b School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou 510006, China;
c Lingnan Medical Research Center, The first Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510006, China;
d Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry (CAF), Nanjing 210042, China
Luminol, chemically known as 5-amino-2, 3-dihydro-1, 4-phthalazinedione, is the most commonly used chemiluminescent substance in biochemical analysis both in bench studies and clinical applications [1, 2]. Luminol can react with reactive oxygen species (ROS) such as H2O2, peroxide anion (O2•−) and hydroxyl radical (•OH) to form an oxidized intermediate, which further decomposes to generate 3-aminophthalic acid and light (Scheme S1 in Supporting information) [3-5]. On these bases, any analyte leading to the generation of ROS is appropriate to be analyzed by luminol-based CL assays [6-11]. Another well-known application is that luminol is used as CL indicator to identify and image bloodstains over other colored substances during criminal investigation [12, 13]. More importantly, luminol and its derived reagents have been extensively applied as CL substrates of HRP in the most common bioanalytical techniques including WB, enzyme-linked immunosorbent assay (ELISA) and so on [14-19]. Although widely used, luminol and its derived reagents are still suffering from drawbacks, such as short luminescence time and weak intensity in some cases. This fact has prompted a lot of attempts to design improved luminol reagents, including searching for advanced catalysts and enhancers, synthesizing new derivatives [20-25]. Among all, chemical derivatization on luminol is most attractive as it can potentially offer new CL substances with improved luminescence properties.
Current methods for chemical modification of luminol mainly rely on two strategies as follow: (1) tuning electron-donating effect by substitution on the phenyl ring to form analogues, potentially with improved CL efficiency [26-28]; and (2) introducing other functional groups to the site of C5-NH2 to generate multifunctional luminescent probes [29-31]. Although many derivatives have been made by following the strategies above, most analogues have not been practically used due to some limitations. For example, previous research found that proton transfer between the amino and carbonyl groups is essential for emitting light, that is why modification on C5-NH2 usually leads to decreased CL efficiency [32]. lt is known that keto-enol isomerization exists on luminol, and basic conditions can stabilize the enol forms. However, there is almost no report on discovering novel luminescent substances upon modification on the hydroxyl groups of enol forms. Most recently, Singh et al. proposed that C1-O-alkylation on luminol by alkyl halides is appreciable under basic media, and this O-alkylated luminol exhibited increased fluorescence upon excitation [33]. With this regard, a fluorescence assay has been developed to detect sulfur mustard (SM). However, the CL properties have not been evaluated in their research.
Herein, series of luminol derivatives with C1-O-etherification and C1-O-esterification have been prepared through a simple synthetic route (Scheme 1). The potentials for these analogues to detect hemin, bloodstain and HRP have been examined. Some O-esterificated analogues exhibited improved CL properties in comparison with luminol itself. Based on the discovery that O-etherification can greatly suppress CL signal, a luminol-O-β-d-glucose conjugate has been prepared and evaluated as a CL probe for sensing β-Glu.
|
Download:
|
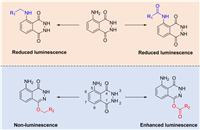
| Scheme 1. Chemical derivatization on luminol and the influences to luminescence property. R1 and R2 correspond to alkyl, aryl groups and others. | |
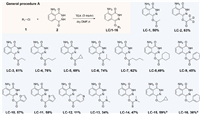
Esterification on luminol with various acyl chlorides was firstly performed at room temperature using dimethylformamide (DMF) as solvent and triethylamine (TEA) as base. Etherification was performed through similar conditions using alkyl halides as starting materials (Scheme S2 in Supporting information). As shown in Scheme 2, sixteen analogues (LC-1 to LC-16) have been prepared with isolated yields ranging from 11% to 83%. Since acylation and alkylation always occur at C1-O group under the proposed conditions, C5-N modification was conducted following a two-step procedure, using 3-aminophthalic acid (3) as starting material. Moderate conversions were observed and the products were listed in Table S1. All the synthetic products have been characterized by NMR and high-resolution mass spectrometry (HRMS). Particularly, the structures of LC-5, LC-8 and LC-15 were further confirmed by X-ray crystallographic analysis with the CCDC No. 2107419, 2107427 and 2107420, respectively.
