b School of Public Health, Wuhan University, Wuhan 430071, China
RNA molecules contain plenty of chemical modifications that play important roles in RNA metabolism [1]. Over 150 naturally occurring RNA modifications have been identified to be present in various RNA species, including ribosomal RNAs (rRNAs), transfer RNA (tRNA), messenger RNA (mRNA), and microRNA (miRNA) [1-4]. The modifications in RNA greatly expand the diversity of RNA molecules. These RNA modifications have been revealed to modulate the secondary structure, folding, stability and degradation of RNA molecules [5]. Many of these modifications in RNA are dynamically added and removed by writer and eraser proteins, which has been considered as a new layer of RNA modification-mediated regulation of a wide range of biological processes [6].
Ribosomes are composed of small subunit rRNA (SSU rRNA), large subunit rRNA (LSU rRNA), and ribosomal proteins [7]. rRNAs provide the structural framework of ribosomes and play critical roles in protein translation [7]. In ribosome biogenesis, rRNAs acquire various chemical modifications in their transcription and subsequent maturation process [8]. The changes in the rRNA modifications can influence the structure as well as catalytic activity of ribosomes and therefore alter the efficiency and accuracy of translation, which confers an additional regulatory layer in gene expression [8]. It has been reported that mammal cytosolic rRNAs contain more than 20 types of modifications [9], while Escherichia coli (E. coli) rRNAs contain 17 kinds of modifications (Table S1 in Supporting information) [10].
Despite the high diversity of the RNA modifications observed in nature, only a limited set of chemical modifications have been characterized in plant rRNAs. In plants, it has been reported that 10 kinds of modifications are present in the plant SSU of ribosomes (18S rRNA), including N6-methyladenosine (m6A), 5-methylcytidine (m5C), N7-methylguanosine (m7G), N1-methyladenosine (m1A), pseudouridine (ѱ), 2′-O-methyladenosine (Am), 2′-O-methylcytidine (Cm), 2′-O-methylguanosine (Gm), 2′-O-methyluridine (Um), and 1-methylguanosine (m1G) [11-15]. As for the plant LSU of ribosomes (25S rRNA), 9 kinds of modifications have been identified, i.e., Am, Cm, Gm, Um, ѱ, m6A, m5C, m1A and m1G (Table S1) [11-16]. These modifications are involved in various plant physiology processes, including plant development and stress response [17]. However, so far, there are still lacks of systematic and comprehensive profiling of modifications in plant 18S rRNA and 25S rRNA, which is partially due to the challenge in obtaining highly pure rRNAs.
The study of RNA modifications has made huge progresses in the past decades due to the technological advancements [18-20]. For instance, the approaches of two-dimensional thin layer chromatography [21], dot blots with antibodies specific to given modification [22], capillary electrophoresis or liquid chromatography-mass spectrometry, and chemical labeling-mass spectrometry have been used to determine modifications in RNA [23-30]. In addition, some methods, such as chemical labeling/treatment, immunoprecipitation, and enzymatic digestion in combination of high-throughput sequencing, also have been developed to map modifications in RNA [31-33]. These methods greatly promote the functional investigation of RNA modifications.
In this study, we aimed to systemically profile and characterize modifications in plant rRNAs. We initially chose the model plant of Arabidopsis thaliana (A. thaliana) for the investigation. In the RNA pool of eukaryotic cells, although mRNA only accounts for a small fraction (< 5%) [34], it may still affect the purity of isolated A. thaliana rRNAs. In addition, it is worth noting that the A. thaliana tissues might contain trace level of bacterial contamination. In this regard, obtaining highly pure rRNA is critical to determinate new modifications in A. thaliana rRNAs since contamination from other RNA species or from bacteria may cause the false positive identification. Here we developed a method to purify rRNAs by successive isolation with different strategies, including (1) polyA-based mRNA depletion and (2) agarose gel electrophoresis-based purification (Fig. 1A). Generally, after the depletion of the A. thaliana mRNA from total RNA, agarose gel electrophoresis was further employed to separate and purify the 18S rRNA and 25S rRNA of A. thaliana, which could remove the potential contamination of E. coli rRNAs from the isolated A. thaliana rRNAs.
