Exosomes, which are nanosized phospholipid bilayer extracellular vesicles (30–100 nm), can be secreted by nearly all eukaryotic cells [1]. Notably, tumor exosomes carry many cancerous bioinformation, which not only mediate cell-to-cell communication but also regulate the microenvironment to promote cancer metastasis and tumor growth [2, 3]. Therefore, tumor exosomes have become new and reliable biomarkers for nondestructive early cancer diagnosis [4]. To date, various technologies based on different signal generation mechanisms have been reported for the detection of exosomes. Despite the impressive progress already made, most of these methods still have certain restrictions. Conventional methods, such as nanoparticle tracking analysis (NTA), and atomic force microscopy, have poor selectivity for detecting cancerous exosomes [5-7]. Other approaches, such as flow cytometry, fluorescence, and electrochemical methods, have been explored for sensitive detection of tumor exosomes as well [8-16]. However, these detection techniques unavoidably required expensive and cumbersome instruments, extensive operation processes, and technical steps, which greatly hindered their widespread applications. Hence, it is still technically challenging to explore simple, easy to operate, and portable methods to determine cancer-specific exosomes with high sensitivity and good selectivity.
Nowadays, many portable devices have been developed, such as thermometers, barometers, and personal glucose meters [17-19]. Among them, the thermometers have been widely utilized for environmental monitoring and medical diagnostics owning to their intrinsic merits of low cost, convenient operation, rapid analysis, and excellent portability. Photothermal conversion materials (PCMs) can effectively convert the NIR light into heat when irradiated by a near-infrared (NIR) laser. Thus, PCMs mediated photothermal effects have been widely used in most temperature-based detection strategies [20-27]. Nevertheless, most of the reported photothermal methods were immunoassays, in which the use of antibodies was expensive and unstable. Furthermore, the preparation of some PCMs typically needed tedious procedures and harsh experimental conditions. More importantly, the variation of temperature-induced by a single PCM was limited, thus affecting the detection sensitivity and accuracy. For the sake of addressing these issues, herein, we try to construct a facile and reliable photothermal sensing strategy for the highly sensitive and selective detection of tumor exosomes. To the best of our knowledge, detection of tumor exosomes by using temperature as the output signal is scarcely reported, and some drawbacks in terms of "turn-off" detection mode and relatively low sensitivity also limited their wide applications [28, 29].
Among different PCMs, MnO2 has attracted attention due to its high photothermal conversion performance, high stability, simple fabrication, low cost, and simple to functionalize [30]. To date, nanozymes have gained much attention and numerous nanomaterials have shown outstanding enzyme-like activity [31-36]. Especially, MnO2-based nanoparticles have been reported to exhibit good oxidase-like activity that can catalyze the oxidation of TMB to generate oxidized TMB (oxTMB), which has been proven to have a robust photothermal property [37]. In addition, IR780, as one of the cyanine dyes, has strong absorption in the NIR region and higher photo-conversion efficiency to produce heat upon NIR irradiation, which has been widely explored for photothermal therapy as well [38]. Inspired by the aforementioned works, the system consisting of MnO2, IR780, and TMB is expected to show outstanding photothermal performance, which will be favorable for the development of photothermal sensors with high sensitivity.