|
Download:
|
| Scheme 2. Scope of C1-O-modification and the products. Reaction conditions: luminol (1.0 mmol), acyl/alkyl chlorides 1 (1.0 mmol), TEA (3.0 mmol), r.t., 5 h. Isolated yield. a50 ℃, 3 h. | |
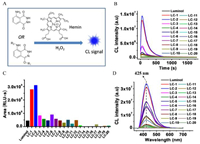
Luminol has been extensively used as an indicator in the identification and imaging of bloodstains during criminal investigations. Iron protoporphyrin IX (Fe-PPIX) and its protein bound forms (such as hemoglobin) are the major species in blood that can catalyze the oxidation of luminol in the presence of H2O2 [34-36]. To test CL properties of the synthetic analogues in the detection of Fe-PPIX and blood, a hemin (Fe3+-PPIX) reaction system was applied, in which hemin was used as the catalyst and H2O2 as the oxidants (Fig. 1A). As indicated from Fig. 1B, improved luminescence performances were observed on several synthetic ester derivatives in comparison with luminol. Among them, the acetyl product LC-1 and the deuterated acetyl product LC-2 have the highest luminous efficiency, with largely increased intensity and longer luminescence time. The integrated areas of LC-1 and LC-2 under the response curves (0–750 s) reach to 6.27-fold and 6.98-fold to that of luminol, respectively. The luminescence performances of LC-3 and LC-6 are also better than luminol, but they are not so significant in comparison with LC-1 and LC-2. Other ester derivatives have similar or weaker luminescence to luminol (Fig. 1C). In a further study, we found that both LC-1 and LC-2 exhibited greater CL emitting than luminol under the pHs ranging from 9.0 to 11.0 (Fig. S1 in Supporting information).
|
Download:
|
| Fig. 1. (A) A schematic figure shows light emitting from luminol/H2O2 in the presence of hemin as catalyst. (B) CL response curves (intensity versus time/s) of luminol and the synthetic derivatives. (C) Integral area under the response curves from Fig. 1B. (D) CL spectra of luminol and the derivatives. BR buffer (40 mmol/L) at pH 9.0 was used as the reaction medium, hemin at 0.1 µmol/L, CL substrates at 250 µmol/L, H2O2 at 500 µmol/L were used for response curve collection, while, hemin at 0.1 µmol/L, CL substrates at 500 µmol/L, H2O2 at 20 mmol/L were used for the collection of CL spectra. | |
It has been indicated that steric effect and electron-donating ability of the substituent on the aromatic ring can influence light emission significantly, but it largely depends on which position has been substituted [28, 37-39]. For example, dual-substitution at both para- and ortho-positions to C5-NH2 group of luminol can offer analogues with improved CL responses under mild pHs [39]. However, there is less knowledge about the modification on the heterocyclic ring and its consequence on light emitting. To investigate the possible mechanism, the reaction solutions of LC-2 and luminol have been analyzed by high performance liquid chromatography (HPLC). As found from Fig. S2 (Supporting information), the reaction between LC-2 and H2O2 upon catalyzed by hemin is fast as many 3-aminophthalic acid products have generated within 1 min. In contrast, only a small portion of luminol has converted to product even after 25 min. A possible reaction mechanism has been proposed in Fig. S3 (Supporting information). Fe-PPIX has limited stability during the catalytic progress as the radicals can also attack the catalyst, and catalyst deactivation will be significant if radicals cannot be effectively consumed by other substances [40]. This could probably explain the differences observed between LC-2 and luminol. The enol form of luminol in basic condition was thought important to be attacked by ROS. Esterification on OH group is able to stabilize luminol at enol form, which might be one of the reasons why several luminol ester derivatives have higher luminous efficiencies. However, larger substitutes can result to steric hindrance and solubility issues, which greatly affect the reactivity. In contrast, it's found that N-derivatives LC-17, LC-18, LC-19 and LC-20 have weak luminescence (Figs. 1B and C), which is consistent with previous observation that amino group modification will significantly reduce the CL efficiency of luminol [32]. In addition, CL spectra show that both N-modification and O-modification do not lead to wavelength shift in comparison with luminol, and all of them peaked at around 425 nm (Fig. 1D).
