b School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China
β-Mannopyranosyl units are important components of numerous natural bioactive products. High stereoselective β-mannopyranosylation has been considered one of the most challenging problem in the field of glycochemistry, due to the anomeric effect and steric hindrance from the 1,2-cis configuration [1, 2]. In the past several years, a variety of strategies have been reported to solve this problem, including intramolecular aglycon delivery (IAD) [3, 4], hydrogen-bonding aglycone delivery (HAD) [5, 6], 4,6-tethered strategy [7-11], C-5 carboxylate strategy [12], 2,6-lactone-bridged methods [13, 14] and other approaches [15-19]. Among these methods, the 4,6-O-benzylidene tethered strategy and C-5 carboxylate strategy have been widely used in the synthesis of natural products and oligosaccharides containing β-mannanoside [20-24]. In this paper, we reported a catalytic, efficient and concise strategy to construct β-mannosidic linkages with a donor which could be readily prepared.
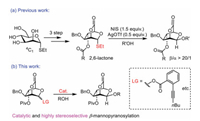
In 2019, a novel protocol for stereoselective construction of β-mannosides was developed by our group using a 2,6-lactone-bridged thiomannosyl donor through the remote acyl-group participation as well as the steric effect of O-4 substituent (Fig. 1a) [14]. In that paper, we demonstrated that 2,6-lactone-bridged mannosyl imidate donors were difficult to be prepared in large scale, due to the sensitivity of the bridged lactone to bases and therefore the formation of a tricyclic product containing 1,3-dioxolane ring via intramolecular reaction. Thus, thioglycosyl donors were used for the stereoselective β-mannosylation. However, stoichiometric NIS (N-iodosuccinimide) and subcatalytic amount of AgOTf are required to activate the thioglycosyl donor due to decreased activity of the bridged lactone motif. Considering that the 2,6-lactone-bridged mannosyl donor possess superiority in high stereoselective β-mannosylation, we envision that the glycosylation of 2,6-lactone-bridged mannosyl donor with proper leaving group could realize mild, catalytic and high β-stereoselective mannosylation (Fig. 1b). Given that the alkynoates [25-27], especially the ortho-alkynylbenzoate developed by Yu and co-workers [26, 27] have been widely used in glycosylation in recent years, we explore 2,6-lactone-bridged mannosyl donors with an alkynoate as leaving group for β-mannopyranosylation (Fig. 1).
|
Download:
|
| Fig. 1. 2,6-Lactone-bridged mannopyranosyl donor for glycosylation. | |
The glycosyl donors were prepared on the base of our reported work [14]. The building block 1 was first prepared through 3 steps from mannose (Scheme 1). Then, the hydroxyl group at the C-4 was protected with bulky Piv group for its best performance in remote participation in our previous work [14]. After treatment of compound 2 with Pd(PPh3)4 in AcOH, the hemiacetals 3 was afforded. And then, the 2,6-lactone-bridged mannosyl alkynoates 4-1 and 4-2 were readily afford by one-step esterification of 3 with the corresponding acyl chlorides in about 90% yield.
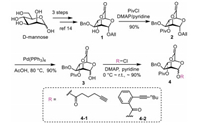
|
Download:
|
| Scheme 1. Preparation of glycosyl donor 4-1 and 4-2. | |
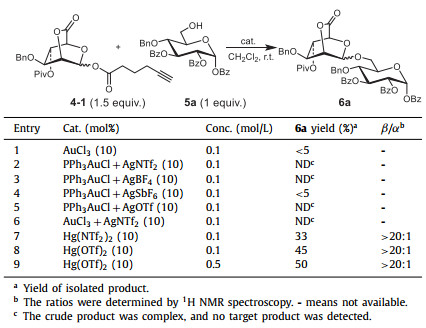
With the above glycosyl donors in hand, we initiated our studies by investigating the coupling reaction between 4-1 and acceptor 5a (Table 1). At first, the reaction was optimized in the presence of the Au(Ⅰ) or Au(Ⅲ) which are typically used to activate alkynyl group in organic synthesis [28] and glycosylation [27]. Unfortunately, only minor or even no product was afforded (Table 1, entries 1–6). Considering that Hg(Ⅱ) catalysts have been successfully explored to catalyze n–pentenyl-type glycosides for glycosylation in our previous work [29], Hg(Ⅱ) catalysts were thus examined in this work (Table 1, entries 7 and 8). The results showed that both Hg(NTf2)2 and Hg(OTf)2 can catalyze the reaction with high β-selectivity and Hg(OTf)2 (Table 1, entry 8) exhibited better catalytic activity than Hg(NTf2)2 (Table 1, entry 7). However, owing to incomplete consumption of acceptor 5a in the presence of both Hg(NTf2)2 and Hg(OTf)2, the glycosylation provided the corresponding product only in 33% and 45% yield respectively. When 10% Hg(OTf)2 was used and the reaction concentration was increased from 0.1 to 0.5 mol/L, no obvious improvement was observed (Table 1, entry 9). It's speculated that the strong electron-withdrawing character of bridged lactone may decrease the activation of the glycosyl donor. Thus, Yu's ortho-alkynylbenzoate method [26, 27] was chosen for the next investigation.