|
Download:
|
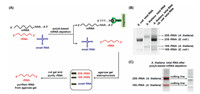
| Fig. 1. The developed method for the purification of plant 18S rRNA and 25S rRNA. (A) Schematic illustration of the proposed method by a successive isolation with different strategies, including polyA-based mRNA depletion and agarose gel electrophoresis-based purification. PMP, paramagnetic particles. (B) Separation of RNA from A. thaliana and from E. coli by agarose gel electrophoresis. (C) Purification of the 18S rRNA and 25S rRNA of A. thaliana. | |
With the proposed purification method, we then isolated the 18S rRNA and 25S rRNA of A. thaliana by agarose gel electrophoresis after polyA-based mRNA depletion. The results showed that A. thaliana 18S and 25S rRNA and E. coli 16S and 23S rRNA could be well separated in 1.2% agarose gel (Fig. 1B). The bands of the 18S rRNA and 25S rRNA of A. thaliana were then cut and purified (Fig. 1C). We utilized qPCR to evaluate the purity of isolated 18S rRNA and 25S rRNA (potentially presence of E. coli 16S rRNA and 23S rRNA). To this end, the PCR amplification efficiencies of E. coli 16S rRNA and 23S rRNA were firstly examined (Table S2 in Supporting information). The results demonstrated that 92.0% and 105.6% amplification efficiencies toward E. coli 16S rRNA and 23S rRNA were achieved (Fig. S1A and B in Supporting information), suggesting the real-time qPCR was capable of the quantitative evaluation of the purity of isolated rRNAs. The qPCR results showed that the purity of the isolated 18S rRNA and 25S rRNA of A. thaliana were 99.70% and 99.96%, respectively (Figs. S1C–F), indicating highly pure plant 18S rRNA and 25S rRNA.
With the highly pure A. thaliana rRNAs, we next profiled the modifications in A. thaliana 18S rRNA and 25S rRNA. In this respect, a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method for the simultaneous detection of totally 57 modified nucleosides and 4 canonical nucleosides were established (Table S3 in Supporting information). Among these 61 nucleosides, there are 11 groups of isomers or nucleosides with similar molecular weights (m1A, m2A, m6A, m8A and m1I; io6A (cis) and io6A (trans); m1G, m2G, m6G and m7G; m3C, m5C, m3U and m5U; f5C and m4,4C; s2U, s4U, s2C and ho5U; hm5U, mo5U and m5s2U; Cm and Um; ca5C and ac4C; m5Um, m5Cm and m4Cm; f5Cm and m4,4Cm) that have the same multiple reaction monitoring (MRM) mass transition. However, under the optimized LC and MS conditions, all the 61 nucleosides can be well differentiated and determined (Fig. S2 and Table S4 in Supporting information), which provided the essential platform for profiling of modifications in rRNAs.
The extracted 18S rRNA and 25S rRNA with high purity from leaves of A. thaliana were digested into nucleosides and analyzed by LC-ESI-MS/MS. The confirmation of detected RNA modifications was based on the consistence of retention times and MS2 spectra between the detected modifications and nucleoside standards. With these criteria, 10 kinds of modifications (Am, Cm, Gm, Um, ѱ, m6A, m5C, m7G, m2,2G, and m6,6A) were detected from 2 µg of 18S rRNA by comparing to the 57 nucleoside standards (Figs. 2A and B and Fig. S3 in Supporting information). Similarly, 12 kinds of modifications were detected from 2 µg of 25S rRNA, including Am, Cm, Gm, Um, ѱ, m6A, m5C, m1A, m2,2G, m6,6A, m7G, and m3U (Figs. 2C–F and Fig. S4 in Supporting information). In addition, we separately added the standards of aforementioned modifications to the digested nucleosides from 18S rRNA and 25S rRNA of A. thaliana. The spiked nucleoside standards showed the same retention times as those detected in A. thaliana (Fig. 2, Figs. S3 and S4). We also checked these modifications in water or the sample with only enzymes and omitting rRNA. It can be seen that they were all undetectable in these controls, which excluded the possibility that the detected modifications were from the contamination of digestion enzymes (Fig. 2, Figs. S3 and S4).

