b Faculty of Science, Kunming University of Science and Technology, Kunming 650500, China;
c State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
The magnetism of carbon nanomaterials, such as nanographene (NG), has attracted extensive attention due to its high magnitudes of spin-wave stiffness [1], weak spin-orbit coupling [2], hyperfine couplings [3] and large spin coherence lifetimes [4], which hold promise for spintronics [5,6]. The open-shell [7,8] structures of magnetic NGs with high reactivity results from the presence of unpair electrons (radicals), which make it challenging in solution-based synthesis and magnetic ground state measurement [9,10]. However, as an alternative method, on-surface synthesis under ultra-high vacuum has emerged as a powerful flexible synthetic toolkit in recent years [11]. Current research focus of carbon magnetism inducing involve four aspects: NG or graphene nanoribbons (GNR) with spin-polarized zigzag edges states [12-15], sublattice imbalance as guided by Lieb's theorem for bipartite lattices or defects induced systems [16-20], NGs with non-Kekulé structure formed by the topological frustrated [21] or size-induced Coulomb repulsion between valence electrons [22-26], and non-benzenoid rings imbedded NG [27,28].
The total spin quantum number (S) of NGs might be engineered in four ways. Firstly, artificially precursors transformed into open-shell structure by directly cyclodehydrogenation [16,17,19,21,24,29-33] to induce the magnetism. Secondly, the radical positions of NGs could be passivated by H atoms released from cyclodehydrogenation, which will modulate S from 0 to 1/2 [21] and 1 to 1/2 [19]. In this situation, the S could be recovered by applying bias to remove the redundant H atoms. Thirdly, quenching the spin by creating single C–metal bond, for example, bounding the radical positions to the elbow area of Au(111) surface [21] by applying positive voltage pulse [34]. Lastly, the spin on/off could be switched due to the charge transfer by changing adsorption positions [35-37]. Recently, an ingenious co-deposition method had been employed to induce magnetism in boron atoms doped graphene nanoribbon [38].
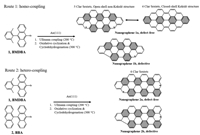
Here, we induce the co-deposition method into the engineering of nanographene and synthesize plenty products with different magnetic ground states by homo-coupling and hetero-coupling between precursors. We demonstrate this engineering strategy by co-depositing two precursors (precursor 1, 9-bromo-10-(2,6-dimethylphenyl)anthracene, BDMPA [23]; precursor 2, 10-bromo-9,9′-bianthracene, BBA [39]) on Au(111) substrate. As illustrated in route 1 of Fig. 1, surface-catalyzed Ullmann homo-coupling and afterwards cyclodehydrogenation reactions (300 ℃, 6 ℃/min) of precursor 1 lead to the formation of NG 1a (defect-free). Simultaneously, a NG 1b endowed with a pentagonal terminus resulting from losing a methyl group in the precursor 1 could also be formed during the annealing process. According to chemical structures and Lieb's theorem [20], the total spin of 1a is 0. The pentagonal ring in NG 1b renders a non-Kekulé system with an unpaired electron, that means a S = 1/2 grounds state of NG 1b. NG 1a can be described as either an open-shell non-Kekulé structure with 5 Clar sextets or a closed-shell Kekulé structure with 4 Clar sextets, respectively. This competition from the aromaticity of 5 Clar sextets is higher than 4 Clar sextets one, while the open-shell structure is more reactive than the closed-shell one. In addition, DFT calculation show that the real structure of NG 1a is still ambiguous (Table S1 in Supporting information) as the energies of open-shell and closed-shell structures are almost the same. When missing a methyl group in the precursor 1, a pentagonal topological defect in NG 1b raises a result of sublattice-imbalance, which manifests as a Kondo resonance. In another way, route 2, surface-catalyzed Ullmann hetero-coupling of the precursor 1 and the precursor 2 and afterwards cyclodehydrogenation cyclization reactions (300 ℃, 6 ℃/min) lead to formations of NG 2a and NG 2b. NG 2a, a defect-free structure, possesses a triplet ground state (S = 1). NG 2b, similar with NG 1b, formed by missing a methyl group, has a doublet ground state (S = 1/2).