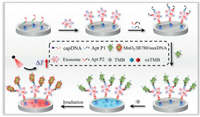
Herein, we reported a facile and portable photothermal aptasensor for directly capturing and quantifying tumor exosomes based on the MnO2/IR780 nanozyme-based synergistic temperature signal amplification system. In portable detection, it is significant to amplify the detection signal to realize the highly sensitive determination of targets. In this work, MnO2/IR780 nanosheets (MnO2/IR780 NSs) were prepared by the simple one-step method, which not only acted as an excellent photothermal conversion nanomaterial for producing great heat signal but also was used as an oxidase enzyme for catalyzing TMB to generate oxTMB with a strong photothermal property, thus conjoining with MnO2/IR780 NSs to enhance the temperature signal to result in increased sensitivity. Moreover, to further improve the detection sensitivity, increasing the amount of MnO2/IR780 NSs modified on exosomes is regarded as another effective method. It has been reported that CD63 and mucin 1 (MUC1) are in overexpressed on numerous cancerous exosomes [39], and thus dual-aptamer recognition is promising to increase the attached amount of MnO2/IR780 NSs. The principle of the multiple amplified photothermal detection of tumor exosomes was shown in Scheme 1, in which MCF-7 cell-derived exosome was used as the model target. In detail, four DNA components are employed in the detection system (Table S1 in Supporting information): biotinylated capture probe (capDNA) that includes CD63 aptamer sequence, aptamer probe 1 (Apt P1) that includes MUC1 aptamer sequence and a linker ssDNA S1, aptamer probe 2 (Apt P2) that includes CD63 aptamer sequence and the linker S1, and an auxiliary DNA (auxDNA) that was partly complementary to the sequences of linker S1. When the MCF-7 cell-derived exosome was efficiently captured by capDNA modified interface, Apt P1 and Apt P2 were simultaneously attached to the surface of exosomes. Then, MnO2/IR780 NSs conjugated with the auxDNA would be adsorbed on the exosomes through the DNA hybridization reaction between S1 and the auxDNA probe. After being further introduced TMB into the detection system, TMB would be oxidized by MnO2/IR780 NSs to form oxTMB. As a result, the temperature noticeably elevated upon NIR laser irradiation. Consequently, highly sensitive detection of tumor exosomes was readily achieved. Additionally, this method can directly detect tumor exosomes in complex culture medium and serum samples, thus holding great promise in nondestructive early cancer diagnosis.
|
Download:
|
| Scheme 1. Schematic diagram of the portable photothermal aptasensor for tumor exosomes detection. | |
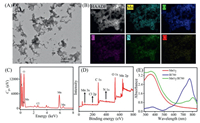
The morphology of MnO2/IR780 NSs was studied by transmission electron microscopy (TEM). As shown in Fig. 1A, the as-prepared MnO2/IR780 displayed an amorphous sheet-like nanostructure with a size of about 100–200 nm. To further explore the elemental distribution of the MnO2/IR780, high-angle annular dark-field-scanning transmission electron microscopy (HAADF-STEM) imaging was carried out (Fig. 1B). The uniform distribution of Mn (blue-green zone), O (blue zone), C (pink zone), N (green zone), and Cl (yellow) elements in MnO2/IR780 were observed in mapping images. The presence of Mn, O, C, N and Cl elements was also observed by the X-ray spectroscopy analysis (Fig. 1C). Additionally, the high-resolution X-ray photoelectron spectroscopy (XPS) was performed. The peaks representing Mn, O, C, N and Cl could be observed in Fig. 1D and Fig. S1 (Supporting information). Moreover, the absorption of MnO2/IR780 NSs at 380 nm and 800 nm were attributed to the MnO2 and IR780 absorbance, respectively (Fig. 1E). It could be observed that the absorption of IR780 in MnO2/IR780 NSs was shifted, which possibly resulted from the aggregates of IR780 in MnO2/IR780 NSs. Additionally, MnO2, IR780, and MnO2/IR780 NSs presented different colors (Fig. S2 in Supporting information). Taken together, all these results confirmed the successful preparation of MnO2/IR780.
