b Affiliated Hospital of Guilin Medical University, Guilin 541001, China
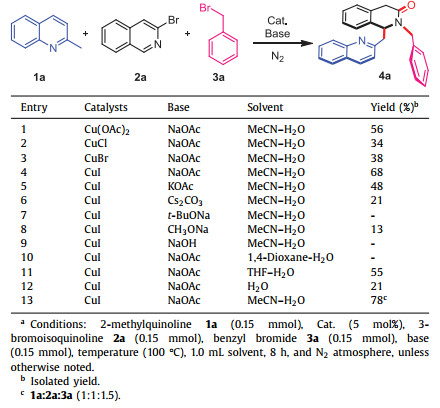
The isoquinolinone skeleton is the core structure present in many functional compounds having various biological activities [1]. For example, Gliquidone A (Fig. 1) is an oral antidiabetic drug for treating type 2 diabetes [2], while Minalrestat B [3] and isoquinoline-1, 3-diones C [4] are used as aldose reductase and tyrosyl DNA phosphodiesterase Ⅱ inhibitors, respectively. In addition, compounds D and E are potent inhibitors of HIV-1 integrase [5, 6] and MDM2 [7, 8], respectively (Fig. 1).
|
Download:
|
| Fig. 1. Examples of bioactive isoquinolinone molecules. | |
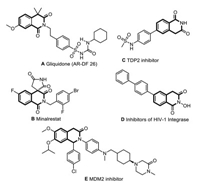
Because of the interesting biological activities of these compounds, considerable efforts have been devoted to synthesizing isoquinolinone scaffolds with different substitution patterns. For example, intermolecular cyclization [9-13] and intramolecular cyclization [14, 15] have been applied to construct the tetrahydroisoquinolinone skeleton. In addition, the strategies of tetrahydroisoquinoline ring site functional group modification [16-18] and isoquinoline dearomatisation have attracted the interest of many organic chemists due to their high site selectivity. For isoquinoline dearomatisation, oxidizing isoquinolinium salts has emerged as an exceptionally reliable and direct strategy for synthesising isoquinolinone derivatives. In this regard, the excellent examples reported by He [19], Fu [20], Huang [21], Peng [22] and Luo [23] involved the C1 carbonylation of isoquinoline (Scheme 1a). Dearomatisation of isoquinolines often occurs at the C1 position upon adding different nucleophiles, and such as silyl phosphites [24], indole [25], phenylacetylene [26], ethers [27]. However, realisation of one-pot functionalization and carbonylation at C1 and C3 positions, respectively, of 3-haloisoquinoline is difficult.
|
Download:
|
| Scheme 1. Functionalization of isoquinolines. | |
2-Alkylquinolines are widely available important biological compounds that can be applied as a drug or candidate drug in medicinal chemistry research [28-30]. Many researchers have successfully introduced 2-alkylquinolines in organic molecules to obtain relevant bioactive molecules [21-33]. However, the direct introduction of 2-alkylquinoline structure is challenging because activating the sp3C–H bond is difficult [34-37]. As a part of our continuing research on the construction of functional N-heterocycles [38-40], herein we report a multi-component coupling method that involves one-pot construction of new C–N, C–C and C=O bonds to synthesize isoquinolinone skeletons bearing 2-methylquinoline groups in one pot (Scheme 1b).
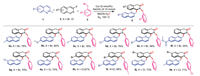
In this study, we initially focused on developing a more efficient catalyst system for combining 2-methylquinoline 1a, 3-bromoisoquinoline 2a, and benzyl bromide 3a as a model reaction (Table 1). First, we tested different copper catalysts in MeCN–H2O solvent under nitrogen atmosphere at 100 ℃ for 8 h. CuI showed the best catalytic effect, with a 68% yield of product 4a (entries 1–4). In the screening of various bases, NaOAc demonstrated the best results (entries 4–9). Moreover, efficiency of the target reaction was significantly dependent on the solvent used. Thus, solvent screening was performed (entries 10–12), the results confirming that MeCN–H2O (6:1, v/v, entry 13) showed the best results.
|
|
Table 1 Screening conditions.a |
After determining the optimal conditions, we first explored the range of N-heteroarene substrates. It is established that 2-methylquinolines bearing different substituent groups can easily react with 3-haloisoquinolines (mainly bromo and chloro analogues); the results are summarized in Scheme 2. 2-Methylquinolines with different substituents, including electron-donating (-Me and -OMe) and electron-withdrawing groups (-F, -Cl, -Br and -CO2Me), were successfully transformed into isoquinolinone derivatives 5 (5a–5g) in moderate to high yields. Remarkably, this catalytic system is also suitable for converting 3-chloro-isoquinoline, providing various isoquinolinones in good yields.