|
Download:
|
| Fig. 1. Synthetic routes toward NGs 1a, 1b, 2a and 2b. Route 1: precursor 1 undergoes homo-coupling into the defect-free NG 1a and defective 1b. Route 2: precursors 1 and 2 hetero-coupling into the defect-free NG 2a and defective 2b. | |
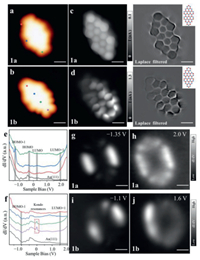
After depositing the precursor 1 on Au(111) surface and subsequent cyclodehydrogenation, the NGs 1a and 1b were obtained (Fig. 2). Figs. 2a and b show the STM images of NGs 1a and 1b, respectively. The NG 1a can be clearly recognized from the STM image via the two-lobe-like feature at both triangular termini (Fig. 2a), while the NG 1b possesses such feature only at one of the triangular ends (Fig. 2b). The structures are verified by our BR-STM images and corresponding chemical structure displayed in Figs. 2c and d. The additional states are visible near non-defect area in Fig. 2d. To elucidate the experimental electronic properties, we employed STS characterizations of the NG 1a (Fig. 2e) and 1b (Fig. 2f). For the NG 1a, the STS depicts several electronic resonances labeled as HOMO-1 (–1.35 V), LUMO+1 (+2.0 V) and a pair of electronic resonances marked as frontier molecular orbitals. The absence of any spin enhanced state near Fermi level from BR-STM as well as the inelastic spin excitation signal in a higher energy resolution dI/dV spectroscopy (Fig. S1 in Supporting information) alone with calculated energy (Table S1) which may illustrate a closed-shell structure of NG 1a. The precise energy positions of LUMO and HOMO are identified to be –0.3 V and +0.21 V, respectively, which reveals a frontier gap of 0.51 eV for NG 1a. The closed-shell nature of NG 1a by the frontier maps (Fig. S1b in Supporting information) with different shapes and symmetries. The spatial maps of electronic state distribution of HOMO-1 (Fig. 2g), and LUMO+1 (Fig. 2h) reveal similar results as reported before [18]. For NG 1b, the STS mainly shows two obvious electronic resonances in Fig. S2b (Supporting information), and labeled as HOMO-1 (–1.1 V) and LUMO+1 (+1.6 V) in Fig. 2f. Figs. 2i and j show the spatial maps of electronic states distribution of HOMO-1 and LUMO+1 and the additional maps are displayed in Fig. S3 (Supporting information). The dI/dV spectra in Fig. 2f depict the zero-energy states (ZESs) near the defect free terminus (the purple and green lines in Fig. 2f) which correspond to the additional states in BR-STM (Fig. 2d). To amplify on the ZESs of NG 1b, we performed low-energy range dI/dV spectra in Fig. S4 (Supporting information). The ZES appears when the tip position is close to defect free area and the resonance fits well with the Frota function [40]. These results indicate that the ZES in NG 1b is attributed to the Kondo resonances [16-19], [30,41].
