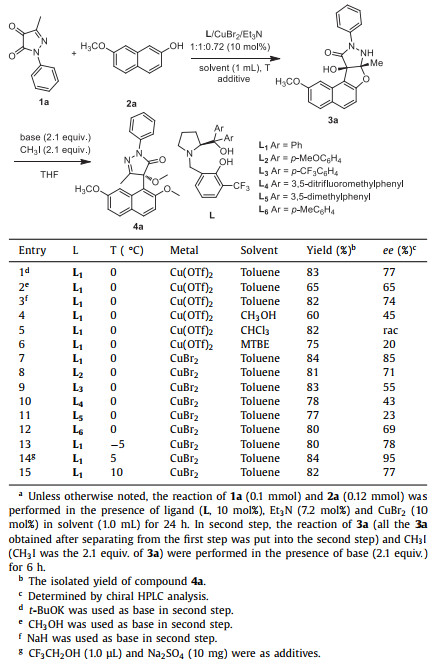
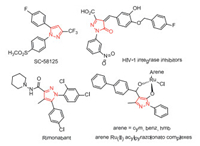
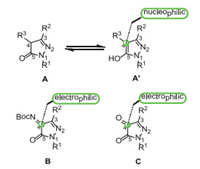
In the past few decades, pyrazole derivatives were widely used in the pharmaceutical industry [1-4]. They are the core structures existing in cox-2 inhibitors SC-58,125 [5], antiobesity drug Rimonabant [6], anti-lung cancer drugs [7], HIV-1 integrase inhibitors [8], arene-Ru(Ⅱ) acylpyrazolonato complex, etc. (Fig. 1) [9]. Pyrazole-5-one derivatives bearing multiple reaction sites can be widely utilized for the asymmetry synthesis of pyrazole derivatives [10-17]. For instance, the C-4, 5-OH positions of pyrazole-5-one (A') are nucleophilic while the C-3 position of pyrazole-5-one (A') is electrophilic. When pyrazole-5-one was converted into ketimine (B) and pyrazolone (C), the reaction polarity was switched, that is, the C-3 and C-4 positions are electrophilic (Fig. 2). Taking advantage of the modifiable characteristics of pyrazole derivatives, the synthetic chemists developed various efficient methods to synthesize the chiral pyrazoles derivatives with different functions [18-26]. Kesavan's group [27] successfully realized the asymmetric reaction of pyrazolones with isatylidine β, γ-unsaturated α-ketoester by virtue of the nucleophilicity of C-4 position. In 2017, Enders's group [28] reported an enantioselective synthesis of furanonaphthopyrazolidinone derivatives, obtaining the C-4 substituted pyrazole derivatives with high yields and excellent stereoselectivities. Recently, Wu's group [29] developed the enantioselective alkynylation reaction of terminal alkynes with pyrazole-4, 5-diones under Lewis acid catalysis to give the corresponding products with excellent yields and enantioselectivities (Scheme 1).
|
Download:
|
| Fig. 1. Some biologically active pyrazole derivatives. | |
|
Download:
|
| Fig. 2. Reactive centers of pyrazolin-5-one derivatives. | |
|
Download:
|
| Scheme 1. Asymmetric synthesis of pyrazole derivatives. | |
As far as we know, Friedel-Crafts reaction has emerged as one of the most commonly strategies for the construction of C—C bonds [30-34]. To the best of our knowledge, there are few studies on the asymmetric Friedel-Crafts reaction of pyrazolones [28, 35]. Usually, pyrazolone derived ketimine was most commonly used for the synthesis of pyrazole derivatives. Moreover, almost all the reactions containing pyrazolone skeletons were catalyzed with organocatalyst [36-41]. However, under the use of the chiral Lewis acid catalysts, the Friedel-Crafts reaction of pyrazolone involved was rarely reported. Therefore, the asymmetry Friedel-Crafts reaction containing pyrazolone is still challenging. It is of great research significance to enrich the methods and the catalytic system to prepare chiral pyrazolone derivatives. Herein, we envisioned to build chiral pyrazolone derivatives via a Lewis acid catalyzed Friedel-Crafts reaction by virtue of L-Cu complex developed in our laboratory.
As far as we know, β-naphthol has been demonstrated to be excellent donor of Friedel-Crafts reaction [42-45]. β-naphthol has two nucleophilic reaction sites and the regioselectivity of the β-naphthol reaction is very important. Based on the previous research results in our group [46-50, we assumed that the asymmetric Friedel-Crafts reaction between β-naphthol and pyrazole-4, 5-diones could be catalyzed by the chiral L-Cu complex. The reaction went through an intermediate structure containing dihydronaphthofuran ring. The compounds containing dihydronaphthofuran moiety presented various biological activity [51-53]. To our delight, the reaction can be carried out under mild conditions to provide a series of chiral pyrazole-4, 5-diones derivatives with high yields and excellent enantioselectivities. More importantly, these derivatives have one tetrasubstituted stereocenters (Scheme 1). It is delightful that the regioselectivity and stereoselectivity of reacting pyrazole-4, 5-diones with β-naphthol through the L-Cu complex was effectively controlled.