|
|
Table 1 Optimization of reaction conditions for glycosylations of donor 4-1 with acceptors 5a. |
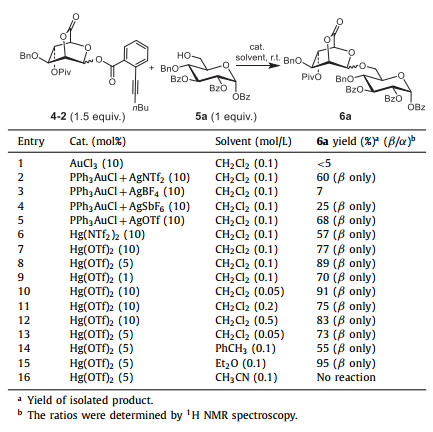
Initially, coupling reaction between ortho-alkynylbenzoate donor 4-2 and acceptor 5a was conducted using the typical Au catalyst as promotor. Gratifyingly, 6a with exclusive stereoselectivities was afforded when the reaction was catalyzed by Ph3PAuCl (0.1 equiv.) and AgNTf2 (or AgSbF6) (0.1 equiv.) (Table 2, entries 2 and 4). However, the acceptor 5a was not yet completely consumed, leading to the formation of the glycosyl product in 60% yield for AgNTf2 and in 25% yield for AgSbF6 respectively. Furthermore, when Ph3PAuCl (0.1 equiv.) and AgOTf (0.1 equiv.) (Table 2, entry 5) was used to calalyze the reaction, the yield for the target disaccharide was only increased to 68%. According to the aforementioned results, Hg(Ⅱ) (Table 1, entries 7–9) exhibited better activity than Au(Ⅰ) or Au(Ⅲ) (Table 1, entries 1–6) for the activation of the alkynoate. Therefore, Hg(NTf2)2 and Hg(OTf)2 were applied to catalyze the reaction (Table 2, entries 6 and 7). Again, Hg(OTf)2 displayed better catalytic activity in comparison with Hg(NTf2)2, and 77% yield was afforded when the reaction was conducted in CH2Cl2 at concentration of 0.1 mol/L (Table 2, entry 7). Thus, Hg(OTf)2 was considered as the optimal catalyst for the further studies of glycosyl condition. Then, the amount of catalyst, reaction concentration and solvents were screened for the optimal glycosylation condition. It was shown that 89% yield was obtained for using 5% catalyst (Table 2, entry 8) at a reaction concentration of 0.1 mol/L, and 91% yield for using 10% catalyst (Table 2, entry 10) at a reaction concentration of 0.05 mol/L. To reduce the usage of Hg(Ⅱ), the amount of catalyst was determined as 5% for the following studies. At last, different solvents (including toluene, ether, and acetonitrile, Table 2, entries 14–16) were also examined, and the yield was further increased to 95% when using Et2O as the solvent (Table 2, entry 15). Generally, Et2O was considered to be helpful for construction of α-configuration glycosides. However, the selectivity was not influenced in this work, demonstrating the strong tendency to β-configuration of 2,6-lactone-bridged mannosyl donor.
