b Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, School of Science, Westlake University, Hangzhou 310024, China
Pyrroles represent one of the most important class of five-membered heterocycles that are prevalent in a number of biologically active natural products [1-3] and synthetic pharmaceuticals (Fig. 1) [4-7]. For instance, Lipitor, containing a tetrasubstituted pyrrole core, is one of the most best-selling drugs in synthetic pharmaceutical history by Pfizer and primarily used to lower blood cholesterol and treat cardiovascular disease. Thus many synthetic methods [8-11] toward pyrrole rings have been developed, including name reactions such as Paal-Knorr reactions. These methods suffered from accessing pyrrole containing sensitive functional groups and the inability to provide pyrrole ring with high flexibility. Therefore development of new and efficient methods to build polysubstituted pyrrole ring from readily available starting material under mild reaction conditions is highly desirable. Intermolecular cycloaddition reaction provided a modular approach to construct multisubstituted pyrroles and various [4 + 1] [12-18], [3 + 2] [19-36], and [2 + 2 + 1] [37-47] cycloaddition strategies have been developed [48-53]. Herein, we reported a new gold catalyzed [3 + 2] cycloaddition reaction of terminal alkynes with 1, 2-diaza-1, 3-dienes to construct tetrasubstituted pyrroles. This reaction featured by using a neighboring hydroxymethyl as a directing group through addition/cycloaddition/elimination cascade, which are totally different with traditional cyclcometallation approach in directing group assisted C-H activations. To the best of our knowledge, such kind of directing approach has not been reported in literatures.
|
Download:
|
| Fig. 1. Representative pyrrole-containing natural products and pharmaceuticals. | |
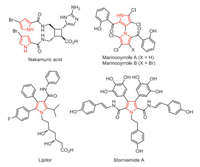
In recent years, homogeneous gold catalysis has witnessed an exponential growth in organic synthesis. Gold catalysts serving as π-acids can activate C-C multiple bonds and trigger subsequent cascade reactions to assemble complex structures [54-71]. Recently we [72-75] and other groups [76-80] developed an efficient gold and metal Lewis acid combination strategy to construct complex fused, spiro and bridged rings (Scheme 1A). Gold-catalyzed 5-exo-dig cyclization of an alkynyl alcohol generating an active exocyclic enolether intermediate (M), subsequent Lewis aicd catalyzed inverse-electron-demand hetero-Diels-Alder reaction with electron deficient unsaturated ketone ester or ortho-quinone methides could form various polycycles in one step with high reaction efficiency. Following this concept, we wish to extend this reaction mode to 1, 2-diaza-1, 3-dienes. We reasoned that the [3 + 2] type reaction of the electron-deficient 1, 2-diaza-1, 3-dienes with our gold-catalyzed in situ formed enol ether M would form the spirocycle N (Scheme 1B). Indeed, when we performed this reaction, the target sprio product N was not observed, but another polysubstituted pyrrole product was isolated, which constitutes a [3 + 2] cycloaddition between alkyne and azadiene to synthesize pyrroles, and thus the neighboring hydroxymethyl group serve as an important directing group. In this communication, we report the preliminary results of this serendipity.
|
Download:
|
| Scheme 1. Gold-catalyzed cycloisomerization/cycloaddition cascade leading to polycycles. | |
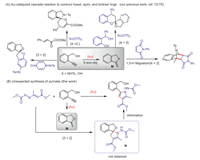
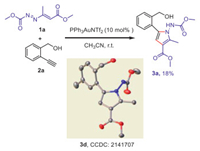
Following previous Au-catalytic strategy, 1, 2-diaza-1, 3-diene 1a and alkynyl alcohol 2a were chose as the model substrates to test the viability of this hypothesis (Scheme 2). When the reaction was performed with Ph3PAuNTf2 as the catalyst, a new product was isolated in 18% yield. To our great surprise, it is not the expected spiro adduct N, but a tetrasubstituted pyrrole 3a. The structure was carefully characterized by NMR and mass analysis, and finally confirmed by the single X-ray analysis (Scheme 2). Inspired by these results, we continued to optimize reaction conditions to develop an efficient catalytic system toward pyrroles (Table 1, for details, see Supporting information). Various transition-metal catalysts were screened at room temperature, copper(I) and Ag(I) could not deliver any products, and the reactants remained unreacted (entries 1 and 2, Table 1). Then various gold catalysts were tested (entries 3-7), and when 10 mol% of (tBu2PhO)3PAuNTf2 was used, the target pyrrole was isolated in highest 86% yield (entry 7), which is the optimized conditions. Lowering the catalyst loading to 5 mol%, much lower 53% yield was observed (entry 8). The effect of solvents was examined (entries 9-12) and acetonitrile proved to be the most favorable solvent. Decreasing or increasing the reaction temperature all led to decreased yield (entries 13 and 14).