|
Download:
|
| Fig. 2. Determination of modifications in 18S rRNA and 25S rRNA of A. thaliana. (A, B) The extracted-ion chromatograms of detected new modifications of m2,2G and m6,6A from 2 µg of 18S rRNA, 18S rRNA with spiked standards, H2O blank, enzyme blank, 6 ng of E. coli 16S rRNA, and the nucleoside standards. (C-F) The extracted-ion chromatograms of modifications of m2,2G, m6,6A, m7G, and m3U from 2 µg of 25S rRNA, 25S rRNA with spiked standards, H2O blank, enzyme blank, 0.8 ng of E. coli 23S rRNA, and the nucleoside standards. | |
It is worth noting that, among the 10 kinds of modifications (Am, Cm, Gm, Um, ѱ, m6A, m5C, m7G, m2,2G and m6,6A) detected in plant 18S rRNA, 2 kinds of modifications of m2,2G and m6,6A are the newly discovered modifications that have not been reported in previous studies (Table S5 in Supporting information). As for the 25S rRNA, 4 kinds of modifications (m7G, m3U, m2,2G and m6,6A) are the newly identified ones besides the other 8 kinds of modifications (Am, Cm, Gm, Um, ѱ, m6A, m5C and m1A) that have been previously reported in 25S rRNA of plants (Table S6 in Supporting information). We next carefully evaluated the effect of the potential contamination of E. coli rRNAs in the determination of new modifications in 18S and 25S rRNA of A. thaliana. The qPCR analysis showed that the potential contamination of E. coli 16S rRNA was less than 0.3%, i.e. less than 6 ng of E. coli 16S rRNA in 2 µg of A. thaliana 18S rRNA (2 µg × 0.3% = 6 ng). We analyzed the modifications from 6 ng of E. coli 16S rRNA. The results suggested that no signal was observed from 6 ng of E. coli 16S rRNA (Figs. 2A and B and Fig. S3), indicating that the detected modifications indeed came from 18S rRNA, but not from the potential trace level contamination of E. coli 16S rRNA. As for the 25S rRNA of A. thaliana, the potential contamination of E. coli 23S rRNA was less than 0.04%. Again, no signal was observed from 0.8 ng of E. coli 23S rRNA (2 µg × 0.04% = 0.8 ng) (Figs. 2C–F and Fig. S4). These results demonstrated that the purity of the isolated rRNA could meet the requirement for the identifications of new modifications in both 18S rRNA and 25S rRNA of A. thaliana.
We further employed the high-resolution MS analysis to validate the new modifications detected in 18S rRNA and 25S rRNA of A. thaliana. The retention times of new modifications detected in the rRNAs of A. thaliana were similar to the nucleoside standards (Fig. 3). Moreover, the parent ions and product ions (m/z shown in blue) of the detected new modifications in the 18S rRNA or 25S rRNA of A. thaliana were identical to their corresponding theoretical values (m/z shown in red) as well as to those of the nucleoside standards (Fig. 3), further confirming these detected new modifications. Taken together, with the isolated highly pure rRNAs, we identified 2 kinds of new modifications of m2,2G and m6,6A in 18S rRNA, and 4 kinds of new modification of m2,2G, m6,6A, m7G and m3U in 25S rRNA of A. thaliana.