|
Download:
|
| Scheme 2. Substrate scope of N-heteroaromatics. Standard conditions: 2-methylquinolines 1 (0.15 mmol), 3-haloisoquinolines 2 (0.15 mmol) benzyl bromide 3 (0.225 mmol), CuI (5 mol%), NaOAc (0.15 mmol) and MeCN-H2O (6:1, v/v) at 100 ℃ for 8 h under N2 atmosphere. | |
Next, we studied the scope and applicability of alkyl halides (Scheme 3). Various benzyl bromides bearing electron-withdrawing and electron-donating groups reacted easily, furnishing isoquinolinones 6a–6n in moderate to good isolated yields. In addition, benzyl chloride was found to be suitable for participating in this reaction and affording isoquinolinones in a higher yield (4a). The yields obtained from benzyl bromides with electron-donating groups (-Me and -OMe) were higher than those from benzyl bromides with electron-withdrawing groups (-NO2 and -CF3). The structure of 6d was determined by single-crystal X-ray diffraction analysis. Furthermore, a variety of disubstituted benzyl bromides (3,5-OMe2 and 2,4-F2) were investigated to expand the scope of this method (6k and 6l), which can be converted to 1-substituted-3-isoquinolinones under standard conditions. Interestingly, 2-bromomethylnaphthalene can also participate in the reaction to furnish the target product in a moderate yield of 68% (6m).
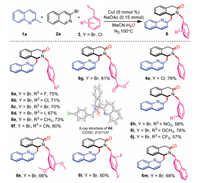
|
Download:
|
| Scheme 3. Substrate scope of alkyl halides. Standard conditions: 2-methylquinoline 1a (0.15 mmol), 3-bromoisoquinoline 2a (0.15 mmol.), alkyl halides 3 (0.225 mmol), CuI (5 mol%), NaOAc (0.15 mmol), and MeCN–H2O (6:1, v/v) at 100 ℃ for 8 h under N2 atmosphere. | |
Additionally, to prove the potential application of the method described herein, we determined that different substituted phenylacetylenes, 3-bromoisoquinoline, and benzyl bromide could afford 1-alkynyl-3-isoquinolinone in one pot under standard conditions (Scheme 4a). This synthetic method can also be applied to the later functionalization of complex molecules. For example, the 5-(4′-(bromomethyl)-[1,1′-biphenyl]-2-yl)-1-trityl-1H (BrOTBN) tetrazole is a crucial intermediate for synthesising the antihypertensive drug losartan [41, 42]. We employed BrOTBN as the reaction substrate to form the C–N bond to construct a new nitrogen heterocycle by the one-pot three-component method (Scheme 4b).

|
Download:
|
| Scheme 4. Potential application of the developed method. | |
Several control experiments were conducted (Scheme 5) to study the mechanism of this reaction. The model reaction was interrupted after 3 h to analyze the intermediates formed. Product 4a, 2a-1, and 2a' were detected in 52%, 8%, and 0% yields, respectively (Scheme 5a). The reaction of 2a-1 with 2-methylquinoline 1a under the standard conditions did not give product 4a (Scheme 5b). When 3-bromoisoquinoline and isoquinoline salts reacted with 2-methylquinoline, respectively the isoquinoline salt did not react, thereby confirming the crucial role played by 3-bromo group in the initiation of this reaction (Schemes 5c and d). H2O labelling experiments were also performed to prove the role of water in the reaction. In the D2O labelling experiment, deuterium was incorporated into the tetrahydroisoquinoline unit to produce 4a-d, indicating the transfer of the D atom from D2O to isoquinoline (Scheme 5e). In addition, the proportion of 18O-labelled product 4a-18O increased when H216O was replaced with 18O-labelled water for the reaction, as determined by high-resolution mass spectrometry (Scheme 5f). This observation indicated that the oxygen atoms in isoquinolinone were mainly derived from H2O. However, no significant decrease in yield was observed when 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) was added to the reaction system. Hence, it was concluded that no radical-mediated process was involved.
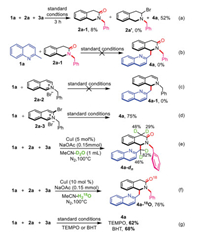
|
Download:
|
| Scheme 5. Control experiments. | |
Thus, based on the above-mentioned results, a reasonable mechanism was proposed (Scheme 6). Initially, 3-bromoisoquinoline reacts with benzyl bromide to form N-alkylisoquinoline salt 2a-3. Under the action of OAc−, water forms HOAc and OH−; nucleophilic attack of the hydroxide ion on 2a-3 removes Br to form intermediate 2a-4, which is isomerized to a more stable intermediate 2a-5. Finally, 2-methylquinoline isomerizes nucleophilic enamine under alkaline conditions [43-45] and undergoes nucleophilic addition with 2a-5 to obtain the target product 4a.