|
Download:
|
| Scheme 2. Initial formation of the unexpected pyrrole 3a and crystal structure of 3d. | |
|
|
Table 1 Reaction condition optimization.a |
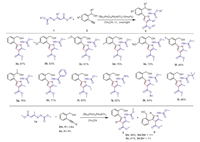
With the optimized reaction conditions in hand, the substrate scope was explored (Scheme 3). Diverse substituted alkynyl alcohols and 1, 2-diaza-1, 3-dienes were prepared and subjected to the standard reactions, a large variety of polysubstituted pyrroles were synthesized in good yields. Electron-donating groups such as methyl (3d) and methoxyl (3b and 3c), and electron-withdrawing groups such as fluoro (3f) on the aromatic ring are all compatible with the reaction. Adding one methyl on the directing hydroxymethyl group does not affect this reaction (3g). Various ester, ketone, amide functionalized diaza-1, 3-dienes are all suitable substrates, giving good yields of the corresponding pyrroles (3h-3l). Internal alkynes 2m and 2n were also viable in this reaction, affording a mixture of two regioisomers, and the ration is almost 1:1.
|
Download:
|
| Scheme 3. Scope of gold-catalyzed cascade reaction to the tetrasubstituted pyrroles. | |
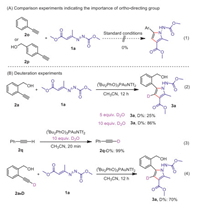
To gain insights into the reaction mechanism, we conducted several control experiments. Simple phenylacetylene or move the hydroxymethyl group to the para position, no desired products were observed, indicating the importance of this ortho directing effect of hydroxymethyl group (Scheme 4A). To further figure out the addition/elimination process, we carried out deuteration experiments (Scheme 4B). When the reaction was performed under standard condition with extra 5 equiv. of D2O, the hydrogen on pyrrole ring was 25% deuterated, and 86% deuterated if 10 equiv. of D2O was added (reaction 2). Control experiments of phenylacetylene under the same conditions indicating there is a fast H-D exchange process through an alkynyl gold intermediate (reaction 3). If deuterated substrate 2a-D was subjected to standard reaction under anhydrous conditions, the isolated product 3a was 70% deuterated (reaction 4).
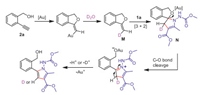
Based on these experiments and previous literatures, a plausible mechanism was proposed in Scheme 5. Au-catalyzed 5-exo-dig cyclization gives alkenyl gold intermediate, and subsequent proton deauration affords electron-rich enol ether intermediate M. The cycloaddition of M with 2a, produced spiro polycycle N. Stepwise nucleophilic addition of M to 1a and subsequent cyclization route could not be excluded. Then a Lewis acid promoted C-O bond cleavage forming a carbon cation, and aromatization elimination of proton afforded pyrrole product 3a. Both H+ and D+ was possibly eliminated, which accord with the deuteration results in (Scheme 4, reactions 2 and 4). Herein, the aromatization is the major driving force for the elimination reactions.