|
Download:
|
| Fig. 1. (A) TEM image of MnO2/IR780 NSs. (B) HAADF-STEM image and corresponding elemental mapping of Mn, O, C, N and Cl elements for the MnO2/IR780. EDX analysis (C) and XPS spectrum (D) of MnO2/IR780. (E) UV–vis absorption spectra of MnO2, IR780, and MnO2/IR780. | |
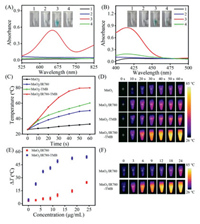
Next, the oxidase-like property of the as-prepared MnO2/IR780 NSs was explored. As shown in Fig. 2A, no obvious absorption at 652 nm was observed for free TMB or MnO2/IR780 NSs and both of them were colorless. However, when MnO2/IR780 NSs were added to the TMB solution, a distinct blue color with a maximum absorbance at 652 nm was observed, which is the typical characteristic of oxTMB. The absorbance at 652 nm dropped greatly and no noticeable color change occurred after adding MnO2/IR780 NSs into the N2-purged TMB solution, implying that dissolved oxygen played an important role in the oxidation reaction of TMB catalyzed by MnO2/IR780 NSs. Additionally, another model substrate, ABTS displayed obvious absorption at 417 nm after the addition of MnO2/IR780 NSs (Fig. 2B). Meanwhile, the oxidation products of TMB and ABTS showed obvious color changes. MnO2/IR780 NSs showed > 85% of the relative activity in a wide pH range from 3.0 to 7.0 (Fig. S3 in Supporting information). Furthermore, we can find that the absorption of TMB at 652 nm progressively enhanced with the increasing concentration of MnO2/IR780 NSs (Fig. S4 in Supporting information), suggesting the catalytic oxidation was concentration-dependent. It is worth noting that the absorbance rapidly elevated and tended to be stable after 30 s, demonstrating the oxidation reaction was very fast and MnO2/IR780 NSs was a good oxidase-like enzyme. Remarkably, oxTMB also had a strong absorption at 808 nm and the absorbance at 808 nm of MnO2/IR780-TMB system exhibited a good linear relationship with the concentration of MnO2/IR780 (Fig. S5 in Supporting information), confirming that the MnO2/IR780-TMB system was very suitable for NIR-driven photothermal conversion.
|
Download:
|
| Fig. 2. UV-vis spectra and photographs of TMB (A) and ABTS (B) catalyzed oxidation by MnO2/IR780 NSs (7.5 µg/mL), respectively. 1 TMB or ABTS, 2 MnO2/IR780, 3 MnO2/IR780 + TMB or ABTS, 4 MnO2/IR780 + N2 + TMB or ABTS. Temperature change curves (C) and real-time infrared thermal images (D) of MnO2, MnO2-TMB, MnO2/IR780, and MnO2/IR780-TMB solution upon laser irradiation. Temperature increase changes (E) and real-time infrared thermal images (F) of MnO2/IR780 and MnO2/IR780-TMB at different concentrations under laser irradiation for 60 s. | |
The photothermal effect of MnO2 NSs was examined and the results indicated that MnO2 NSs had a good photothermal effect (Figs. S6–S8 in Supporting information). Next, the photothermal effect of MnO2/IR780 NSs was evaluated. As shown in Fig. S9 (Supporting information), the temperature of MnO2/IR780 sharply elevated after irradiation 60 s, which was significantly higher than that of MnO2. The results validated that the MnO2/IR780 NSs exhibited a stronger photothermal effect than that of MnO2 NSs and MnO2 NSs + free IR780 (Fig. S10 in Supporting information), which was probable that the absorption of IR780 in MnO2/IR780 NSs was red-shifted. Particularly, the photostability of IR780 in MnO2/IR780 NSs was higher than that of IR780 alone (Fig. S11 in Supporting information). In addition, the temperature raised quickly as the concentration of MnO2/IR780 NSs increased (Fig. S12 in Supporting information). The temperature of MnO2/IR780 NSs increased progressively with the increasing of laser energy as well (Fig. S13 in Supporting information). Fig. S14 (Supporting information) showed that the photothermal stability of MnO2/IR780 NSs was favorable. Taken together, all these results demonstrated that MnO2/IR780 NSs have excellent photothermal properties.
Encouraged by the aforementioned satisfactory outcomes, we intended to further explore the temperature changes of the MnO2/IR780-mediated TMB oxidation reaction solutions upon laser irradiation. As shown in Figs. 2C and D, a noticeable temperature rise was detected for MnO2/IR780-TMB system. Obviously, the temperature increase of MnO2/IR780-TMB system was much higher than that of the MnO2-TMB solution under the same conditions. Moreover, Figs. 2E and F displayed that the temperature variations of the MnO2/IR780-TMB solution were significantly higher than those of MnO2/IR780 alone. Also, the temperature of MnO2/IR780-TMB solution progressively elevated as the concentration of MnO2/IR780 increased (Fig. S15 in Supporting information). Thus, all these results clearly demonstrated that MnO2/IR780-TMB system was very beneficial to establishing biosensors for the quantitative determination of analytes with high sensitivity.
