b Beijing Key Laboratory of Drug Targets Identification and Drug Screening, National Center for Pharmaceutical Screening, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
Rhein (Rhe) (Scheme 1) is a bioactive constituent of anthraquinone, isolated from Polygonaceae plants such as Rhubarb, Polygonum Multiflorum and Polygonum Cuspidatum [1, 2]. Numerous studies have demonstrated that Rhe could alleviate the severity of joint inflammatory responses and bone destruction in arthritis [[3-6]. For instance, diacerein, a metabolic precursor of Rhe, has been used in the clinical treatment of osteoarthritis [7]. Besides, Rhe is well recognized for its anti-tumor [8], anti-viral [9], anti-diabetic nephropathy [10], antibacterial [11] and other biological activities [[12-14], especially effective in anti-inflammations [15]. Despite its good curative effects in inflammatory responses and wide pharmaceutical activities, the application of Rhe still remains limited due to its poor solubility [16, 17], which is the key factor affecting oral bioavailability [18]. Additionally, poor solubility may lead to poor drug stability and is not conducive to the development of acceptable formulations [19]. Hence, it is essential to improve the solubility of Rhe for its development and application.
|
Download:
|
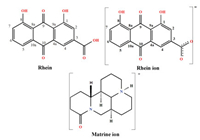
| Scheme 1. The molecular structure of rhein molecule, rhein ion and matrine ion. | |
The previously reported methods to improve the solubility of Rhe include structural modification and pharmaceutical formulations, such as rhein metal complex [20], rhein salt formation [21], rhein ester derivatives [22], rhein solid dispersion [23], rhein cyclodextrin inclusion [24]. As a new class of solid drugs, pharmaceutical cocrystal [25, 26], consists of API and CCF in a fixed stoichiometric ratio through non-covalent interactions, offers an alternative approach to improve the solubility and oral bioavailability of poorly soluble drugs [27, 28], while also develop stable and acceptable formulations [29, 30]. Z. Chi et al. reported rhein-l-arginine cocrystal [31], rhein-l-lysine cocrystal [32], which exhibited the improvement in solubility and oral bioavailability compared with pure Rhe. The cocrystal above-mentioned have enhanced the solubility of Rhe to some extent, but no single crystal structure has been reported for the formation of the eutectic.
The present work developed an innovative Rhe-Mat cocrystal with improved solubility and bioavailability. It is worth mentioning that the structure of Rhe-Mat cocrystal was obtained and the SXRD results indicated an asymmetric unit of the cocrystal consists of two rhein molecules, two rhein ions, two matrine ions, and one water molecule. Additionally, the prepared Rhe-Mat cocrystal was characterized by different characterization methods including SXRD, PXRD, TGA and DSC. Above all, the formation mechanism of Rhe-Mat cocrystal was elucidated by molecular surface electrostatic potential (MSEP), which could help to understand the interaction pattern between Rhe and Mat, providing guidance and reference for the screening and preparation of cocrystal of other anthraquinones. The results indicated that the Rhe-Mat cocrystal exhibited the better dissolution, and the higher absorption rate and peak blood concentrations compared with Rhe. Therefore, it can be concluded that present work offered a significant advantage in the improvement of the solubility and oral bioavailability of poorly insoluble drugs.
The Rhe-Mat cocrystal was obtained by slurrying method and solution vaporization method. Slurrying method: 1 mmol Rhe (284.22 mg) and 1 mmol Mat (248.36 mg) was dissolved in 6 mL of ethanol, stirring continuously for 24 h at 25 ℃. Subsequently, the obtained suspension was filtered and the Rhe-Mat cocrystal solid was harvested after drying in a 40 ℃ vacuum drying oven for 12 h. Solution vaporization method: weigh 500 mg of the obtained powder sample and dissolve it in 20 mL of 95% ethanol solution to obtain clear and transparent brown solution, which was filtered and the filtrate was placed at 25 ℃ for static culture about 30 days to harvest high-quality crystals.
The PXRD experiment was used to confirm the formation of Rhe-Mat cocrystal. As shown in Fig. S1 (Supporting information), the characteristic diffraction peaks at 2θ of 10.0°, 10.9°, 17.7°, 19.4°, 21.9°, 27.7° for rhein and 7.1°, 11.8°, 17.8°, 19.9°, 23.4° for matrine disappeared, and new characteristic peaks appeared at 8.8°, 10.9°, 12.7°, 14.6°, 22.5°, 25.2°, 26.4° in the pattern of Rhe-Mat cocrystal. Besides, compared with the physical mixture profile, the cocrystal showed significant differences in the number and intensity of characteristic diffraction peaks, which could serve as a proof of new crystalline phase formation. Additionally, the PXRD experiment was conducted to confirm the purity of Rhe-Mat cocrystal, and the results showed that the pattern of Rhe-Mat overlapped with the simulated one from the SXRD analysis, indicating the high purity of Rhe-Mat cocrystal.