|
Download:
|
| Fig. 2. STM images and electronic structures of NGs 1a and 1b. (a, b) STM images of NG 1a (c) and 1b (d) on Au(111) (V = −500 mV, I = 200 pA, 3 × 3 nm2). (c, d) BR-STM images (left panel) and Laplace filtered images (right panel) of NGs 1a and 1b (V = 2 mV, 2 × 2 nm2). dI/dV spectroscopy on NG 1a (e) and 1b (f) (V = −2 V, I = 350 pA, Vrms = 15 mV). (g, h) Experimental dI/dV maps of HOMO-1 and LUMO+1 for NG 1a (I = 350 pA, Vrms = 15 mV, 3 × 3 nm2). (i, j) Experimental dI/dV maps of HOMO−1 and LUMO+1 for NG 1b (I = 350 pA, Vrms = 15 mV, 3 × 3 nm2). | |
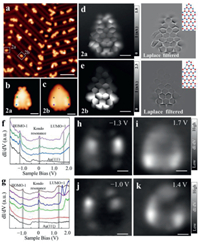
To engineer the NG magnetism, we co-deposited the precursor 1 and the precursor 2 onto the Au(111) substrate. After annealing process, two new products (NGs 2a and 2b) were observed, as shown in Fig. 3a. NGs 2a and 2b are marked by solid white rectangles (Fig. 3a). The difference between NGs 2a and 2b can be distinguished from zoom-in highly-resolved STM images (Figs. 3b and c). Two-lobe like local density state distribution shown in Fig. 3b corresponds to the triangular end of NG 2a, while the pentagonal defect terminus of NG 2b shows a faint feature (Fig. 3c). To gain bond-resolved images of NGs 2a and 2b, we performed high resolution STM characterizations by attaching a CO molecule at the apex of tip. Figs. 3d and e depict bond-resolved STM (BR-STM) images of NGs 2a and 2b, respectively, unambiguously confirming the defect-free feature of NG 2a and the pentagonal defect feature of NG 2b. Both BR-STM images show the feature with additional states, indicating the Kondo resonance enhanced state distributions [18,19]. Moreover, the BR-STM image for NG 2a shows the feature with additional state around whole structure, while only the bottom part of BR-STM image for NG 2b shows the feature with additional state. The difference means that the Kondo resonance distributions of NG 2a and NG 2b [35,42-44]. dI/dV spectra of NGs 2a and 2b shown in Figs. 3f and g shed light on the ZESs existence as well as energy positions of HOMO-1 and LUMO+1. The distributions of ZESs for NGs 2a and 2b fit well with the electronic-state enhanced area of BR-STM images in Figs. 3d and e. dI/dV maps at –1.3 V, and +1.7 V for NG 2a (Figs. 3 h and i) and –1.0 V, and +1.4 V for NG 2b (Figs. 3j and k) reveal spatial distributions of electronic states.
|
Download:
|
| Fig. 3. STM images and electronic structures of NGs 2a and 2b. (a) Large-scale STM image of NGs 2a and 2b on Au(111) (V = −1 V, I = 30 pA. 40 × 40 nm2). High-resolution STM images of NGs 2a (b) and 2b (c) (V = 2 mV, I = 200 pA. 3 × 3 nm2). BR-STM images (left panel) with CO functionalized tip for NG 2a (d) and 2b (e) and corresponding Laplace-filtered images (V = 2 mV, 2.5 × 2.5 nm2). dI/dV spectra of NGs 2a (f) and 2b (g) (V = –2.0 V, I = 350 pA). (h, i) Experimental dI/dV maps of HOMO-1 and LUMO+1 of NG 2a (I = 350 pA, Vrms = 15 mV). (j, k) Experimental dI/dV maps of HOMO-1 and LUMO+1 of NG 2b (I = 350 pA, 3 × 3 nm2). | |
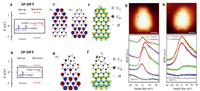
To further investigate the electronic structures and magnetic properties of NGs 2a and 2b, we performed SP-DFT calculations. Compared with antiferromagnetic (AFM), the NG 2a is further verified to be ferromagnetic (FM) as preferable energy (Table S2 in Supporting information). Figs. 4a and b present the SP-DFT calculated energy spectra of NGs 2a and 2b, which show single electron occupied frontier orbitals SOMOs and SUMOs of the non-degenerated energy level ψ2 and ψ3 of NG 2a and the energy level ψ2 of NG 2b respectively, triggering spin polarizations. According to the calculated energy level, magnetic ground states of NGs 2a and 2b are S = 1 and S = 1/2 respectively. The calculated spin-polarized wave functions of the SOMOs of NGs 2a and 2b are displayed in Figs. 4c and d, respectively. ψ2↑ and ψ3↑ of the NG 2a exhibit dominant localization at the top and the bottom terminus with slight overlap. ψ2↑ of the NG 2b only exhibit dominant localization at the bottom area. The spin density distribution of the triplet ground state of NG 2a shown in Fig. 4e is around the whole CB atoms, while the doublet ground state of NG 2b in Fig. 4f is around at the bottom CB atoms of the NG 2b. Furthermore, the calculated spin density distributions fit well with the experimental BR-STM results (Figs. 3d and e). Low-energy interval dI/dV spectra at the marked positions (Figs. 4g and h) reveal the existence of Kondo resonances. The distributions of Kondo resonance fit well with the calculate spin density state distributed area. The Kondo peak positions are slight away from Fermi level and the shapes are slightly asymmetric, which can be attributed to the charge transfer and the hybridization with the substrate [35,36,45]. As shown in Fig. 4g, compared with the terminal ends, the Kondo peak acquired in middle positions of NG 2a are positively shifted (Table S3 in Supporting information), which may indicate a stronger charge transfer area at the middle part of NG 2a.