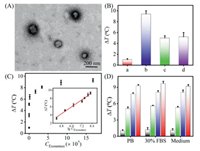
The isolated MCF-7 cells-derived exosomes were characterized by TEM and NTA. In Fig. 3A, it could be seen that the collected exosomes are spherical vesicles with diameters ranging from 90 nm to 120 nm, which matched well with the morphology previously reported. The size distribution of NTA characterization was consistent with that observed in TEM (Fig. S16 in Supporting information). Next, the feasibility of the as-proposed strategy was investigated. It could be clearly found that the dual aptamer recognition strategy was noticeably better than that of only one aptamer recognition (Fig. 3B and Fig. S17 in Supporting information), which could importantly improve the detection sensitivity.
|
Download:
|
| Fig. 3. (A) TEM characterization of the MCF-7 cell-derived exosomes. (B) The temperature elevation of the sensing method under different conditions. (a) No aptamer, (b) Apt P1 + Apt P2, (c) Apt P1, and (d) Apt P2. (C) Relationship between the values of ΔT and different exosome concentrations. Inset shows the linear correlation between the ΔT value and the logarithmic of the exosome concentration. (D) ΔT value of the photothermal sensor in response to different amounts of MCF-7 cell-derived exosomes under different conditions. Blank bars, green bars, blue bars, and red bars stand for 0, 1.68 × 105, 3.36 × 107, and 1.68 × 108 particles/mL, respectively. | |
To achieve the best performance, various important experimental parameters were systematically optimized, including capDNA concentration, incubation time of exosomes, and MnO2/IR780/auxDNA (Figs. S18–S20 in Supporting information). Under the optimum conditions, the performance of the as-proposed photothermal sensing methods for the detection of exosomes was studied. As demonstrated in Fig. 3C and Fig. S21 (Supporting information), ΔT continually enhanced with the increasing concentration of exosomes. Noteworthily, the photothermal aptasensor displayed an excellent linear relationship between ΔT and the logarithm of exosome concentration varying from 1.68 × 104 particles/mL to 1.68 × 108 particles/mL. The detection limit was determined to be 5.1 × 103 particles/mL according to the standard 3σ rule, which was comparable or superior to those of the most previous strategies for exosome determination (Table S2 in Supporting information). The likely explanation for such low detection limit includes four main reasons: the increased amount of MnO2/IR780 NSs modified on target exosomes by dual aptamer recognition, the excellent photothermal performance of MnO2/IR780 NSs, the outstanding oxidase-like activity of MnO2/IR780 NSs for catalyzed oxidation of TMB, and the superior photothermal property of oxTMB. More significantly, compared to those previously developed exosome assays, the method we established here presented the excellent merits of portability, robust practicality, and easy operation.
Furthermore, the selectivity of the photothermal sensor was studied by detecting other exosomes secreted from HL-7702 cells. Absolutely, a much higher ΔT was detected for MCF-7 cells than that of the HL-7702 cells with the same exosome concentration (Fig. S22 in Supporting information), implying the excellent specificity for tumor exosome detection. Thus, these results well confirmed that the as-developed photothermal sensor was able to differentiate exosomes derived by various cell lines. Moreover, the applicability of the photothermal biosensor was explored by analyzing tumor exosomes in complex biological matrix. After adding MCF-7 cell-derived exosomes with different concentrations into the culture medium and 30% diluted exosome-free fetal bovine serum (FBS), the temperature changes of the sensor were recorded respectively. As depicted in Fig. 3D, the observed ΔT in culture medium and 30% diluted FBS were roughly the same as those of in PB buffer solution with the same number of exosomes. Overall, all of these results validated that the as-developed photothermal biosensor holds great promise the application in complex biological environments.