Thermal behaviors of Rhe-Mat cocrystal were investigated by DSC and TGA. As shown in Fig. S2 (Supporting information), DSC patterns of Rhe and Mat revealed endothermic peaks at 328 ℃ and 79 ℃, 90 ℃, respectively. Distinctively, a peak at 171 ℃ is different from the melting points of Rhe and Mat, demonstrating the formation of Rhe-Mat cocrystal. In addition, the peak at 74 ℃ corresponds to the peak of solvent water. Meanwhile, the mass loss in the TGA pattern of Rhe-Mat cocrystal (Fig. S3 in Supporting information) was 1.23%, equivalent to one water molecule, which in good agreement with the SXRD analysis.
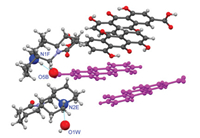
The SXRD experiment indicated that Rhe-Mat cocrystal crystallize in the P1 space group and an asymmetric unit of consists of two rhein ions (A, B), two rhein molecules (C, D), two matrine ions (E, F) and one water molecule (W) (Fig. 1). As shown in Fig. S4 (Supporting information), rhein ion A formed a dimer with rhein molecule C by a planar D1 1(2) H-bond (O6C—H6C⋯O6A, d: 2.570 Å, θ: 168.04°); two dimers were firmly connected by R2 2(10) hydrogen-bonded cycle (O1C—H1C⋯O3A, d: 2.911 Å, θ: 120.45°; O1A-H1A⋯O3C, d: 2.865 Å, θ: 118.63°) to form tetramer, and so on, to form the first layer structure. Meanwhile, rhein ion B interacted with rhein molecule D by planar D1 1(2) H-bond (O6D-H6D⋯O6B, d: 2.514, θ: 166.86°) to form dimers, which were assembled by planar D1 1(2) H-bond (O1D-H1D⋯O3B, d: 2.852, θ: 115.92°) to form the second layer structure. The different layer structures are almost parallel and π⋯π stacking interactions can be observed between the neighboring rhein molecules.
|
Download:
|
| Fig. 1. The asymmetric unit and hydrogen bond contacts of Rhe-Mat cocrystal. | |
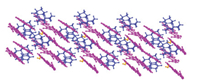
In the layered structure, the O—H proton of Rhe transferred to Mat and formed N1F-H1F⋯O5B (d: 2.756 Å, θ: 167.89°), N2E-H2E⋯O1W (d: 2.732 Å, θ: 160.82°), O1W -H1W⋯O5A (d: 2.695 Å, θ: 116.28°) ionic bond, respectively. The N1F-H1F⋯O5B hydrogen bond formed between the N1F of matrine ion F and the O5B of rhein ion B. However, due to the limitation of steric hindrance, matrine ion E cannot directly form a hydrogen bond with rhein ion A (Fig. S5 in Supporting information). Therefore, water molecules are required to act as a "bridge" connection, that is, the N2E of matrine first connected with the O1W of water molecules and formed N2E-H2E⋯O1W hydrogen bond, and then the O1W, forming the three-dimensional layer structure, which extended along [010] direction (Fig. 2). The crystallographic parameters and the bond lengths and angles of hydrogen bonding are shown in Tables S1 and S2 (Supporting information), respectively.
|
Download:
|
| Fig. 2. Three-dimensional layered structure of Rhe-Mat cocrystal. | |
The exploration of Rhe-Mat cocrystal formation mechanism are based on the principle that the structure of a crystal is determined by hierarchial organization of function group interactions [33]: The strongest H-bond is formed between the best H-bond donor and the best H-bond acceptor, the next best H-bond donor interacts with the next best H-bond acceptor in the absence of steric constraints, until all of the interaction sites are satisfied [34]. The H-bond interaction sites αi and βj can be defined by all the local maxima and minima on the MSEP map.
The MSEP was calculated by Gaussian 09 to elucidate the formation mechanism of Rhe-Mat cocrystal. For each compound, gas phase ab initio calculations were used to identify maxima and minima mapped onto the 0.002 Bohr/Å3 electron density isosurface [35]. The MEPS were converted into the corresponding H-bond parameters αi and βj using Eqs. 1 and 2 [36].