|
Download:
|
| Fig. 4. SP-DFT calculated magnetic properties of NGs 2a and 2b. (a, b) SP-DFT calculated energy spectra of NGs 2a and 2b. DFT calculated spin-polarized wave functions of the SOMO of NG 2a (c) and 2b (d). SP-DFT simulated spin density distributions of NG 2a (e) and 2b (f). gray and black balls represent carbon atoms from two different sublattice. The spin density marked by cyan and yellow represent two different spin directions. Experimental low-energy ranges dI/dV spectra of the Kondo resonances detected in 2a (g) and 2b (h), with fitting using the Frota function [40]. | |
There are two obvious additional details, when taking a deeper analysis of the Kondo resonance by comparing NGs 2a and 2b. Firstly, the Kondo resonance acquired at both ends of NG 2a and around NG 2b shows obvious Kondo state difference, where NG 2a possesses smaller resonance amplitude and linewidth corresponding to the underscreen effect of S = 1 system [19,46] and NG 2b shows a complete wash out of the magnetic moment of S = 1/2. Secondly, the spatial variation of the Kondo peak when STS is measured at different lateral positions on the NG 2a which is different from a shorter NG 3 (Fig. S6 in Supporting information). Combining these Kondo resonance differences and calculated wave function results, we concluded two opening explanations for the NG 2a. As the longer distance of two spins compared with NG 3, they have negligible direct coupling leading the S = 1/2 Kondo feature [19,21] in the middle area and indirect spin coupling or exchanging at both ends of NG 2a leading the under screened Kondo feature. Another possibility is that Kondo peak differences are result from relative position differences corresponding to the spin center and underneath gold substrate [47].
We demonstrate an efficient synthetic strategy to engineer and enrich the magnetic NG products by co-depositing diverse precursors on the Au(111) substrate. Two homo-coupled products (NG 1a and NG 1b) acquired by Ullmann coupling and cyclodehydrogenation reactions of precursor 1 and two hetero-coupled products (NG 2a and NG 2b) co-deposited by precursor 1 and precursor 2 have been successfully fabricated. Defective NG 1b hosts spins of S = 1/2 resulting from a sublattice imbalanced carbon skeleton. The magnetism for defect-free NG 2a and defective NG 2b host spins of S = 1/2 and S = 1, respectively. Our work successfully realizes the magnetism engineering and enrichment by inducing another precursor and offers a valid opportunity via the co-deposition to extend the magnetism engineering of NG on metal substrate.
Declaration of competing interestThe authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 61901200), the National Recruitment Program for Young Professionals (No. 132310976002), the Yunnan Fundamental Research Projects (Nos. 2019FD041, 202101AV070008, 202101AW070010 and 202101AU070043), the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB30000000), and the Dongguan Innovation Research Team Program. The numerical calculations in this paper have been done on Hefei advanced computing center.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.04.048.