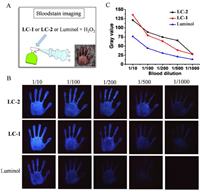
The good performances of LC-1 and LC-2 on hemin detection encouraged us to further apply them for bloodstain imaging. To do this, LC-1, LC-2 and luminol were mixed with H2O2 to form the imaging solutions separately (Fig. 2A). The imaging solutions were then sprayed against the bloodstains through a small spray bottle (15 mL), and the CL imaging was taken immediately by a smart phone. As shown in Figs. 2B and C, the CL imaging reagents containing LC-1 or LC-2, individually, show great advantages over luminol in bloodstain imaging. Upon 1000 times dilution, LC-1 and LC-2 can still show obvious CL signal, whereas, luminol can no longer perform effective imaging. The result implies that imaging solutions with LC-1 or LC-2 have excellent application prospects in criminal investigations. LC-5 and luminol with similar performance in hemin detection also have comparable capabilities in bloodstain imaging. Whereas, LC-15 and LC-19 does not show the ability to image bloodstains (Fig. S5 in Supporting information).
|
Download:
|
| Fig. 2. (A) Schematic diagram shows bloodstain imaging using CL solutions. (B) The bloodstain images taken by a smartphone upon detection with CL solution containing luminol, LC-1 and LC-2, respectively. (C) Quantitative analysis of CL intensity from Fig. 2B, please refer to Fig. S4 for details. | |
HRP is well-known as an important enzyme for in vitro diagnosis (IVD), and luminol is the classic CL substrate of HRP. The catalytic center of HRP protein is a Fe-PPIX, which catalyzes the oxidation of luminol following the mechanism similar to free hemin [41, 42]. With these regards, we conducted HRP detection using LC-1, LC-2, LC-3 and LC-6 that have exhibited excellent responses to hemin (Fig. 3A). It can be seen from Fig. 3B that LC-2 shows obvious advantages over other tested compounds in sensing HRP, as indicated by higher intensity and longer emission time. The integral area of LC-2 under the response curve is 18.2-fold to luminol. LC-1 and LC-6 also have enhanced performances than luminol, but they are far from LC-2 (Fig. S6 in Supporting information). It is known that CL signal from luminol can be dramatically enhanced using enhancers in real applications.
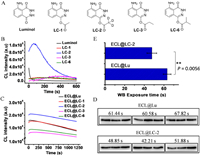
|
Download:
|
| Fig. 3. (A) Chemical structures of the substrates tested for HRP sensing. (B) CL response curves of luminol and the synthetic derivatives (all at 200 µmol/L) upon reaction with HRP (10 ng) and H2O2 (5 mmol/L) in 10 mmol/L Tris–HCl solution (pH 8.5). (C) CL response curves of ECL solution with the CL substrate (50 µmol/L) upon reaction with HRP (1 ng) and H2O2 (5 mmol/L), in the presence of enhancers (SPTZ 1 mmol/L, MORP 2 mmol/L). (D) WB analysis of GAPDH, GAPDH (MC4) mouse mAb, goat anti-mouse IgG (H + L)-HRP were used as the first and secondary antibodies respectively. (E) The exposure times used to take the images in Fig. 3D. P < 0.01 was set as extreme significant. | |
3-(10-Phenothiazinyl) propane-1-sulfonate (SPTZ) together with 4- morpholinopyridine (MORP) is recognized as one of the best enhancers for HRP/Luminol system [43], which was mixed together with luminol or its derivatives to prepare enhanced chemiluminescence (ECL) solutions. As expected, all ECL systems show greatly enhanced CL performances than those without enhancers as indicating by higher intensity and longer luminescence time. ECL@LC-2 is the most effective one among all (Fig. 3C). WB imaging was then conducted using the newly prepared ECL solutions, following the procedure described in supporting information. The first antibody was normally diluted (5000 times) before WB, while, the HRP-labeled secondary antibody was diluted for 1 million times from the commercial kits to look into the sensitivity. As illustrated in Fig. 3D, imaging solution using LC-2 as ECL substrate worked very well in WB imaging. Under auto exposure mode, 47.64 s on average was needed for ECL@LC-2 to take a reliable image, while 63.28 s was needed for ECL@Lu to take an image with similar qualities. The exposure time for ECL@LC-2 is extremely significant shorter than ECL@Lu (P = 0.0056) (Fig. 3E). The result indicated that ECL reagents made from LC-2 shows advantages in WB imaging over luminol.