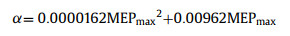
|
(1) |
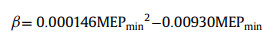
|
(2) |
MEPmin and MEPmax (kJ/mol) are the local minima and maxima on the MEPS, respectively.
The MSEP maps of all ingredients of Rhe-Mat cocrystal were intuitively drawn in Fig. S6 (Supporting information). As shown in Fig. S6, the red region distributed over hydrogen indicates the positive electrostatic potential region, whereas the black region located around the oxygen linked to carbon atoms of carbanyl groups represents negative electrostatic potential region. Specifically, the local first minima of rhein ion B interacted with the first maxima of matrine ion F, forming the N1F-H1F⋯O5B (d: 2.756 Å, θ: 167.89°) ionic bond (Fig. S7 in Supporting information). Owning to the limitation of steric hindrance (Fig. S8 in Supporting information), matrine ion E cannot directly form a hydrogen bond with rhein ion A. Therefore, the local first minima of rhein ion A interacted with the local first maxima of water, and subsequently the latter interacted with the local first minima of matrine ion E, forming the O1W-H1W⋯O5A (d: 2.695 Å, θ: 116.28°), N2E-H2E⋯O1W (d: 2.732 Å, θ: 160.82°) hydrogen bond, respectively. Furthermore, it should be pointed out that the introduction of the water molecules resulted in the incensement of local electrostatic potential, which, in turn, induced local polarity enhancement for the most of the aggregations, which is conducive to the dissolvability improvement of the Rhe-Mat cocrystal [37].
The stability of Rhe-Mat cocrystal was tested according to Chinese Pharmacopoeia [39]. As shown in Fig. S9 (Supporting information), the Rhe-Mat cocrystal was stable at high temperature and illumination conditions while part of it transformed into Rhe under high humidity after 10 days, which indicated that Rhe-Mat cocrystal was deliquescent and should be kept in sealed and dry conditions.
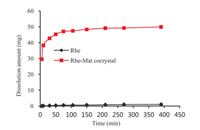
It is well recognized that the improved solubility may lead to the increased bioavailability [38]. The dissolution assessment of Rhe and Rhe-Mat cocrystal were conducted by dissolution tester. As expected, the results of the dissolution experiments indicated that the dissolution rate and solubility of Rhe were remarkably improved in form of Rhe-Mat cocrystal (Fig. 3). In specific, the Rhe-Mat cocrystal (55.47 µg/mL) was about 92.44% higher than that of Rhe (1.13 µg/mL) in terms of the sample added to media in pure water. Moreover, the maximum concentration of Rhe-Mat cocrystal was approximately 50-fold higher than Rhe, which can be maintained for over 6 h, indicating enhanced bioavailability and thereby suitable for the development of cocrystal drugs to increase the solubility-limited bioavailability.
|
Download:
|
| Fig. 3. Powder dissolution profiles of Rhe and Rhe-Mat cocrystal in pure water. | |
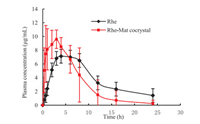
The pharmacokinetic experiments were performed to evaluate the oral bioavailability of Rhe-Mat cocrystal in rhesus monkeys. In specific, Rhesus monkeys were administrated at a single oral dose of 15 mg/kg Rhe and Rhe-Mat cocrystal, respectively. The pharmacokinetic parameters of Rhe and Rhe-Mat cocrystal were shown in Tables S3 and S4 (Supporting information), respectively. It can be seen from Fig. 4 that the Cmax of Rhe and Rhe-Mat cocrystal were 8.20 and 10.38 µg/mL, respectively, which indicated that the peak blood concentration of Rhe-Mat cocrystal was higher than that of Rhe. Moreover, the Tmax of Rhe and Rhe-Mat cocrystal were 5.67 and 2.95 h, respectively, demonstrated that the absorption rate of Rhe-Mat cocrystal was faster than Rhe.