|
Download:
|
| Fig. 3. Identification of new modifications in 18S rRNA and 25S rRNA of A. thaliana by high-resolution mass spectrometry. (A, B) The extracted-ion chromatograms and product ion spectra from the m2,2G and m6,6A standards (above) and 18S rRNA of A. thaliana (below). (C–F) The extracted-ion chromatograms and product ion spectra from the m2,2G, m6,6A, m7G, and m3U standards (above) and 25S rRNA of A. thaliana (below). | |
We next quantitatively measured the detected modifications (Am, Cm, Gm, Um, ѱ, m6A, m5C, m7G, m2,2G, m6,6A, m1A and m3U) in A. thaliana 18S rRNA and 25S rRNA. Various amounts of nucleoside standards and fixed amounts of isotope internal standard (rC-13C5) were mixed to construct the calibration curves by plotting the peak area ratios (nucleosides/rC-13C5) against the amounts of nucleosides. The results indicated that good linearities were achieved and the coefficients of determination (R2) were higher than 0.99 (Table S7 in Supporting information). The limits of detection (LODs) of the detected modifications ranged from 0.1 fmol to 318.5 fmol (Table S7). It can be seen that two uridine modifications (Um and ѱ) have higher LODs than other modifications, which is due to the low ionization efficiencies of uridine modifications in LC-ESI-MS/MS analysis. The relative errors (REs) and intra and inter-day relative standard deviations (RSDs) were calculated to evaluate the accuracy and precision of the method. The results showed the REs and RSDs were less than 11.0% and 13.5%, respectively (Table S8 in Supporting information), demonstrating good accuracy and precision of the developed method.
With the validated LC-ESI-MS/MS method, we quantified the levels of these detected modifications in 18S rRNA and 25S rRNA of different A. thaliana tissues, including leaves, stems and flowers (Fig. S5A in Supporting information). As the aforementioned results, Am, Cm, Gm, Um, ѱ, m6A, m5C, m7G, m2,2G and m6,6A were detected in 18S rRNA of A. thaliana leaves, stems and flowers; Am, Cm, Gm, Um, ѱ, m6A, m5C, m7G, m2,2G, m6,6A, m1A and m3U were detected in the 25S rRNA of A. thaliana leaves, stems and flowers. As for the newly identified modifications in 18S rRNA of A. thaliana leaves, stems and flowers, the measured contents of m2,2G and m6,6A ranged from 0.0040% to 0.0069% (m2,2G/G) and from 0.1922% to 0.2126% (m6,6A/A), respectively (Figs. S5B and C). As for the newly identified modifications in 25S rRNA, the quantification results of these modifications of m2,2G (0.0037%−0.0050%, m2,2G/G), m6,6A (0.0199%−0.0347%, m6,6A/A), m7G (0.0031%−0.0056%, m7G/G), and m3U (0.04792%−0.1253%, m3U/U), were all obtained from different tissues of A. thaliana (Figs. S5D–G). We also measured the contents of other modifications that were previously reported present in A. thaliana (Fig. S6 in Supporting information). The quantification results showed that the measured levels of these modifications were comparable to the previous studies (Tables S5 and S6).
We next investigated whether these newly detected modifications are prevalent in rRNAs of plants. In this respect, we examined these modifications in rRNAs of different plants, including rice and perennial ryegrass. Similarly to the A. thaliana sample, the results showed that 10 modifications, including m2,2G and m6,6A, were detected in 18S rRNA of rice and perennial ryegrass (Figs. S7 and S8 in Supporting information); while 12 modifications, including m7G, m3U, m2,2G and m6,6A, were detected in 25S rRNA of rice and perennial ryegrass (Figs. S9 and S10 in Supporting information). Quantification of these modifications in 18S rRNA and 25S rRNA of rice and perennial ryegrass are shown in Fig. S11 (Supporting information). It can be seen that the levels of these new modifications were comparable in different plant species (Figs. S5 and S11). Collectively, the results suggested that these newly identified modifications are prevalent in rRNAs of different plant species.