|
|
Table 2 Optimization of reaction conditions for glycosylations of donor 4-2 with acceptors 5a. |
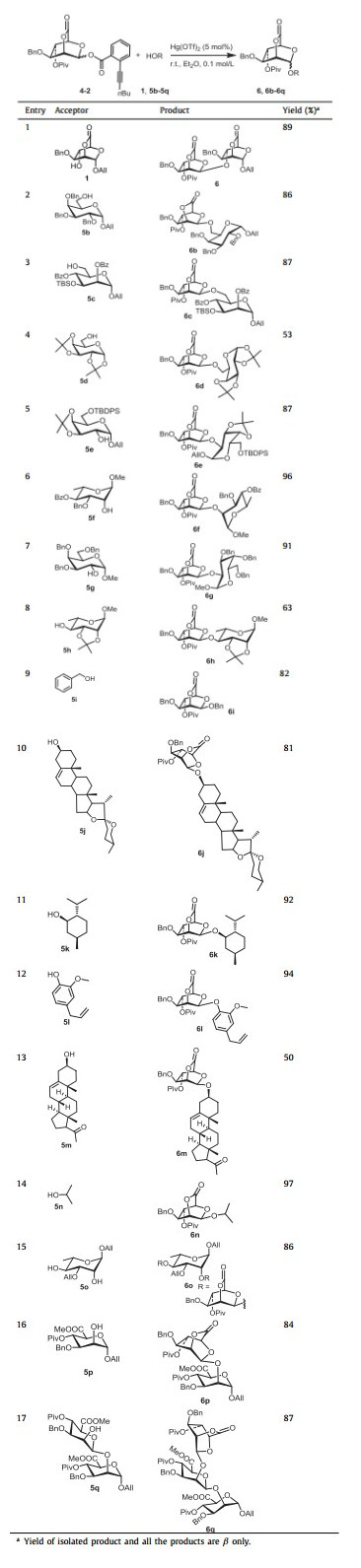
With the optimized condition in hand, the scope of this reaction was then explored. With 4-2 as the donor, glycosylation with primary and secondary hydroxyl acceptors was conducted. To our delight, the glycosylation of 4-2 with all acceptors proceeded smoothly, providing the corresponding glycosides with β-configuration in good yield (Table 3). Although it was reported that acceptor with less reactivity usually led to increase in α-selectivity [31, 32], the highly stereoselective β-mannopyranosylation performance remained, when the alcohol with less nucleophilicity (represented by 1) was used as acceptor. Interestingly, when acceptor 5o with two free hydroxyl groups was used, the catalytic dose (5% Hg(OTf)2) was still sufficient to ensure the smooth glycosylation (Table 3, entry 15), indicating the strong efficacy of Hg(OTf)2 for activating ortho-hexynylbenzoate. In addition, the glycosylation condition was also suitable for saponins (Table 3, entry 10), demonstrating the potential of this protocol for the synthesis of β-mannosyl saponins. However, when the acid-sensitive protecting group ketal was used (in 5d, 5e, 5h), the corresponding yield (Table 3, entries 4, 5, 8) was slightly affected due to the Lewis acidic character of Hg(Ⅱ) catalysts. It should be emphasized, β−1,6-, β−1,4-linked mannobioses units, which are enormously existed in nature [30], can be readily constructed in high yield (Table 3, entries 1 and 3). More importantly, the lactone can be feasibly attacked by base with high nucleophilicity (CH3COO−Na+) and the resulting product (such as 5p, 5q) with free hydroxyl groups at C-2 can be used as acceptor for further glycosylation. Thus, the β−1,2-linked oligomannosyl moiety, which is an important component of the cell wall of Candida albicans [33], could be readily constructed as 6p and 6q (Table 3, entries 16 and 17). After several manipulations (including reduction and deprotection) of 6p and 6q, the resultant products could be used as antigens for investigation of anti-bacterial vaccine. All of those results demonstrated that our strategy has a wide range of substrate applicability.
|
|
Table 3 Glycosylations of donor 4-2 with acceptors 1 and 5b-5q. |
In conclusion, a mild and catalytic method for highly stereoselective β-mannosylation of various alcohols with a 2,6-lactone-bridged mannopyranosyl ortho-hexynylbenzoate as donor has been developed. The reaction proceeded smoothly to give the corresponding product in high yield with exclusive stereoselectivities, demonstrating its potential application in the synthesis of natural products containing β-mannosyl structural unit.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21472119, 22107061), the Science and Technology Program of Shaanxi Province, China (No. 2018ZDXM-GY-152). All of the financial supports are gratefully acknowledged.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.071.
| [1] |
K. Sasaki, K. Tohda, Tetrahedron Lett. 59 (2018) 496-503. DOI:10.1016/j.tetlet.2017.12.077 |
| [2] |
S.S. Nigudkar, A.V. Demchenko, Chem. Sci. 6 (2015) 2687-2704. DOI:10.1039/C5SC00280J |
| [3] |
G. Stork, G. Kim, J. Am. Chem. Soc. 114 (1992) 1087-1088. DOI:10.1021/ja00029a047 |
| [4] |