| [1] |
D.M. Edwards, M.I. Katsnelson, J. Phys. Condens. Matter. 18 (2006) 7209-7225. DOI:10.1088/0953-8984/18/31/016 |
| [2] |
O.V. Yazyev, Rep. Prog. Phys. 73 (2010) 056501. DOI:10.1088/0034-4885/73/5/056501 |
| [3] |
B. Trauzettel, D.V. Bulaev, D. Loss, G. Burkard, Nat. Phys. 3 (2007) 192-196. DOI:10.1038/nphys544 |
| [4] |
O.V. Yazyev, M.I. Katsnelson, Phys. Rev. Lett. 100 (2008) 047209. DOI:10.1103/PhysRevLett.100.047209 |
| [5] |
Y.W. Son, M.L. Cohen, S.G. Louie, Nature 444 (2006) 347-349. DOI:10.1038/nature05180 |
| [6] |
W. Han, R.K. Kawakami, M. Gmitra, J. Fabian, Nat. Nanotechnol. 9 (2014) 794-807. DOI:10.1038/nnano.2014.214 |
| [7] |
Y. Morita, S. Suzuki, K. Sato, T. Takui, Nat. Chem. 3 (2011) 197-204. DOI:10.1038/nchem.985 |
| [8] |
W. Zeng, J. Wu, Chem 7 (2021) 358-386. DOI:10.1016/j.chempr.2020.10.009 |
| [9] |
D. Small, V. Zaitsev, Y. Jung, et al., J. Am. Chem. Soc. 126 (2004) 13850-13858. DOI:10.1021/ja046770i |
| [10] |
K. Goto, T. Kubo, K. Yamamoto, et al., J. Am. Chem. Soc. 121 (1999) 1619-1620. DOI:10.1021/ja9836242 |
| [11] |
J. Cai, P. Ruffieux, R. Jaafar, et al., Nature 466 (2010) 470-473. DOI:10.1038/nature09211 |
| [12] |
J. Li, S. Sanz, M. Corso, et al., Nat. Commun. 10 (2019) 1-10. DOI:10.1038/s41467-018-07882-8 |
| [13] |
J. Jung, A.H. MacDonald, Phys. Rev. B 79 (2009) 235433. DOI:10.1103/PhysRevB.79.235433 |
| [14] |
K. Nakada, M. Fujita, G. Dresselhaus, M.S. Dresselhaus, Phys. Rev. B 54 (1996) 17954-17961. DOI:10.1103/PhysRevB.54.17954 |
| [15] |
J. Fernandez-Rossier, J.J. Palacios, Phys. Rev. Lett. 99 (2007) 177204. DOI:10.1103/PhysRevLett.99.177204 |
| [16] |
N. Pavlicek, A. Mistry, Z. Majzik, et al., Nat. Nanotechnol. 12 (2017) 308-311. DOI:10.1038/nnano.2016.305 |
| [17] |
S. Mishra, D. Beyer, K. Eimre, et al., J. Am. Chem. Soc. 141 (2019) 10621-10625. DOI:10.1021/jacs.9b05319 |
| [18] |
S. Mishra, D. Beyer, R. Berger, et al., J. Am. Chem. Soc. 142 (2020) 1147-1152. DOI:10.1021/jacs.9b09212 |
| [19] |
J. Li, S. Sanz, J. Castro-Esteban, et al., Phys. Rev. Lett. 124 (2020) 177201. DOI:10.1103/PhysRevLett.124.177201 |
| [20] |
E.H. Lieb, Phys. Rev. Lett. 62 (1989) 1201-1204. DOI:10.1103/PhysRevLett.62.1201 |
| [21] |
S. Mishra, D. Beyer, K. Eimre, et al., Nat. Nanotechnol. 15 (2020) 22-28. DOI:10.1038/s41565-019-0577-9 |
| [22] |
E. Turco, S. Mishra, J. Melidonie, et al., J. Phys. Chem. Lett. 12 (2021) 8314-8319. DOI:10.1021/acs.jpclett.1c02381 |
| [23] |
S. Mishra, X. Yao, Q. Chen, et al., Nat. Chem. 13 (2021) 581-586. DOI:10.1038/s41557-021-00678-2 |
| [24] |
S. Mishra, J. Melidonie, K. Eimre, et al., Chem. Commun. 56 (2020) 7467-7470. DOI:10.1039/D0CC02513E |