|
Download:
|
| Scheme 4. Control experiments. | |
|
Download:
|
| Scheme 5. Proposed mechanism. | |
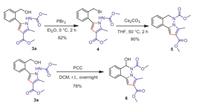
To further highlight the synthetic utility of our reported method, we carried out product derivatizations. The hydroxymethyl group is not only a directing group, but also a very useful synthetic handles for further transformations (Scheme 6). Treatment of 3a with PBr3 led to the formation of 4 in 82% yield, subsequent Cs2CO3 promoted cyclization gave tricyclic heterocycle 5 in 90% yield. Alternatively, PCC oxidation of 3a generated tricyclic hemiaminal heterocycle 6 in 78% yield.
|
Download:
|
| Scheme 6. Synthetic elaborations of product. | |
In summary, we have established the first Au-catalyzed cycloaddition of alkynes with azadienes to access tetrasubstituted pyrroles. It is noteworthy that the employment of an ortho hydroxymethyl group as a directing group is key to the success of this reaction. The directing group participates in the reaction with an addition/elimination process, which are totally different with traditional cyclometallation pathway. This type of directing effect enriched the chemistry of organometallics mediated transformations, and offer new opportunities in organic synthesis. Further application of this strategy is going on in our lab and will be reported in due course.
Declaration of competing interestThere are no conflicts of interests to declare.
AcknowledgmentsWe are grateful for the financial support from the Natural Science Foundation of China and Shandong Province (Nos. 21971149, 92156007, ZR2019ZD45, ZR2020KB005), and the Fundamental Research Funds of Shandong University. We thank the Analytical Center of Shandong University where carrying out the NMR, mass spectra and X-ray crystallographic analysis.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.05.002.
| [1] |
D.P. O'Malley, K. Li, M. Maue, A.L. Zografos, P.S. Baranet, J. Am. Chem. Soc. 129 (2007) 4762-4775. DOI:10.1021/ja069035a |
| [2] |
C.C. Hughes, A. Prieto-Davo, P.R. Jensen, W. Fenical, Org. Lett. 10 (2008) 629-631. DOI:10.1021/ol702952n |
| [3] |
D.L. Boger, C.W. Boyce, M.A. Labroli, C.A. Sehon, Q. Jin, J. Am. Chem. Soc. 121 (1999) 54-62. DOI:10.1021/ja982078+ |
| [4] |
C. Teixeira, F. Barbault, J. Rebehmed, et al., Bioorg. Med. Chem. 16 (2008) 3039-3048. DOI:10.1016/j.bmc.2007.12.034 |
| [5] |
M. Biava, G.C. Porretta, D. Deidda, et al., Bioorg. Med. Chem. 12 (2004) 1453-1458. DOI:10.1016/j.bmc.2003.12.037 |
| [6] |
M. Protopopova, E. Bogatcheva, B. Nikonenko, et al., Med. Chem. 3 (2007) 301-316. DOI:10.2174/157340607780620626 |
| [7] |