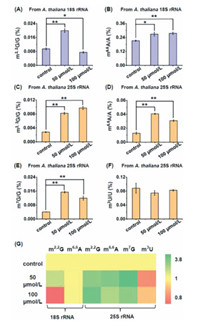
Previous studies demonstrated RNA methylation could be directly or indirectly influenced by various environmental stresses [35, 36]. Cd is a widely distributed carcinogen in soil and over-accumulation of Cd in plant cells could affect various physiological processes of plants [37, 38]. Here we evaluated the effect of Cd stress on RNA modifications, including the newly identified ones, in 18S rRNA and 25S rRNA of A. thaliana. We treated A. thaliana with various concentrations of Cd (0, 50 and 100 µmol/L) for 7 days and then harvested the seedlings of A. thaliana for rRNA isolation and purification according to aforementioned rRNA isolation method. We found that treatment of A. thaliana with 50 µmol/L and 100 µmol/L of Cd could lead to significant increase in the levels of m6,6A in 18S rRNA, and m2,2G, m6,6A, and m7G in 25S rRNA (Fig. 4). Treatment of A. thaliana with 50 µmol/L Cd could lead to significant increase in the level of m2,2G in 18S rRNA, but 100 µmol/L Cd treatment would lead to the decreased level of m2,2 G in 18S rRNA (Fig. 4A). The content of m3U in A. thaliana 25S rRNA showed unobvious change upon Cd treatment (Fig. 4F). Similarly, the levels of other modifications in A. thaliana rRNAs were also affected by Cd stress, with majority of these modifications showing increased levels upon Cd stress (Fig. 4G and Fig. S12 in Supporting information). We speculated that Cd treatment would cause the changed activity or expression of enzymes responsible for the formation and removal of modifications, which eventually leads to the altered levels of modifications in plant rRNA. The underlying mechanisms of the altered levels of rRNA modifications upon Cd stress are required further investigation in future study. Nevertheless, these results indicated that RNA modifications in A. thaliana rRNAs play physiological roles in response to Cd stress.
|
Download:
|
| Fig. 4. Effect of Cd stress on the levels of modifications in A. thaliana 18S rRNA and 25S rRNA. (A, B) Levels of m2,2G and m6,6A in 18S rRNA of A. thaliana upon Cd stress with different concentrations. (C–F) Levels of m2,2G, m6,6A, m7G and m3U in 25S rRNA of A. thaliana upon Cd stress with different concentrations. * P < 0.05; ** P < 0.01. Error bars represent standard deviation. (n = 3). (G) Heatmap showing the contents of modifications in 18S rRNA and 25S rRNA of A. thaliana upon Cd stress with different concentrations. | |
In summary, we developed an rRNA isolation strategy through polyA-based mRNA depletion and agarose gel electrophoresis-based purification. Gratifyingly, this purification method is amenable for obtaining highly pure plant 18S rRNA and 25S rRNA, which enables the precise profiling and identification of modifications in plant rRNAs. Profiling and characterization of modifications by LC-ESI-MS/MS analysis showed that 10 and 12 kinds of modifications were present in the 18S rRNA and 25S rRNA of plants, respectively. Significantly, 2 kinds of modifications of m2,2G and m6,6A in 18S rRNA, and 4 kinds of modifications of m2,2G, m6,6A, m3U and m7G in 25S rRNA, were firstly discovered to be widely present in plants. In addition, we observed that Cd exposure led to significant changes of the levels of modification in both 18S rRNA and 25S rRNA of A. thaliana, indicating that the modifications in plant rRNAs should play critical roles in response to environmental stress. Future study on identifying the methyltransferases and demethylases of these newly identified modifications in plant rRNAs may benefit the understanding of the functions of these new modifications in plant rRNAs.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgmentsThe work is supported by the Fundamental Research Funds for the Central Universities (No. 2042021kf0212) and the National Natural Science Foundation of China (Nos. 22074110 and 21721005). We thank Prof. Zhulong Chan (College of Horticulture and Forestry Sciences, Huazhong Agricultural University, China) for providing perennial ryegrass leaves.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.05.045.