In summary, we have reported a facile portable photothermal aptasensor for directly capturing and quantifying tumor exosomes on the basis of MnO2/IR780 nanozyme. Through the dual aptamer recognition strategy and MnO2/IR780-TMB multiple amplified system, the as-proposed sensor displayed high sensitivity and selectivity for the detection of tumor exosomes. A detection limit for tumor-derived exosomes as low as 5.1 × 103 particles/mL was obtained, which was comparable or superior to most other approaches. Furthermore, this assay exhibited outstanding selectivity to discriminate the cancer cell-derived exosomes from normal cell-derived exosomes and could efficiently analyze the target exosomes spiked in biological samples. More significantly, compared to those previously established exosome assays, our method showed the excellent merits of portability, robust practicality, and easy operation. Overall, the as-developed photothermal aptasensor holds great potential in exosome-related clinical diagnostics and biochemical study.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 22174083 and 22076090), Shandong Provincial Natural Science Foundation (No. ZR2020ZD37), Special Foundation for Taishan Scholar of Shandong Province (No. TSQN202103093), Shandong Province Higher Educational Program for Young Innovation Talents, and the Research Foundation for Distinguished Scholars of Qingdao Agricultural University (No. 6651119006). Technical support was provided by Central Laboratory of Qingdao Agricultural University.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.06.030.
| [1] |
G. Ailuno, S. Baldassari, F. Lai, T. Florio, G. Caviglioli, Cells 9 (2020) 2569. DOI:10.3390/cells9122569 |
| [2] |
Y.H. Soung, S. Ford, V. Zhang, J. Chung, Cancers 9 (2017) 8 (Basel).
|
| [3] |
X. Li, Y. Wang, Q. Wang, et al., Cancer Lett. 435 (2018) 55-65. DOI:10.1016/j.canlet.2018.07.037 |
| [4] |
J. Wang, Y. Liu, W. Sun, et al., Cancer Biomark. 21 (2018) 805-812. DOI:10.3233/CBM-170738 |
| [5] |
V. Sokolova, A.K. Ludwig, S. Hornung, et al., Colloid Surf. B 87 (2011) 146-150. DOI:10.1016/j.colsurfb.2011.05.013 |
| [6] |
S. Sharma, M. LeClaire, J.K. Gimzewski, Nanotechnology 29 (2018) 132001. DOI:10.1088/1361-6528/aaab06 |
| [7] |
T.S. Lyu, Y. Ahn, Y.J. Im, et al., PLoS One 16 (2021) e0231994. DOI:10.1371/journal.pone.0231994 |
| [8] |
B.A. Brown, X. Zeng, A.R. Todd, et al., Anal. Chem. 92 (2020) 3285-3292. DOI:10.1021/acs.analchem.9b05173 |
| [9] |
G. Li, N. Zhu, J. Zhou, et al., J. Mater. Chem. B 9 (2021) 2709-2716. DOI:10.1039/D0TB02894K |
| [10] |
Q.M. Feng, P. Ma, Q.H. Cao, Y.H. Guo, J.J. Xu, Chem. Commun. 56 (2020) 269-272. DOI:10.1039/C9CC08051A |
| [11] |
F. He, J. Wang, B.C. Yin, B.C. Ye, Anal. Chem. 90 (2018) 8072-8079. DOI:10.1021/acs.analchem.8b01187 |
| [12] |
X. Yin, T. Hou, B. Huang, L. Yang, F. Li, Chem. Commun. 55 (2019) 13705-13708. DOI:10.1039/C9CC07253E |
| [13] |