W. Tian, B. Li, D. Tian, W. Tang, Chin. Chem. Lett. 33 (2022) 197-200. DOI:10.1016/j.cclet.2021.06.091 |
| [8] |
M.W. Rommi, S.F. MacDonald, Can. J. Chem. 48 (1970) 1689-1697. DOI:10.1139/v70-279 |
| [9] |
L. Knorr, Ber. Dtsch. Chem. Ges. 17 (1884) 1635-1641. DOI:10.1002/cber.18840170220 |
| [10] |
D.H.R. Barton, S.Z. Zard, J. Chem. Soc. Chem. Commun. (1985) 1098-1100. |
| [11] |
W.S. Bishop, J. Am. Chem. Soc. 6 (1945) 2261-2262. DOI:10.1021/ja01228a502 |
| [12] |
K. Luo, S. Mao, K. He, et al., ACS Catal. 10 (2020) 3733-3740. DOI:10.1021/acscatal.9b05360 |
| [13] |
P. Daw, S. Chakraborty, J.A. Garg, Y. Ben-David, D. Milstein, Angew. Chem. Int. Ed. 55 (2016) 14373-14377. DOI:10.1002/anie.201607742 |
| [14] |
R.L. Sahani, R.S. Liu, Angew. Chem. Int. Ed. 56 (2017) 1026-1030. DOI:10.1002/anie.201610665 |
| [15] |
B. Emayavaramban, M. Sen, B. Sundararaju, Org. Lett. 19 (2017) 6-9. DOI:10.1021/acs.orglett.6b02819 |
| [16] |
C.G. Overberger, L.C. Palmer, B.S. Marks, N.R. Byrd, J. Am. Chem. Soc. 77 (1955) 4100-4104. DOI:10.1021/ja01620a040 |
| [17] |
W. Geng, W.X. Zhang, W. Hao, Z. Xi, J. Am. Chem. Soc. 134 (2012) 20230-20233. DOI:10.1021/ja308950d |
| [18] |
Y. Shan, L. Su, D. Chen, Chin. Chem. Lett. 32 (2021) 437-440. DOI:10.1016/j.cclet.2020.04.041 |
| [19] |
O.A. Attanasi, G. Favi, F. Mantellini, G. Moscatelli, S. Santeusanio, Adv. Synth. Catal. 353 (2011) 1519-1524. DOI:10.1002/adsc.201100094 |
| [20] |
O.V. Larionov, A. DeMeijere, Angew. Chem. Int. Ed. 44 (2005) 5664-5667. DOI:10.1002/anie.200502140 |
| [21] |
M. Gao, C. He, H. Chen, et al., Angew. Chem. Int. Ed. 52 (2013) 6958-6961. DOI:10.1002/anie.201302604 |
| [22] |
J. Liu, Z. Fang, Q. Zhang, Q. Liu, X. Bi, Angew. Chem. Int. Ed. 52 (2013) 6953-6957. DOI:10.1002/anie.201302024 |
| [23] |
D. Zhao, S. Vsquez-Céspedes, F. Glorius, Angew. Chem. Int. Ed. 54 (2015) 1657-1661. DOI:10.1002/anie.201410342 |
| [24] |
F. Kallmeier, B. Dudziec, T. Irrgang, R. Kempe, Angew. Chem. Int. Ed. 56 (2017) 7261-7265. DOI:10.1002/anie.201702543 |
| [25] |
J. Xu, A.P. Green, N.J. Turner, Angew. Chem. Int. Ed. 57 (2018) 16760-16763. DOI:10.1002/anie.201810555 |
| [26] |
G.J. Mei, X. Tang, Y. Tasdan, Y. Lu, Angew. Chem. Int. Ed. 59 (2020) 648-652. DOI:10.1002/anie.201911686 |
| [27] |
S. Kamijo, C. Kanazawa, Y. Yamamoto, J. Am. Chem. Soc. 127 (2005) 9260-9266. DOI:10.1021/ja051875m |
| [28] |
J.Y. Liao, P.L. Shao, Y. Zhao, J. Am. Chem. Soc. 137 (2015) 628-631. DOI:10.1021/ja511895q |
| [29] |
G.M. Torres, J.S. Quesnel, D. Bijou, B.A. Arndtsen, J. Am. Chem. Soc. 138 (2016) 7315-7324. DOI:10.1021/jacs.6b02314 |
| [30] |
O.A. Attanasi, L. DeCrescentini, G. Favi, J. Org. Chem. 69 (2004) 2686-2692. DOI:10.1021/jo0349072 |
| [31] |