| [1] |
P. Boccaletto, F. Stefaniak, A. Ray, et al., Nucleic Acids Res. 50 (2020) D231-D235. DOI:10.1093/nar/gkab1083 |
| [2] |
Y. Dai, C.B. Qi, Y. Feng, et al., Anal. Chem. 93 (2021) 6938-6946. DOI:10.1021/acs.analchem.0c04630 |
| [3] |
M.Y. Cheng, X.J. You, J.H. Ding, et al., Chem. Sci. 12 (2021) 8149-8156. DOI:10.1039/D1SC01972D |
| [4] |
M.Y. Chen, C.B. Qi, X.M. Tang, et al., Chin. Chem. Lett. 33 (2022) 3772-3776. DOI:10.1016/j.cclet.2021.12.008 |
| [5] |
M. Frye, B.T. Harada, M. Behm, C. He, Science 361 (2018) 1346-1349. DOI:10.1126/science.aau1646 |
| [6] |
I. Barbieri, T. Kouzarides, Nat. Rev. Cancer 20 (2020) 303-322. DOI:10.1038/s41568-020-0253-2 |
| [7] |
J.C. Bowman, A.S. Petrov, M. Frenkel-Pinter, P.I. Penev, L.D. Williams, Chem. Rev. 120 (2020) 4848-4878. DOI:10.1021/acs.chemrev.9b00742 |
| [8] |
J. Jiang, H. Seo, C.S. Chow, Acc. Chem. Res. 49 (2016) 893-901. DOI:10.1021/acs.accounts.6b00014 |
| [9] |
S.K. Natchiar, A.G. Myasnikov, H. Kratzat, I. Hazemann, B.P. Klaholz, Nature 551 (2017) 472-477. DOI:10.1038/nature24482 |
| [10] |
O. Sergeeva, A. Bogdanov, P. Sergiev, Biochimie 117 (2015) 110-118. DOI:10.1016/j.biochi.2014.11.019 |
| [11] |
H.C. Duan, L.H. Wei, C. Zhang, et al., Plant Cell 29 (2017) 2995-3011. DOI:10.1105/tpc.16.00912 |
| [12] |
X. Cui, Z. Liang, L. Shen, et al., Mol. Plant 10 (2017) 1387-1399. DOI:10.1016/j.molp.2017.09.013 |
| [13] |
J. Azevedo-Favory, C. Gaspin, L. Ayadi, et al., RNA Biol. 18 (2021) 1760-1777. DOI:10.1080/15476286.2020.1869892 |
| [14] |
L. Sun, Y. Xu, S. Bai, et al., J. Exp. Bot. 70 (2019) 5089-5600. DOI:10.1093/jxb/erz273 |
| [15] |
C. Enroth, L.D. Poulsen, S. Iversen, et al., Nucleic Acids Res. 47 (2019) e126. DOI:10.1093/nar/gkz736 |
| [16] |
A.L. Burgess, R. David, I.R. Searle, BMC Plant Biol. 15 (2015) 199. DOI:10.1186/s12870-015-0580-8 |
| [17] |
D. Palm, D. Streit, T. Shanmugam, et al., Nucleic Acids Res. 47 (2019) 1880-1895. DOI:10.1093/nar/gky1261 |
| [18] |
C.B. Qi, J.H. Ding, B.F. Yuan, Y.Q. Feng, Chin. Chem. Lett. 30 (2019) 1618-1626. DOI:10.1016/j.cclet.2019.02.005 |
| [19] |
M.D. Lan, B.F. Yuan, Y.Q. Feng, Chin. Chem. Lett. 30 (2019) 1-6. DOI:10.1016/j.cclet.2018.04.021 |