K. Jiang, Y. Wu, J. Chen, et al., Chin. Chem. Lett. 32 (2021) 1827-1830. DOI:10.1016/j.cclet.2020.11.031 |
| [14] |
P. Miao, Y. Tang, Chem. Commun. 56 (2020) 4982-4985. DOI:10.1039/D0CC01817A |
| [15] |
H. Xu, B.C. Ye, TrAC Trend Anal. Chem. 123 (2020) 115773. DOI:10.1016/j.trac.2019.115773 |
| [16] |
S. Hu, X. Fang, G. Liu, et al., Analyst 147 (2022) 48-54. DOI:10.1039/D1AN01825F |
| [17] |
S. Shrivastava, T.Q. Trung, N.E. Lee, Chem. Soc. Rev. 49 (2020) 1812-1866. DOI:10.1039/C9CS00319C |
| [18] |
X. Gao, X. Li, X. Sun, et al., Anal. Chem. 92 (2020) 4592-4599. DOI:10.1021/acs.analchem.0c00018 |
| [19] |
D. Kwon, J. Joo, S. Lee, S. Jeon, Anal. Chem. 85 (2013) 12134-12137. DOI:10.1021/ac403329w |
| [20] |
W. Zhou, J. Sun, X. Li, Anal. Chem. 92 (2020) 14830-14837. DOI:10.1021/acs.analchem.0c03700 |
| [21] |
X. Li, L. Yang, C. Men, et al., Anal. Chem. 91 (2019) 4444-4450. DOI:10.1021/acs.analchem.8b05031 |
| [22] |
X. Liu, L. Zou, X. Yang, et al., Anal. Chem. 91 (2019) 7943-7949. DOI:10.1021/acs.analchem.9b01883 |
| [23] |
L. Pogačnik, M. Franko, Biosens. Bioelectron. 18 (2003) 1-9. DOI:10.1016/S0956-5663(02)00056-8 |
| [24] |
Y. Tao, F. Luo, L. Guo, B. Qiu, Z. Lin, Anal. Chim. Acta 1110 (2020) 151-157. DOI:10.1016/j.aca.2020.03.023 |
| [25] |
T. Dai, Y. Wan, R. Tian, et al., ACS Appl. Bio Mater. 3 (2020) 3260-3267. DOI:10.1021/acsabm.0c00232 |
| [26] |
R. Fu, J. Zhou, Y. Liu, et al., Food Chem. 351 (2021) 129292. DOI:10.1016/j.foodchem.2021.129292 |
| [27] |
S. Hu, L. Tong, J. Wang, X. Yi, J. Liu, Anal. Chem. 91 (2019) 15418-15424. DOI:10.1021/acs.analchem.9b02871 |
| [28] |
N. Cheng, Y. Song, Q. Shi, et al., Anal. Chem. 91 (2019) 13986-13993. DOI:10.1021/acs.analchem.9b03562 |
| [29] |
Y. Zhang, Y. Wei, P. Liu, et al., Anal. Chem. 93 (2021) 11540-11546. DOI:10.1021/acs.analchem.1c02004 |
| [30] |
L. Wang, S. Guan, Y. Weng, et al., ACS Appl. Mater. Interfaces 11 (2019) 6267-6275. DOI:10.1021/acsami.8b20639 |
| [31] |
J. Wu, Z. Wang, X. Jin, et al., Adv. Mater. 33 (2021) 2005024. DOI:10.1002/adma.202005024 |
| [32] |
Y. Chen, L. Jiao, H. Yan, et al., Anal. Chem. 92 (2020) 13518-13524. DOI:10.1021/acs.analchem.0c02982 |
| [33] |
Q. Liang, J. Xi, X.J. Gao, et al., Nano Today 35 (2020) 100935. DOI:10.1016/j.nantod.2020.100935 |
| [34] |
Y. Zhu, J. Wu, L. Han, et al., Anal. Chem. 92 (2020) 7444-7452. DOI:10.1021/acs.analchem.9b05110 |
| [35] |
Y. Huang, J. Ren, X. Qu, Chem. Rev. 119 (2019) 4357-4412. DOI:10.1021/acs.chemrev.8b00672 |
| [36] |
Z. Wei, Y. Yu, S. Hu, X. Yi, J. Wang, ACS Appl. Mater. Interfaces 13 (2021) 16801-16811. DOI:10.1021/acsami.0c21109 |
| [37] |
G. Fu, S.T. Sanjay, W. Zhou, et al., Anal. Chem. 90 (2018) 5930-5937. DOI:10.1021/acs.analchem.8b00842 |
| [38] |
P. Bhattarai, Z. Dai, Adv. Healthc. Mater. 6 (2017) 1700262. DOI:10.1002/adhm.201700262 |
| [39] |
Y. Lyu, D. Cui, J. Huang, et al., Angew. Chem. Int. Ed. 58 (2019) 4983-4987. DOI:10.1002/anie.201900092 |
 2023, Vol. 34
2023, Vol. 34 