S. Michlik, R. Kempe, Nat. Chem. 5 (2013) 140-144. DOI:10.1038/nchem.1547 |
| [32] |
L.W. Qi, J.H. Mao, J. Zhang, B. Tan, Nat. Chem. 10 (2018) 58-64. DOI:10.1038/nchem.2866 |
| [33] |
R.R. Addada, V.R. Regalla, M.R. Vajja, V.N. Vema, V.R. Anna, Tetrahedron Lett. 57 (2016) 2838-2841. DOI:10.1016/j.tetlet.2016.05.025 |
| [34] |
J. Ke, C. He, H. Liu, M. Li, A. Lei, Chem. Commun. 49 (2013) 7549-7551. DOI:10.1039/c3cc43682a |
| [35] |
S. Zhang, Y. Ma, J. Lan, F. Song, J. You, Org. Biomol. Chem. 13 (2015) 5867-5870. DOI:10.1039/C5OB00599J |
| [36] |
C. He, S. Guo, J. Ke, et al., J. Am. Chem. Soc. 134 (2012) 5766-5769. DOI:10.1021/ja301153k |
| [37] |
J.N. Zhu, L.L. Chen, R.X. Zhou, et al., Org. Lett. 19 (2017) 6044-6047. DOI:10.1021/acs.orglett.7b02670 |
| [38] |
X. Wu, P. Zhao, X. Geng, et al., Org. Lett. 20 (2018) 688-691. DOI:10.1021/acs.orglett.7b03821 |
| [39] |
Y. Nishibayashi, M. Yoshikawa, Y. Inada, et al., Angew. Chem. Int. Ed. 42 (2003) 2681-2684. DOI:10.1002/anie.200351170 |
| [40] |
H.C. Chiu, I.A. Tonks, Angew. Chem. Int. Ed. 57 (2018) 6090-6094. DOI:10.1002/anie.201800595 |
| [41] |
Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22 (2020) 118-122. DOI:10.1039/c9gc02657f |
| [42] |
M. Zhang, X. Fang, H. Neumann, M. Beller, J. Am. Chem. Soc. 135 (2013) 11384-11388. DOI:10.1021/ja406666r |
| [43] |
Z.W. Davis-Gilbert, X. Wen, J.D. Goodpaster, I.A. Tonks, J. Am. Chem. Soc. 140 (2018) 7267-7281. DOI:10.1021/jacs.8b03546 |
| [44] |
K. Kawakita, E.P. Beaumier, Y. Kakiuchi, et al., J. Am. Chem. Soc. 141 (2019) 4194-4198. DOI:10.1021/jacs.8b13390 |
| [45] |
Z.W. Gilbert, R.J. Hue, I.A. Tonks, Nat. Chem. 8 (2016) 63-68. DOI:10.1038/nchem.2386 |
| [46] |
W. Liu, H. Jiang, L. Huang, Org. Lett. 12 (2010) 321-315. |
| [47] |
Q.W. Gui, F. Teng, S.N. Ying, Chin. Chem. Lett. 31 (2020) 3241-3244. DOI:10.1016/j.cclet.2020.07.017 |
| [48] |
X. Xin, D. Wang, X. Li, B. Wan, Angew. Chem. Int. Ed. 51 (2012) 1693-1697. DOI:10.1002/anie.201108144 |
| [49] |
L. Huang, Y. Cai, C. Zheng, L.D. Dai, S.L. You, Angew. Chem. Int. Ed. 56 (2017) 10545-10548. DOI:10.1002/anie.201705068 |
| [50] |
V. Estévez, M. Villacampa, J.C. Menéndez, Chem. Soc. Rev. 39 (2010) 4402-4421. DOI:10.1039/b917644f |
| [51] |
V. Estévez, M. Villacampa, J.C. Menéndez, Chem. Soc. Rev. 43 (2014) 4633-4657. DOI:10.1039/C3CS60015G |
| [52] |
X. Chen, Ran Zhao, Z. Liu, et al., Chin. Chem. Lett. 32 (2021) 2305-2308. DOI:10.1016/j.cclet.2021.02.021 |
| [53] |
W. Wang, S. Huang, S. Yan, et al., Chin. J. Chem. 38 (2020) 445-448. DOI:10.1002/cjoc.201900556 |
| [54] |
L. Zhang, J. Sun, S.A. Kozmin, Adv. Synth. Catal. 348 (2006) 2271-2296. DOI:10.1002/adsc.200600368 |
| [55] |