| [20] |
M. Helm, Y. Motorin, Nat. Rev. Genet. 18 (2017) 275-291. DOI:10.1038/nrg.2016.169 |
| [21] |
H. Grosjean, G. Keith, L. Droogmans, Methods Mol. Biol. 265 (2004) 357-391. DOI:10.1385/1-59259-775-0:357 |
| [22] |
L. Shen, Z. Liang, H. Yu, Bio. Protoc. 7 (2017) e2095. |
| [23] |
B. Chen, B.F. Yuan, Y.Q. Feng, Anal. Chem. 91 (2019) 743-756. DOI:10.1021/acs.analchem.8b04078 |
| [24] |
M.Y. Chen, Z. Gui, K.K. Chen, et al., Chin. Chem. Lett. 33 (2022) 2086-2090. DOI:10.1016/j.cclet.2021.08.094 |
| [25] |
C.B. Qi, H.P. Jiang, J. Xiong, B.F. Yuan, Y.Q. Feng, Chin. Chem. Lett. 30 (2019) 553-557. DOI:10.1016/j.cclet.2018.11.029 |
| [26] |
X.J. You, L. Li, T.T. Ji, et al., Chin. Chem. Lett. 34 (2023) 107181. DOI:10.1016/j.cclet.2022.01.074 |
| [27] |
Y.J. Feng, X.J. You, J.H. Ding, et al., Anal. Chem. 94 (2022) 4747-4755. DOI:10.1021/acs.analchem.1c05292 |
| [28] |
F.L. Liu, T.T. Ye, J.H. Ding, et al., Anal. Chem. 93 (2021) 6848-6856. DOI:10.1021/acs.analchem.1c00915 |
| [29] |
Q.Y. Cheng, J. Xiong, C.J. Ma, et al., Chem. Sci. 11 (2020) 1878-1891. DOI:10.1039/C9SC05094A |
| [30] |
X.J. You, T. Liu, C.J. Ma, et al., Anal. Chem. 91 (2019) 10477-10483. DOI:10.1021/acs.analchem.9b01318 |
| [31] |
M. Sakurai, T. Yano, H. Kawabata, H. Ueda, T. Suzuki, Nat. Chem. Biol. 6 (2010) 733-740. DOI:10.1038/nchembio.434 |
| [32] |
K.D. Meyer, Y. Saletore, P. Zumbo, et al., Cell 149 (2012) 1635-1646. DOI:10.1016/j.cell.2012.05.003 |
| [33] |
Z. Zhang, L.Q. Chen, Y.L. Zhao, et al., Sci. Adv. 5 (2019) eaax0250. DOI:10.1126/sciadv.aax0250 |
| [34] |
A.J. Westermann, S.A. Gorski, J. Vogel, Nat. Rev. Microbiol. 10 (2012) 618-630. DOI:10.1038/nrmicro2852 |
| [35] |
C.T. Chan, M. Dyavaiah, M.S. DeMott, et al., PLoS Genet. 6 (2010) e1001247. DOI:10.1371/journal.pgen.1001247 |
| [36] |
J.M. Chu, T.T. Ye, C.J. Ma, et al., ACS Chem. Biol. 13 (2018) 3243-3250. DOI:10.1021/acschembio.7b00906 |
| [37] |
Q. Gu, Z. Chen, W. Cui, et al., Ecotoxicol. Environ. Saf. 147 (2018) 861-871. DOI:10.1016/j.ecoenv.2017.09.054 |
| [38] |
B. Hussain, M.N. Ashraf, S.U. Rahman, et al., Sci. Total Environ. 754 (2021) 142188. DOI:10.1016/j.scitotenv.2020.142188 |
 2023, Vol. 34
2023, Vol. 34 