As found above, O-etherification on luminol can suppress its CL response to hemin, thus implying a promising way in the design of CL assays through signal turn-on manners. These bases inspired us to design CL probes for glycoside hydrolases, as the glycosidic linkage used to construct this sort of probes is exactly an ether bond [44, 45]. With this consideration, a β-Glu probe (Lum-Glc) has been prepared as an example by linking luminol with a d-glucose through C—O bond (Scheme S3 in Supporting information). The enzyme reaction was performed following the procedure described in supporting information. CL signal was recorded through a simple handheld CL meter (Fig. 4A).
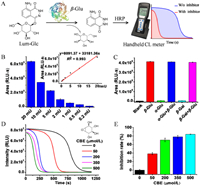
|
Download:
|
| Fig. 4. (A) A schematic diagram illustrating a CL assay for analysis of β-Glu activity, a handheld CL meter was used to record the signal. (B) The integral areas under the response curves of intensity versus time, in the presence of β-Glu at different concentrations; the inset shows an linear relationship. (C) Lum-Glc/HRP/H2O2 system shows excellent selectivity toward β-Glu. (D) The response curves of Lum-Glc/HRP/H2O2 system in the presence of CBE at different concentrations. (E) Inhibition rate of CBE toward β-Glu measured by the newly developed assay. | |
As shown in Fig. 4B, CL signal increases as the concentration of β-Glu increasing, and the integral areas depended linearly on the β-Glu concentration from 0.2 mU to 20 mU (R2 = 0.993). In addition, Lum-Glc exhibited excellent selectivity to β-Glu over other tested glycoside hydrolases, α-glucosidase (α-Glu) and β-galactosidase (β-Gal). As illustrated in Fig. 4C, treated with α-Glu or β-Gal alone cannot lead to CL turn on, whereas, the CL signal can be recovered upon co-treatment with β-Glu. A β-Glu inhibitor screening system has been developed, where conduritol B epoxide (CBE) a well-known inhibitor, was utilized to suppress β-Glu's function [46]. Figs. 4D and E clearly indicate that CBE can suppress the CL signal at CBE concentration dependent manners. 50 µmol/L CBE leads to the decrease of β-Glu activity by 38.43% and 200 µmol/L CBE decreases the activity β-Glu by 70.56%. Therefore, Lum-Glc based CL assay supplied with a simple handheld CL meter offers great opportunities for discovering new β-Glu inhibitors and quantitative measurement of inhibition effect. Moreover, similar design can also be applied to develop analytical assays for other glycoside hydrolases.
Over twenty luminol derivatives have been prepared. It is found for the first time that O-esterification on luminol is able to prepare advanced analogues with improved CL performances. Acetyl and deuterated acetyl analogues (LC-1 and LC-2) exhibited greater potential than luminol in sensing hemin and blood, which have shown the potential for real applications in criminal investigation. LC-2 exhibited improved efficiency in HRP imaging. ECL solution containing LC-2 shows excellent capability for WB imaging. In addition, a CL assay based on a luminol ether derivative (Lum-Glc) has been developed for analyzing β-Glu activity. This study offers not only useful knowledge involving the chemical derivitization of luminol and their CL properties but also important principles for probe design based on luminol and its analogues.
Declaration of competing interestThere are no conflicts to declare.
AcknowledgmentsWe gratefully acknowledge the projects of the Department of Education of Guangdong Province (Nos. 2021ZDZX2030 and 2020ZDZX2017), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110115) and Guangzhou Basic and Applied Basic Research Foundation (No. 202102020548) for funding support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.05.010.