| [25] |
J.I. Urgel, S. Mishra, H. Hayashi, et al., Nat.Commun. 10 (2019) 1-9. DOI:10.1038/s41467-018-07882-8 |
| [26] |
C. Tönshoff, H.F. Bettinger, Angew. Chem. Int. Ed. 49 (2010) 4125-4128. DOI:10.1002/anie.200906355 |
| [27] |
A. Konishi, K. Horii, D. Shiomi, et al., J. Am. Chem. Soc. 141 (2019) 10165-10170. DOI:10.1021/jacs.9b04080 |
| [28] |
S. Mishra, T.G. Lohr, C.A. Pignedoli, et al., ACS Nano 12 (2018) 11917-11927. DOI:10.1021/acsnano.8b07225 |
| [29] |
A. Sánchez-Grande, J.I. Urgel, L. Veis, et al., J. Phys. Chem. Lett. 12 (2021) 330-336. DOI:10.1021/acs.jpclett.0c02518 |
| [30] |
X. Su, C. Li, Q. Du, et al., Nano Lett. 20 (2020) 6859-6864. DOI:10.1021/acs.nanolett.0c02939 |
| [31] |
J. Su, M. Telychko, P. Hu, et al., Sci. Adv. 5 (2019) eaav7717. DOI:10.1126/sciadv.aav7717 |
| [32] |
J. Su, W. Fan, P. Mutombo, et al., Nano Lett. 21 (2020) 861-867. DOI:10.1021/acs.nanolett.0c04627 |
| [33] |
S. Mishra, K. Xu, K. Eimre, et al., Nanoscale 13 (2021) 1624-1628. DOI:10.1039/D0NR08181G |
| [34] |
J. van der Lit, M.P. Boneschanscher, D. Vanmaekelbergh, et al., Nat. Commun. 4 (2013) 2023. DOI:10.1038/ncomms3023 |
| [35] |
Y. Zhao, K. Jiang, C. Li, et al., J. Am. Chem. Soc. 142 (2020) 18532-18540. DOI:10.1021/jacs.0c07791 |
| [36] |
Q. Sun, L.M. Mateo, R. Robles, et al., J. Am. Chem. Soc. 142 (2020) 18109-18117. DOI:10.1021/jacs.0c07781 |
| [37] |
A. Kumar, K. Banerjee, M. Dvorak, et al., ACS Nano 11 (2017) 4960-4968. DOI:10.1021/acsnano.7b01599 |
| [38] |
N. Friedrich, P. Brandimarte, J. Li, et al., Phys. Rev. Lett. 125 (2020) 146801. DOI:10.1103/PhysRevLett.125.146801 |
| [39] |
H. Lee, M. Jo, G. Yang, et al., Dyes Pigm. 146 (2017) 27-36. DOI:10.1016/j.dyepig.2017.06.053 |
| [40] |
H.O. Frota, Phys. Rev. B 45 (1992) 1096-1099. DOI:10.1103/PhysRevB.45.1096 |
| [41] |
M. Ternes, A.J. Heinrich, W.D. Schneider, J. Phys. Condens. Matter. 21 (2009) 053001. DOI:10.1088/0953-8984/21/5/053001 |
| [42] |
Y. Zheng, C. Li, C. Xu, et al., Nat. Commun. 11 (2020) 6076. DOI:10.1038/s41467-020-19834-2 |
| [43] |
Y.Q. Zheng, C. Li, Y. Zhao, et al., Phys. Rev. Lett. 124 (2020) 147206. DOI:10.1103/PhysRevLett.124.147206 |
| [44] |
S. Mishra, G. Catarina, F. Wu, et al., Nature 598 (2021) 287-292. DOI:10.1038/s41586-021-03842-3 |
| [45] |
M. Ternes, New J. Phys. 17 (2015) 063016. DOI:10.1088/1367-2630/17/6/063016 |
| [46] |
W.H. Soe, R. Robles, P. de Mendoza, et al., Nano Lett. 21 (2021) 8317-8323. DOI:10.1021/acs.nanolett.1c02881 |
| [47] |
D. Jacob, R. Ortiz, J. FernȢndez-Rossier, Phys. Rev. B 104 (2021) 075404. DOI:10.1103/PhysRevB.104.075404 |
 2023, Vol. 34
2023, Vol. 34 