A. Fürstner, P.W. Davies, Angew. Chem. Int. Ed. 46 (2007) 3410-3449. DOI:10.1002/anie.200604335 |
| [56] |
A.S.K. Hashmi, M. Rudolph, Chem. Soc. Rev. 37 (2008) 1766-1775. DOI:10.1039/b615629k |
| [57] |
D.J. Gorin, B.D. Sherry, F.D. Toste, Chem. Rev. 108 (2008) 3351-3378. DOI:10.1021/cr068430g |
| [58] |
E. Jiménez-Núñez, A.M. Echavarren, Chem. Rev. 108 (2008) 3326-3350. DOI:10.1021/cr0684319 |
| [59] |
N.T. Patil, Y. Yamamoto, Chem. Rev. 108 (2008) 3395-3442. DOI:10.1021/cr050041j |
| [60] |
S.M.A. Sohel, R.S. Liu, Chem. Soc. Rev. 38 (2009) 2269-2281. DOI:10.1039/b807499m |
| [61] |
A.S.K. Hashmi, Angew. Chem. Int. Ed. 49 (2010) 5232-5241. DOI:10.1002/anie.200907078 |
| [62] |
M. Rudolph, A.S.K. Hashmi, Chem. Soc. Rev. 41 (2012) 2448-2462. DOI:10.1039/C1CS15279C |
| [63] |
F. Wei, C. Song, Y. Ma, et al., Sci. Bull. 60 (2015) 1479-1492. DOI:10.1007/s11434-015-0874-0 |
| [64] |
R. Dorel, A.M. Echavarren, Chem. Rev. 115 (2015) 9028-9072. DOI:10.1021/cr500691k |
| [65] |
L. Liu, J. Zhang, Chem. Soc. Rev. 45 (2016) 506-516. DOI:10.1039/C5CS00821B |
| [66] |
C. Shu, L. Li, T.D. Tan, D.Q. Yuan, L.W. Ye, Sci. Bull. 62 (2017) 352-357. DOI:10.1016/j.scib.2017.01.016 |
| [67] |
P.C. Zhang, Y.L. Li, J. He, et al., Nat. Commun. 12 (2021) 4609. DOI:10.1038/s41467-021-24678-5 |
| [68] |
Z. Yu, B. Ma, M. Chen, et al., J. Am. Chem. Soc. 136 (2014) 6904-6907. DOI:10.1021/ja503163k |
| [69] |
C. Wang, G. Xu, Y. Shao, S. Tang, J. Sun, Org. Lett. 22 (2020) 5990-5994. DOI:10.1021/acs.orglett.0c02083 |
| [70] |
Y. Xu, J. Sun, Org. Lett. 23 (2021) 853-857. DOI:10.1021/acs.orglett.0c04090 |
| [71] |
X. Di, Y. Wang, L. Wu, et al., Org. Lett. 21 (2019) 3018-3022. DOI:10.1021/acs.orglett.9b00537 |
| [72] |
S. Zhang, B. Cheng, S. Wang, et al., Org. Lett. 19 (2017) 1072-7075. DOI:10.1021/acs.orglett.7b00090 |
| [73] |
M. Liang, S. Zhang, J. Jia, et al., Org. Lett. 19 (2017) 2526-2529. DOI:10.1021/acs.orglett.7b00804 |
| [74] |
Teng Q, J. Qi, L. Zhou, Z. Xu, C.H. Tung, Org. Chem. Front. 5 (2018) 990-993. DOI:10.1039/C7QO01005B |
| [75] |
X. Wang, S. Dong, Z. Yao, et al., Org. Lett. 16 (2014) 22-25. DOI:10.1021/ol4033286 |
| [76] |
J. Li, L. Lin, B. Hu, et al., Angew. Chem. Int. Ed. 55 (2016) 6075-6078. DOI:10.1002/anie.201601701 |
| [77] |
S. Ge, W. Cao, T. Kang, et al., Angew. Chem. Int. Ed. 58 (2019) 4017-4021. DOI:10.1002/anie.201812842 |
| [78] |
J. Gong, Q. Wan, Q. Kang, Adv. Synth. Catal. 360 (2018) 4031-4403. DOI:10.1002/adsc.201800492 |
| [79] |
S. Witzel, A.S.K. Hashmi, J. Xie, Chem. Rev. 121 (2021) 8868-8925. DOI:10.1021/acs.chemrev.0c00841 |
| [80] |
W. Wang, C.L. Ji, K. Liu, et al., Chem. Soc. Rev. 50 (2021) 1874-1912. DOI:10.1039/d0cs00254b |
 2023, Vol. 34
2023, Vol. 34