Initially, 1a and 2a were employed as a model reaction and our chiral L-Cu complex were employed as the catalysts to optimize the reaction conditions (Table 1). First of all, the reaction was carried out in different solvents at 0 ℃. When methanol was employed as the solvent, the enantioselectivity of the product was moderate and low yield was observed (entry 4). When methyl tert-butyl ether and CHCl3 were employed as the solvent, the reaction can afford the desired products with moderate yields but low enantioselectivities (entries 5 and 6). Gratifyingly, when toluene was employed as solvent, the desired product 3a can be obtained with both moderate yield and good enantioselectivity (entry 1). However, 3a was unstable in the flash column seperation. The hydroxyl groups of 3a need to be protected in order to obtain the stable product 4a. After optimization, as shown in Table 1 (entries 1–3), 3a was transformed into 4a by virtue of methylation in the presence of t-BuOK. To improve the ee, further screening of the copper salts was also carried out. The counter ions of the copper salts had a great influence on the reaction, perhaps due to the difference of the conductivity, as shown in Tables S3-S5 (Supporting information). It was found that the use of CuBr2 instead of Cu(OTf)2 gave a significant improvement in enantioselectivity in spite of a longer reaction time (entry 7). Afterwards, various ligands were examined in this reaction. We found that the different substituents on the aromatic ring at the pyrrole moiety of the ligands had a great influence on the enantioselectivity (entries 7–12). As a consequence, only ligand 1 led to the highest enantioselectivity (entry 7). Subsequently, the different temperature was screened in this reaction since the temperature can affect the ee value obviously (entries 7, 13–15). At low temperature of −5 ℃, the reduced enantioselectivity could be observed although the reaction yield could be maintained. When the temperature was increased from 0 ℃ to 5 ℃, the ee value can be increased from 85% to 95% (entry 14). To raise the temperature further resulted in the decrease of the ee value, as shown in entry 15. Therefore, the optimal temperature was determined at 5 ℃. Afterwards, trifluoroethanol and anhydrous sodium sulfate were added as additives to polish this reaction. To our delight, the employment of these two additives resulted in obviously enhancement in the enantioselectivity of the reaction (entry 14). And 3a could be obtained with high yield (93%), excellent ee value (98%) and high dr value (> 20:1). Finally, when the loading of Cu complex was reduced from 10.0 mol% to 5.0 mol% and 2.5 mol%, the ee value was reduced from 95% to 91% and 85% in spite of an almost maintained yield. Therefore, the optimal loading of Cu complex was determined at 10.0 mol%. As a result, the optimal conditions for the reaction can be concluded as follows: the chiral L1CuBr2 complex as the catalyst, toluene as the solvent, Et3N as the base, trifluoroethanol and anhydrous sodium sulfate as the additives, and the reaction being carried out at 5 ℃. Then under −10 ℃, the methylation of 3a was carried out in the presence of t-BuOK and CH3I in THF.
|
|
Table 1 Optimization of the Friedel-Crafts reaction conditions.a |
With the optimal conditions in hand, we began to further investigate the substrate scope of this reaction (Scheme 2). First, the N-1 substituents on pyrazole-4, 5-diones with different aryl groups were examined. It was found that all of these substituents were well tolerated in the reaction regardless of either electronic rich or electronic deficient groups at the para- or meta-position. Gratifyingly, all of 4a-4l with tetrasubstituted chiral center can be obtained with high yields and good ee values. When the meta-substituents on the phenyl ring of 1 was electron-withdrawing groups, such as -Cl, -Br, –OCH3, the corresponding product 4b, 4c and 4e could be easily obtained with moderate yields and high ee values. When the same position was substituted by a methyl group, the corresponding product 4d can be obtained with moderate enantioselectivity. Also, the electron-donating groups at the para-position of the phenyl ring favored this reaction, affording 4g and 4h with excellent ee values of 93% and 90%, respectively.