|
Download:
|
| Scheme 6. Plausible reaction pathway. | |
In summary, we developed an efficient C(sp3)–C(sp2) bond formation method involving the cross-coupling of 2-methylquinolines and in-situ-activated isoquinolines to construct 1,2-disubstituted isoquinolinone derivatives under mild conditions. This multi-component tandem reaction involves constructing new C–N, C=O and C–C bonds in one pot. This method employs an in-situ activation strategy to achieve the trifunctionalisation of 3-haloisoquinoline, which has the advantages of wide substrate adaptability, high atom efficiency, and chemoselectivity.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the Foundation of the National Natural Science Foundation of China (No. 22101212), "Climbing Program" (No. pdjh2021a0505) Special Funds, Science Foundation for Young Teachers of Wuyi University (No. 2019td06), Guangdong Basic and Applied Basic Research Foundation (Nos. 2019A1515110866, 2019A1515110522), and College Students Innovation and Entrepreneurship Training Program of Wuyi University (Nos. 202111349020, 202111349308S) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.032.
| [1] |
B.D. Krane, M. Shamma, J. Nat. Prod. 45 (1982) 377-384. DOI:10.1021/np50022a001 |
| [2] |
L.N. Jia, Y. Yu, M.W Wang, et al., CrystEngComm 21 (2019) 1617-1625. DOI:10.1039/C8CE01848K |
| [3] |
C. Cheng, B. Wan, B. Zhou, et al., Chem. Sci. 10 (2019) 9853-9858. DOI:10.1039/C9SC03406D |
| [4] |
J. kankanala, C. Marchand, M. Abdelmalak, et al., J. Med. Chem. 59 (2016) 2734-2746. DOI:10.1021/acs.jmedchem.5b01973 |
| [5] |
S.K.V. Vernekar, Z. Liu, E. Nagy, et al., J. Med. Chem. 58 (2015) 651-664. DOI:10.1021/jm501132s |
| [6] |
M. Billamboz, F. Bailly, C. Lion, et al., J. Med. Chem. 54 (2011) 1812-1824. DOI:10.1021/jm1014692 |
| [7] |
P. Holzer, K. Masuya, P. Furet, et al., J. Med. Chem. 58 (2015) 6348-6358. DOI:10.1021/acs.jmedchem.5b00810 |
| [8] |
F. Gessier, J. Kallen, E. Jacoby, et al., Bioorg. Med. Chem. Lett. 25 (2015) 3621-3625. DOI:10.1016/j.bmcl.2015.06.058 |
| [9] |
B. Su, M. Deng, Q. Wang, Adv. Synth. Catal. 356 (2014) 977-981. DOI:10.1002/adsc.201300832 |
| [10] |
L. Li, B. Zhou, Y.H. Wang, et al., Angew. Chem. Int. Ed. 54 (2015) 8245-8249. DOI:10.1002/anie.201502553 |
| [11] |
Y. Hu, Z.Q. Shen, H.M. Huang, ACS Catal. 6 (2016) 6785-6789. DOI:10.1021/acscatal.6b01939 |
| [12] |
M. Spallarossa, L. Banfi, A. Basso, et al., Adv. Synth. Catal. 358 (2016) 2940-2948. DOI:10.1002/adsc.201600638 |
| [13] |
D. Kaiser, A. de la Torre, S. Shaaban, et al., Angew. Chem. Int. Ed. 56 (2017) 5921-5925. DOI:10.1002/anie.201701538 |
| [14] |
W. Zhou, Y.X. Zhang, X.D. Nie, et al., J. Org. Chem. 83 (2018) 9879-9889. DOI:10.1021/acs.joc.8b01282 |
| [15] |
L.Q. Lin, S. Fukagawa, D. Sekine, et al., Angew. Chem. Int. Ed. 57 (2018) 12048-12052. DOI:10.1002/anie.201807610 |
| [16] |
Y.Y. Yang, Y.X. Li, C. Cheng, et al., J. Org. Chem. 83 (2018) 3348-3353. DOI:10.1021/acs.joc.7b02961 |
| [17] |
Z. Li, D.S. Bohle, C.J. Li, Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 8928-8933. DOI:10.1073/pnas.0601687103 |