A.A.H. Abdel-Rahman, E.S.H. El Ashry, R.R. Schmidt, Carbohydr. Res. 337 (2002) 195-206. DOI:10.1016/S0008-6215(01)00306-8 |
| [5] |
F. Ding, A. Ishiwata, Y. Ito, Org. Lett. 20 (2018) 4833-4837. DOI:10.1021/acs.orglett.8b01979 |
| [6] |
S.G. Pistorio, J.P. Yasomanee, A.V. Demchenko, Org. Lett. 16 (2014) 716-719. DOI:10.1021/ol403396j |
| [7] |
P. Sun, P. Wang, Y. Zhang, et al., J.Org. Chem. 80 (2015) 4164-4175. DOI:10.1021/acs.joc.5b00140 |
| [8] |
A. Gucchait, A.K. Misra, Org. Biomol. Chem. 17 (2019) 4605-4610. DOI:10.1039/C9OB00670B |
| [9] |
Y. Zhu, B. Yu, Chem. Eur. J. 21 (2015) 8771-8780. DOI:10.1002/chem.201500648 |
| [10] |
M. Heuckendorff, J. Bendix, C.M. Pedersen, M. Bols, Org. Lett. 16 (2014) 1116-1119. DOI:10.1021/ol403722f |
| [11] |
D. Crich, S. Sun, J. Org. Chem. 61 (1996) 4506-4507. DOI:10.1021/jo9606517 |
| [12] |
L.J. van den Bos, J. Dinkelaar, H.S. Overkleeft, G.A. van der Marel, J. Am. Chem. Soc. 128 (2006) 13066-13067. DOI:10.1021/ja064787q |
| [13] |
Y. Hashimoto, S. Tanikawa, R. Saito, K. Sasaki, J. Am. Chem. Soc. 138 (2016) 14840-14843. DOI:10.1021/jacs.6b08874 |
| [14] |
H. Xu, L. Chen, Q. Zhang, et al., Chem. Asian J. 14 (2019) 1424-1428. DOI:10.1002/asia.201801740 |
| [15] |
H. Elferink, R.A. Mensink, P.B. White, T.J. Boltje, Angew. Chem. Int. Ed. 55 (2016) 11217-11220. DOI:10.1002/anie.201604358 |
| [16] |
M. Tanaka, J. Nashida, D. Takahashi, K. Toshima, Org. Lett. 18 (2016) 2288-2291. DOI:10.1021/acs.orglett.6b00926 |
| [17] |
N. Nishi, J. Nashida, E. Kaji, D. Takahashi, K. Toshima, Chem. Commun. 53 (2017) 3018-3021. DOI:10.1039/C7CC00269F |
| [18] |
G. Hodosi, P. Kováč, J. Am. Chem. Soc. 119 (1997) 2335-2336. DOI:10.1021/ja964021y |
| [19] |
H. Nguyen, D. Zhu, X. Li, J. Zhu, Angew. Chem. Int. Ed. 55 (2016) 4767-4771. DOI:10.1002/anie.201600488 |
| [20] |
K. Ohara, C.C. Lin, P.J. Yang, et al., J. Org. Chem. 78 (2013) 6390-6411. DOI:10.1021/jo4005266 |
| [21] |
D. Crich, A. Banerjee, Q. Yao, J. Am. Chem. Soc. 126 (2004) 14930-14934. DOI:10.1021/ja047194t |
| [22] |
S.L. Tang, N.L. Pohl, Org. Lett. 17 (2015) 2642-2645. DOI:10.1021/acs.orglett.5b01013 |
| [23] |
M.T. Walvoort, H. van den Elst, O.J. Plante, et al., Angew. Chem. Int. Ed. 51 (2012) 4393-4396. DOI:10.1002/anie.201108744 |
| [24] |
D. Pan, L. Zhang, Q. Hua, Y. Yang, Org. Biomol. Chem. 17 (2019) 6174-6177. DOI:10.1039/C9OB01254K |
| [25] |
H. Imagawa, A. Kinoshita, T. Fukuyama, H. Yamamoto, M. Nishizawa, Tetrahedron Lett. 47 (2006) 4729-4731. DOI:10.1016/j.tetlet.2006.04.114 |
| [26] |
Y. Li, Y. Yang, B. Yu, Tetrahedron Lett. 49 (2008) 3604-3608. DOI:10.1016/j.tetlet.2008.04.017 |
| [27] |
W. Li, B. Yu, Chem. Soc. Rev. 47 (2018) 7954-7984. DOI:10.1039/C8CS00209F |
| [28] |
Z. Lu, T. Li, S.R. Mudshinge, B. Xu, G.B. Hammond, Chem. Rev. 121 (2021) 8452-8477. DOI:10.1021/acs.chemrev.0c00713 |
| [29] |
Y. Zu, C. Cai, J. Sheng, et al., Org. Lett. 21 (2019) 8270-8274. DOI:10.1021/acs.orglett.9b03038 |
| [30] |
M. Yamabhai, S. Sak-Ubol, W. Srila, D. Haltrich, Crit. Rev. Biotechnol. 36 (2016) 32-42. DOI:10.3109/07388551.2014.923372 |
| [31] |
S. van der Vorm, T. Hansen, J.M.A. van Hengst, et al., Chem. Soc. Rev. 48 (2019) 4688-4706. DOI:10.1039/C8CS00369F |
| [32] |
C.W. Chang, M.H. Lin, C.K. Chan, et al., Angew. Chem. Int. Ed. 60 (2021) 12413-12423. DOI:10.1002/anie.202013909 |
| [33] |
N. Shibata, Y. Okawa, Chem. Pharm. Bull. 58 (2010) 1386-1390. DOI:10.1248/cpb.58.1386 |
 2022, Vol. 33
2022, Vol. 33