then, we generated a statistical graph (Figs. 4c and d) of the cellular energetic costs and 2-NBDG fluorescence of many groups. 2-NBDG and ATP/ADP have the same trend. With decreasing width, energetic costs and glucose uptake increase, indicating that cells respond to various microchannels through glucose uptake and energy production. During the migration process, a high ATP/ADP ratio indicates that more energy is consumed, which in turn means that more glucose uptake is required. This increased glucose uptake on the narrow microchannels can be used to compensate for energetic consumption, because the intercellular force on the narrow microchannels requires high actomyosin contractility and intercellular tensile forces. Cells was adapted to different environments by acquiring dynamic energetic costs.
In conclusion, we investigated intercellular mechanical properties under microchannels of different widths and observed the influence of cell migration characteristics, such as intercellular forces, cell orientation and energy, reflecting different migration behaviors. In narrow microchannels, cell orientation is relatively uniform, and cells move in the direction of cell migration. By contrast, cell orientation in the wide microchannel is heterogeneous. Moreover, a higher intercellular force is present in the narrow microchannels, and actomyosin is more contractible, thereby requiring more energetic cost and glucose uptake. The cell orientation is more orderly on narrow microchannels, because actomyosin contraction leads to greater intercellular force, energetic costs, and glucose uptake in highly confined environments. Through these evidence, we proved that we can use an intercellualr force probe to study the effect of space restriction on epithelial cell migration. Our findings provide unique insights into the ability of mechanical force adjustment to adapt to the environment in microchannels of different widths.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 22034003), Excellent Research Program of Nanjing University (No. ZYJH004), Fundamental Research Funds for the Central Universities (No. 020514380181), State Key Laboratory of Analytical Chemistry for Life Science (No. 5431ZZXM2002), and Program B for Outstanding PhD candidate of Nanjing University (No. 201702B052).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.107789.
| [1] |
D.E. Ingber, FASEB J. 20 (2006) 811-827. DOI:10.1096/fj.05-5424rev |
| [2] |
B. Ladoux, R.M. Mège, Nat. Rev. Mol. Cell Biol. 18 (2017) 743-757. DOI:10.1038/nrm.2017.98 |
| [3] |
P.K. Viji Babu, C. Rianna, U. Mirastschijski, et al., Sci. Rep. 9 (2019) 12317. DOI:10.1038/s41598-019-48566-7 |
| [4] |
M. Gómez-González, E. Latorre, M. Arroyo, et al., Nat. Rev. Phys. 2 (2020) 300-317. DOI:10.1038/s42254-020-0184-6 |
| [5] |
X. Hu, F.M. Margadant, M. Yao, et al., Protein Sci. 26 (2017) 1337-1351. DOI:10.1002/pro.3188 |
| [6] |
E.A.R. Morris, S. Bodin, B. Delaval, et al., Bio. Cell 109 (2017) 210-221. DOI:10.1111/boc.201700001 |
| [7] |
S. Jain, V.M.L. Cachoux, G.H.N.S. Narayana, et al., Nat. Rev. Phys. 16 (2020) 802-809. DOI:10.1038/s41567-020-0875-z |
| [8] |
Y. Inoue, M. Suzuki, T. Watanabe, et al., Biomech. Model. Mech. 15 (2016) 1733-1746. DOI:10.1007/s10237-016-0794-1 |
| [9] |
J.W. Song, L.L. Munn, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 15342-15347. DOI:10.1073/pnas.1105316108 |
| [10] |
H. Zang, X. Li, Phys. Rev. E 101 (2020) 032406. DOI:10.1103/PhysRevE.101.032406 |
| [11] |
G.M. Allen, K.C. Lee, E.L. Barnhart, et al., Cell Syst. 11 (2020) 286-299. DOI:10.1016/j.cels.2020.08.008 |
| [12] |
S. Yokoyama, T.S. Matsui, S. Deguchi, Biochem. Bioph. Res. Co. 482 (2017) 975-979. DOI:10.1016/j.bbrc.2016.11.142 |
| [13] |
S.R.K. Vedula, H. Hirata, M.H. Nai, et al., Nat. Mater. 13 (2014) 87-96. DOI:10.1038/nmat3814 |
| [14] |
E. Bazellières, V. Conte, A. Elosegui-Artola, et al., Nat. Cell Biol. 17 (2015) 409-420. DOI:10.1038/ncb3135 |
| [15] |
Z. Liu, J.L. Tan, D.M. Cohen, C.S. Chen, Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 9944-9949. DOI:10.1073/pnas.0914547107 |
| [16] |
G. Charras, A.S. Yap, Curr. Biol. 28 (2018) R445-R457. DOI:10.1016/j.cub.2018.02.003 |
| [17] |
A. Ray, O. Lee, Z. Win, et al., Nat. Commun. 8 (2017) 14923. DOI:10.1038/ncomms14923 |
| [18] |
C.M. Nelson, R.P. Jean, J.L. Tan, et al., Proc. Natl. Acad. Sci. U. S. A. 102 (2005) 11594-11599. DOI:10.1073/pnas.0502575102 |
| [19] |
J.T. Parsons, A.R. Horwitz, M.A. Schwartz, Nat. Rev. Mol. Cell Biol. 11 (2010) 633-643. DOI:10.1038/nrm2957 |
| [20] |
X.H. Wang, F. Yang, J.B. Pan, et al., Anal. Chem. 92 (2020) 16180-16187. DOI:10.1021/acs.analchem.0c03935 |
| [21] |
B. Zhao, C. O'Brien, A.P.K.K.K. Mudiyanselage, et al., J. Am. Chem. Soc. 139 (2017) 18182-18185. DOI:10.1021/jacs.7b11176 |
| [22] |
Y. Chang, Z. Liu, Y. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 2901-2904. DOI:10.1021/jacs.5b11602 |
| [23] |
V.P.Y. Ma, K. Salaita, Small 15 (2019) 1900961. DOI:10.1002/smll.201900961 |
| [24] |
S.R.K. Vedula, M.C. Leong, T.L. Lai, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 12974-12979. DOI:10.1073/pnas.1119313109 |
| [25] |
W. Xi, S. Sonam, T. B. Saw, et al., Nat. Commun. 8 (2017) 1517. DOI:10.1038/s41467-017-01390-x |
| [26] |
R. Mayor, S. Etienne-Manneville, Nat. Rev. Mol. Cell Biol. 17 (2016) 97-109. DOI:10.1038/nrm.2015.14 |
| [27] |
J.G. Peacock, B.A. Couch, A.J. Koleske, Cytoskeleton 67 (2010) 666-675. DOI:10.1002/cm.20479 |
| [28] |
Y. Li, L. Yao, Y. Mori, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 23894-23900. DOI:10.1073/pnas.1907625116 |
| [29] |
M. Tantama, J.R. Martínez-François, R. Mongeon, et al., Nat. Commun. 4 (2013) 2550. |
| [30] |
J. Zhang, F. Goliwas Kayla, W. Wang, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 7867-7872. DOI:10.1073/pnas.1809964116 |
| [31] |
M.R. Zanotelli, A. Rahman-Zaman, J.A. VanderBurgh, et al., Nat. Commun. 10 (2019) 4185. DOI:10.1038/s41467-019-12155-z |
| [32] |
T. Isogai, J.S. Park, G. Danuser, Nat. Cell Bio. 19 (2017) 591-593. |
 2023, Vol. 34
2023, Vol. 34 