| [18] |
Z. Li, C.J. Li, J. Am. Chem. Soc. 127 (2005) 3672-3673. DOI:10.1021/ja050058j |
| [19] |
L.Y. Xie, Y. Duan, L.H. Lu, et al., ACS Sustain. Chem. Eng. 5 (2017) 10407-10412. DOI:10.1021/acssuschemeng.7b02442 |
| [20] |
Y.H. Jin, L.Y. Ou, H.J. Yang, et al., J. Am. Chem. Soc. 139 (2017) 14237-14243. DOI:10.1021/jacs.7b07883 |
| [21] |
G.J. Wang, W.Y. Hu, Z.L. Hu, et al., Green Chem. 20 (2018) 3302-3307. DOI:10.1039/C8GC01488D |
| [22] |
Y.K. Zhou, W. Liu, Z.M. Xing, et al., Org. Chem. Front. 7 (2020) 2405-2413. DOI:10.1039/D0QO00663G |
| [23] |
L.G. Bai, Y. Zhou, X. Zhuang, et al., Green Chem. 22 (2020) 197-203. DOI:10.1039/C9GC03629F |
| [24] |
A.R. Choudhury, S. Mukherjee, Chem. Sci. 7 (2016) 6940-6945. DOI:10.1039/C6SC02466A |
| [25] |
M. Zhang, W.S. Sun, G.M. Zhu, et al., ACS Catal. 6 (2016) 5290-5294. DOI:10.1021/acscatal.6b01693 |
| [26] |
X. Kou, Q.Y. Zhao, Z.H. Guan, Org. Chem. Front. 7 (2020) 829-833. DOI:10.1039/D0QO00041H |
| [27] |
Q. Sun, Y.Y. Zhang, J. Sun, et al., Org. Lett. 20 (2018) 987-990. DOI:10.1021/acs.orglett.7b03751 |
| [28] |
J.P. Michael, Nat. Prod. Rep. 25 (2008) 166-187. DOI:10.1039/B612168N |
| [29] |
A.G. Myers, N.J. Tom, M.E. Fraley, et al., J. Am. Chem. Soc. 119 (1997) 6072-6094. DOI:10.1021/ja9703741 |
| [30] |
Y. Abe, H. Kayakiri, S. Satoh, et al., J. Med. Chem. 41 (1998) 4062-4079. DOI:10.1021/jm980300f |
| [31] |
F. Wang, C. Luo, G. Deng, et al., Green Chem. 16 (2014) 2428-2431. DOI:10.1039/c4gc00038b |
| [32] |
Z. Tan, H. Zhao, C. Zhou, et al., J. Org. Chem. 81 (2016) 9939-9946. DOI:10.1021/acs.joc.6b02117 |
| [33] |
P. Qian, Z. Yan, Z. Zhou, et al., Org. Lett. 20 (2018) 6359-6363. DOI:10.1021/acs.orglett.8b02578 |
| [34] |
P.M. Burton, J.A. Morris, Org. Lett. 12 (2010) 5359-5361. DOI:10.1021/ol102276e |
| [35] |
B. Qian, S.M. Guo, J.P. Shao, et al., J. Am. Chem. Soc. 132 (2010) 3650-3651. DOI:10.1021/ja910104n |
| [36] |
F.F. Wang, C.P. Luo, G.J. Deng, et al., Green Chem. 16 (2014) 2428-2431. DOI:10.1039/c4gc00038b |
| [37] |
H. Komai, T. Yoshino, Matsunaga S, et al., Org. Lett. 13 (2011) 1706-1709. DOI:10.1021/ol200217y |
| [38] |
J.W. Liang, Y.Q. Rao, W.D. Zhu, et al., Org. Lett. 23 (2021) 7028-7032. DOI:10.1021/acs.orglett.1c02323 |
| [39] |
W.Y. Liang, F. Xie, Z.H. Yang, et al., Org. Lett. 22 (2020) 8291-8295. DOI:10.1021/acs.orglett.0c02924 |
| [40] |
Q.L. He, Xie F. C.J.Xia, et al., Org. Lett. 22 (2020) 7976-7980. DOI:10.1021/acs.orglett.0c02910 |
| [41] |
G.J. Griffiths, M.B. Hauck, R. Imwinkelried, et al., J. Org. Chem. 64 (1999) 8084-8089. DOI:10.1021/jo9824910 |
| [42] |
J. Zhao, Y.H. Wang, M. Lou, et al., J. Chem. Res. 37 (2013) 320-322. DOI:10.3184/174751913X13666223718881 |
| [43] |
R.M. Hu, D.Y. Han, N. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 3876-3880. DOI:10.1002/anie.201913400 |
| [44] |
G.Y. Zhang, T. Irrgang, T. Dietel, et al., Angew. Chem. Int. Ed. 57 (2018) 9131-9135. DOI:10.1002/anie.201801573 |
| [45] |
J. Rana, R. Babu, M. Subaramanian, et al., Org. Chem. Front. 5 (2018) 3250-3255. DOI:10.1039/C8QO00764K |
 2022, Vol. 33
2022, Vol. 33