|
Download:
|
| Fig. 4. The plasma concentration-time curve of Rhe and Rhe-Mat cocrystal in rhesus monkeys. | |
The two-way one-sided t-test and the (1–2α)% confidence interval method were used to compare the differences of AUC0-t, AUC0-∞ and Cmax of Rhe and Rhe-Mat cocrystal. The experimental results showed that the confidence interval of AUC0-t, AUC0-∞ and Cmax are 60.3%−117.22%, 48.56%−112.28% and 107.04%−151.91%, respectively, none of which was in the confidence interval of 80%−125%, which could be served as a proof that the oral absorption of Rhe and Rhe-Mat cocrystal in rhesus monkeys was not equivalent in rhesus monkeys. However, the Rhe-Mat cocrystal is a strong base and weak acid cocrystal, and strong acidic environment can destroy the structure of the cocrystal itself, leading to poor absorption. After oral administration, Rhe-Mat cocrystal was destroyed by the strong acidic environment in gastric juice, resulting in a low dose in body circulation, and thus indicating similar PK data to those of Rhe. Therefore, it could be concluded that the pH of the dissolution medium has a significant effect on the dissolution behavior of Rhe-Mat cocrystal.
It is well acknowledged that the bioavailability of orally administered BCS Class II drugs is closely related to the solubility, and thereby the improvement of solubility of drugs is one of the most challenging issues in the oral drug development. At present work, a novel solid form of Rhe-Mat cocrystal has been successfully synthesized and characterized and powder dissolution and pharmacokinetics has been investigated. In addition, the formation mechanism of Rhe-Mat cocrystal was elucidated by molecular surface electrostatic potential (MSEP). Remarkably, the 50-fold increment of solubility in vitro was observed in pure water in the form of Rhe-Mat cocrystal. Moreover, the peak blood concentration of Rhe-Mat cocrystal is higher than that of Rhe, and the absorption rate has been improved as well, indicating that cocrystal formation can be a useful method to improve the solubility and bioavailability of poorly soluble drugs. In conclusion, the present study not only obtained an innovative solid form of rhein for its development and application, and thereby offered significant advantage in developing an acceptable formulation.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThe present work was supported by Drug Innovation Major Project (Nos. 2018ZX09711001–001–015, 2018ZX09711001–003–022) and CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-3–007).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.063.
| [1] |
M.B. Rokaya, P. Maršík, Z. Münzbergová, Biochem. Syst. Ecology. 41 (2012) 83-90. DOI:10.1016/j.bse.2011.11.004 |
| [2] |
S.J. Jang, Y.I. Kuk, J. Plant Diseases Protect. 125 (2018) 451-460. DOI:10.1007/s41348-018-0179-z |
| [3] |
F. Hu, D. Zhu, W. Pei, et al., Int. Immunopharm. 75 (2019) 105780. DOI:10.1016/j.intimp.2019.105780 |
| [4] |
H. Wang, D. Yang, L. Li, et al., Nat. Products Bioprospect. 10 (2020) 445-452. DOI:10.1007/s13659-020-00272-y |
| [5] |
J. Deffaud, M. Kirchmeyer, F. Domagala, et al., Biorheology 45 (2008) 439-455. DOI:10.3233/bir-2008-0484 |
| [6] |
X.D. Cong, M.J. Ding, D.Z. Dai, et al., Inflammation 35 (2012) 1031-1040. DOI:10.1007/s10753-011-9407-4 |
| [7] |
N. Akhter, A.A. Khan, S.B. Ayaz, et al., PAFMJ 65 (2015) 77-80. |
| [8] |
M.X. Tan, Z.F. Wang, Q.P. Qin, et al., Dalton Trans. 49 (2020) 1613-1619. DOI:10.1039/c9dt04594e |
| [9] |