| [1] |
P. Khan, D. Idrees, M.A. Moxley, et al., Appl. Biochem. Biotech. 173 (2014) 333-355. DOI:10.1007/s12010-014-0850-1 |
| [2] |
M. Yang, J. Huang, J. Fan, et al., Chem. Soc. Rev. 49 (2020) 6800-6815. DOI:10.1039/D0CS00348D |
| [3] |
A. Karabchevsky, A. Mosayyebi, A.V. Kavokin, Light Sci. Appl. 5 (2016) e16164. DOI:10.1038/lsa.2016.164 |
| [4] |
Z. Wang, J. Huang, J. Huang, et al., Aggregate 2 (2021) e140. |
| [5] |
M. Vacher, I. Fdez. Galván, B.W. Ding, et al., Chem. Rev. 118 (2018) 6927-6974. DOI:10.1021/acs.chemrev.7b00649 |
| [6] |
T.H. Fereja, S.A. Kitte, W. Gao, et al., Talanta 204 (2019) 379-385. DOI:10.1016/j.talanta.2019.06.007 |
| [7] |
T.H. Fereja, C. Wang, F. Liu, Y. Guan, G. Xu, Analyst 145 (2020) 6649-6655. DOI:10.1039/D0AN01178A |
| [8] |
S.A. Kitte, W. Gao, Y.T. Zholudov, et al., Anal. Chem. 89 (2017) 9864-9869. DOI:10.1021/acs.analchem.7b01939 |
| [9] |
Y. Wu, D. Peng, Z. Qi, et al., Front. Chem. 8 (2020) 601636. DOI:10.3389/fchem.2020.601636 |
| [10] |
Y. Wang, L. Shi, Z. Ye, et al., Nano Lett 20 (2020) 176-183. DOI:10.1021/acs.nanolett.9b03556 |
| [11] |
Y. Liu, Y. Yao, B. Yang, Y.J. Liu, B. Liu, Chin. Chem. Lett. 33 (2022) 2705-2707. DOI:10.1016/j.cclet.2021.09.074 |
| [12] |
S. Polacco, P. Wilson, M. Illes, A.J. Vreugdenhil, T. Stotesbury, Forensic. Chem. 12 (2019) 91-98. DOI:10.1016/j.forc.2019.01.002 |
| [13] |
W. Gao, C. Wang, K. Muzyka, et al., Anal. Chem. 89 (2017) 6160-6165. DOI:10.1021/acs.analchem.7b01000 |
| [14] |
M. Sharafeldin, K. Kadimisetty, K.R. Bhalerao, et al., Anal. Chem. 91 (2019) 7394-7402. DOI:10.1021/acs.analchem.9b01284 |
| [15] |
Z. Zhang, J.H. Lai, K.S. Wu, et al., Talanta 180 (2018) 260-270. DOI:10.1016/j.talanta.2017.12.024 |
| [16] |
Q. Xiao, C.X. Xu, TrAC-Trend. Anal. Chem. 124 (2020) 115780. DOI:10.1016/j.trac.2019.115780 |
| [17] |
Z. Li, L. Wang, Z. Yuan, C. Lu, Chem. Commun. 55 (2019) 679-682. DOI:10.1039/C8CC07598K |
| [18] |
Q. Wang, J. Deng, Y. Chen, Y. Luo, X. Jiang, Chin. Chem. Lett. 31 (2020) 1835-1838. DOI:10.1016/j.cclet.2020.01.028 |
| [19] |
S. He, L. He, B. Liu, et al., Chin. Chem. Lett. 30 (2019) 1031-1034. DOI:10.1016/j.cclet.2019.03.013 |
| [20] |
D.R. Dadadzhanov, I.A. Gladskikh, M.A. Baranov, T.A. Vartanyan, A. Karabchevsky, Sens. Actuators B: Chem. 333 (2021) 129453. DOI:10.1016/j.snb.2021.129453 |
| [21] |
N. Yang, Y. Huang, G. Ding, A. Fan, Anal. Chem. 91 (2019) 4906-4912. DOI:10.1021/acs.analchem.9b01091 |
| [22] |
M. Iranifam, N.R. Hendekhale, H.A.J. Al Lawati, Anal. Methods 10 (2018) 429-438. DOI:10.1039/C7AY02227A |
| [23] |
C.M. Geiselhart, C.W. Schmitt, P. Jockle, H. Mutlu, C. Barner-Kowollik, Sci. Rep. 9 (2019) 14519. DOI:10.1038/s41598-019-51105-z |