L. Cheng, Q. Chen, R. Pi, et al., Eur. J. Pharm. 899 (2021) 173908. DOI:10.1016/j.ejphar.2021.173908 |
| [10] |
G. Wang, Q. Li, D. Chen, et al., Theranostics 9 (2019) 6191. DOI:10.7150/thno.37538 |
| [11] |
J. Azelmat, J.F. Larente, D. Grenier, Archiv. Oral Biol. 60 (2015) 342-346. DOI:10.1016/j.archoralbio.2014.11.006 |
| [12] |
M. Liu, P. Lv, R. Liao, et al., J. Mol. Struct. 1128 (2017) 239-244. DOI:10.3724/sp.j.1118.2017.16136 |
| [13] |
J. Chen, B. Luo, S. Wen, et al., Investig. New Drugs 38 (2020) 755-764. DOI:10.1007/s10637-019-00821-4 |
| [14] |
V. Duraipandiyan, S. Ignacimuthu, M.G. Paulraj, Saudi J. Biolog. Sci. 18 (2011) 129-133. DOI:10.1016/j.sjbs.2010.12.009 |
| [15] |
Y. Gao, X. Chen, L. Fang, et al., Free Rad. Biol. Med. 72 (2014) 104-112. DOI:10.1016/j.freeradbiomed.2014.04.001 |
| [16] |
J. Luo, J. Sun, X. Luo, et al., Drug Develop. Ind. Pharm. 45 (2019) 96-104. DOI:10.1080/03639045.2018.1522326 |
| [17] |
Y. Wei, X. Luo, J. Guan, et al., Drug Develop. Ind. Pharm. 43 (2017) 1885-1891. DOI:10.1080/03639045.2017.1353519 |
| [18] |
H. Feng, Y. Zhu, Z. Fu, et al., Chem. Biol. Drug Design. 90 (2017) 867-872. DOI:10.1111/cbdd.13007 |
| [19] |
B.J. Aungst, J. Pharm. Sci. 106 (2017) 921-929. DOI:10.1016/j.xphs.2016.12.002 |
| [20] |
Z. Fang, L. Hui, C. Hui, et al., Acta Chim. Sin. 69 (2011) 925-930. |
| [21] |
E. Blacher, B. Ben Baruch, A. Levy, et al., Int. J. Cancer. 136 (2015) 1422-1433. DOI:10.1002/ijc.29095 |
| [22] |
X. Zhu, X. Ye, L. Song, et al., Med. Chem. Res. 22 (2013) 2228-2234. DOI:10.1007/s00044-012-0215-7 |
| [23] |
W. Wang, Y. Zhao, X. Liu, et al., West China J. Pharm. Sci. 27 (2012) 32-35. |
| [24] |
S. Petralito, I. Zanardi, A. Memoli, et al., Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 74 (2009) 1254-1259. DOI:10.1016/j.saa.2009.09.056 |
| [25] |
M. Karimi-Jafari, L. Padrela, G.M. Walker, et al., Cryst. Growth Des. 18 (2018) 6370-6387. DOI:10.1021/acs.cgd.8b00933 |
| [26] |
J.M. Chen, S. Li, T.B. Lu, Cryst. Growth Des. 14 (2014) 6399-6408. DOI:10.1021/cg501247x |
| [27] |
Y. Huang, B. Zhang, Y. Gao, et al., Pharm. Sci. 103 (2014) 2330-2337. DOI:10.1002/jps.24048 |
| [28] |
V. Mathur, Y. Satrawala, M.S. Rajput, Int. J. Pharm. Front. Res. 1 (2011) 135-145. |
| [29] |
R. Shaikh, R. Singh, G.M. Walker, et al., Trend. Pharmacol. Sci. 39 (2018) 1033-1048. DOI:10.1016/j.tips.2018.10.006 |
| [30] |
M.L. Cheney, D.R. Weyna, N. Shan, et al., J. Pharm. Sci. 100 (2011) 2172-2181. DOI:10.1002/jps.22434 |
| [31] |
Z. Chi, M. Wang, L. Yang, et al., Anal. Sci. 29 (2013) 661-664. DOI:10.2116/analsci.29.661 |
| [32] |
M. Birer, F. Acartürk, Pharm. Develop. Technol. 26 (2021) 661-672. DOI:10.1080/10837450.2021.1916031 |
| [33] |
J.S. Murray, P. Politzer, J. Mol. Struct. Theochem. 425 (1998) 107-114. DOI:10.1016/S0166-1280(97)00162-0 |
| [34] |
Y. Tsuchiya, K. Kinoshita, H. Nakamura, Protein Engin. Design Select. 19 (2006) 421-429. DOI:10.1093/protein/gzl026 |
| [35] |
D. Musumeci, C.A. Hunter, R. Prohens, et al., Chem. Sci. 2 (2011) 883-890. DOI:10.1039/c0sc00555j |
| [36] |
C.B. Hübschle, S. van Smaalen, J. Appl. Crystal. 50 (2017) 1627-1636. DOI:10.1107/S1600576717013802 |
| [37] |
Y.M. Yu, L.Y. Wang, F.Z. Bu, et al., CrystEngComm 22 (2020) 7992-8006. DOI:10.1039/d0ce01297a |
| [38] |
P. Khadka, J. Ro, H. Kim, et al., Asian J. Pharm. Sci. 9 (2014) 304-316. DOI:10.1016/j.ajps.2014.05.005 |
| [39] |
M.R. Shen, Y. He, S.M. Shi, J. Pharm. Anal. 11 (2021) 155-162. DOI:10.1016/j.jpha.2020.11.008 |
 2023, Vol. 34
2023, Vol. 34 