| [24] |
R. An, S. Wei, Z. Huang, F. Liu, D. Ye, Anal. Chem. 91 (2019) 13639-13646. DOI:10.1021/acs.analchem.9b02839 |
| [25] |
Z. Shi, G. Li, Y. Hu, Chin. Chem. Lett. 30 (2019) 1600-1606. DOI:10.1016/j.cclet.2019.04.066 |
| [26] |
G. Periyasami, L. Martelo, C. Baleizão, M.N. Berberan-Santos, New J. Chem. 38 (2014) 2258-2261. DOI:10.1039/c4nj00364k |
| [27] |
K.O. Sulaiman, A.T. Onawole, D.T. Shuaib, T.A. Saleh, J. Mol. Liq. 279 (2019) 146-153. DOI:10.1016/j.molliq.2019.01.110 |
| [28] |
S. Rink, A. Duerkop, A. Jacobi von Wangelin, M. Seidel, A.J. Baeumner, Anal. Chim. Acta 1188 (2021) 339161. DOI:10.1016/j.aca.2021.339161 |
| [29] |
M.S. Deshmukh, N. Sekar, Dyes Pigm. 113 (2015) 189-199. DOI:10.1016/j.dyepig.2014.08.009 |
| [30] |
S. Bag, J.C. Tseng, J. Rochford, Org. Biomol. Chem. 13 (2015) 1763-1767. DOI:10.1039/C4OB02413C |
| [31] |
A. Pantelia, I. Daskalaki, M.C. Cuquerella, et al., Molecules 24 (2019) 3957. DOI:10.3390/molecules24213957 |
| [32] |
A. Giussani, P. Farahani, D. Martínez-Muñoz, et al., Chem. Eur. J. 25 (2019) 5202-5213. DOI:10.1002/chem.201805918 |
| [33] |
V.V. Singh, V. Kumar, U. Biswas, et al., Anal. Chem. 93 (2021) 1193-1199. DOI:10.1021/acs.analchem.0c04464 |
| [34] |
V. Sharma, R. Kumar, TrAC-Trends Anal. Chem. 107 (2018) 181-195. DOI:10.1016/j.trac.2018.08.006 |
| [35] |
A. Takamura, T. Ozawa, Analyst 146 (2021) 7431-7449. DOI:10.1039/D1AN01637G |
| [36] |
L. Zhao, F. Chen, W. Huang, et al., Sens. Actuators B: Chem. 304 (2020) 127392. DOI:10.1016/j.snb.2019.127392 |
| [37] |
A.G. Griesbeck, Y. Díaz-Miara, R. Fichtler, et al., Chem. Eur. J. 21 (2015) 9975-9979. DOI:10.1002/chem.201500798 |
| [38] |
R.B. Brundrett, E.H. White, J. Am. Chem. Soc. 96 (1974) 7497-7502. DOI:10.1021/ja00831a018 |
| [39] |
T. Mikroulis, M.C. Cuquerella, A. Giussani, et al., J. Org. Chem. 86 (2021) 11388-11398. DOI:10.1021/acs.joc.1c00890 |
| [40] |
M.P. Richards, Antioxid. Redox Signal. 18 (2013) 2342-2351. DOI:10.1089/ars.2012.4887 |
| [41] |
Y. Cheng, M. Cheng, J. Hao, et al., Chem. Sci. 11 (2020) 8846-8853. DOI:10.1039/D0SC02907F |
| [42] |
T. Deng, H. Bao, W. Huang, et al., Dyes Pigm. 173 (2020) 107915. DOI:10.1016/j.dyepig.2019.107915 |
| [43] |
M.M. Vdovenko, V. Papper, R.S. Marks, I.Y. Sakharov, Anal. Methods 6 (2014) 8654-8659. DOI:10.1039/C4AY01906G |
| [44] |
J. Liu, K.A. Schleyer, T.L. Bryan, et al., Chem. Sci. 12 (2021) 239-246. DOI:10.1039/D0SC04872K |
| [45] |
S. Nsanzamahoro, W.F. Wang, Y. Zhang, et al., Anal. Chem. 93 (2021) 15412-15419. DOI:10.1021/acs.analchem.1c03210 |
| [46] |
C.L. Kuo, W.W. Kallemeijn, L.T. Lelieveld, et al., FEBS J. 286 (2019) 584-600. DOI:10.1111/febs.14744 |
 2023, Vol. 34
2023, Vol. 34 