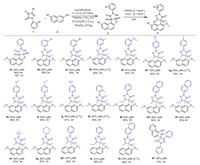
|
Download:
|
| Scheme 2. Scope of the Friedel-Crafts reactions. Unless otherwise noted, all reactions were performed with 1 (0.10 mmol), 2 (0.12 mmol), L1 (10 mol%), Et3N (7.2 mol%), CuBr2 (10 mol%), in toluene (1.0 mL); CF3CH2OH (1.0 μL) and Na2SO4 (10 mg) were as additives; the reaction was conducted at 5 ℃ for 24 h. In second step, the reaction of 3 (all the 3 obtained after separating from the first step was put into the second step) and CH3I (CH3I was the 2.1 equiv. of 3) were performed in the presence of t-BuOK (2.1 mol%) for 6 h. The isolated yields after column chromatography were shown. The ee values were determined by chiral HPLC analysis. The dr value was determined by 1HNMR of the crude product. a NaH was used as base instead of t-BuOK, the reaction was conducted at −10 ℃. b NaH was used as base instead of t-BuOK. c The reaction was performed with L1 (10 mol%), Et3N (7.2 mol%), Cu(OTf)2 (10 mol%), in chlorobenzene (1.0 mL); the reaction was conducted at −10 ℃ for 16 h. | |
In contrast, the substrate bearing electron withdrawing groups at para-position of the phenyl ring, such as -Cl, -CF3, can afford 4f and 4j in 84% and 67% ee values respectively. This indicated that electronic effect of the para-substituents had a great influence on the enantiomeric excess of this reaction. On the other hand, the substrate bearing methyl group at ortho-position of the phenyl ring gave the corresponding product 4k with moderate yield but lower ee values in comparison with that of 4d and 4g. When a methyl group at the para-position was replaced by ethyl group, similarly, the enantioselectivity of 4i was decreased slightly. This can be ascribed to the steric effect.
In order to further broaden the range of substrates, substrate 1 bearing various substituents instead of aryl groups were examined. When the N-1 substituents of substrate 1 were naphthyl, tertbutyl, cyclohexyl groups, the reaction can be carried out smoothly to deliver 4m and 4o-4p with good enantioselectivities (87%−97%). The substrate bearing ethyl group at C-3 position of the pyrazolone moiety gave the corresponding products 4l and 4n with good enantioselectivities of 92% and 90% respectively. The product 4t can be obtained with a reduced ee value (73% ee), perhaps due to the unstability of this product since the produced hydroxyl group was not converted into methoxy group.
As for the substrate 2, without any substituents at the naphthol ring, the desired products 4q-4s were obtained easily with high enantiomeric excess (88%−99%). Moreover, the absolute configuration of the products were confirmed by comparing our experimental data of 4u and 4v with the relevant data 5a and 3b reported in the literature (pages 20–26 in Supporting information) [16].
In order to demonstrate the practical applicability of this reaction, a gram-scale experiment was carried out with amplify in the amount of 4.3 mmol under the standard conditions. It was worth mentioning that 1.1 g of the desired product was finally obtained with a yield of 65% and the ee value of 95% (Scheme 3).
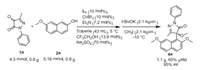
|
Download:
|
| Scheme 3. Gram scale of the Friedel-Crafts reaction. 1a (4.3 mmol), 2a (5.16 mmol), L1 (10 mol%), Et3N (10 mol%), CuBr2 (10 mol%), CF3CH2OH (13.9 mol%) and Na2SO4 (70 mol%) in toluene (43 mL) at 5 ℃. The yield was isolated yield, and the ee values were determined by chiral HPLC analysis. | |
In order to study the reaction mechanism, control experiment was carried out under the standard conditions (Scheme 4). First, when the hydroxyl group of β-naphthol was protected by a methyl group, no product was detected, as shown in Scheme 4a. This implied that the hydrogen atom of hydroxyl group on the β-naphthol was essential to this reaction (Scheme 4b). And the L was synthesized in order to explore the role of the aliphatic hydroxyl group of L. When L was employed as the ligand, the good yield could be maintained while the ee value was dropped to 13%, as shown in Scheme 4c. This implied that the oxygen atom of L could form hydrogen bond with β-naphthol to affect the attack direction of the neucleophile. At the same time, the naphthalene ring was necessary perhaps due to the π-π stacking effect with pyrazole-4, 5-diones, resulting in excellent enantioselectivity. The specific transition state model was proposed, as shown in Fig. 3.
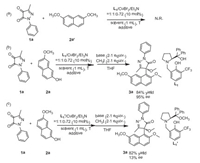
|
Download:
|
| Scheme 4. Control experiments. | |
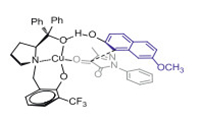
|
Download:
|
| Fig. 3. Proposed transition state of the Friedel-Craft reaction. | |
In conclusion, a copper-catalyzed highly enantioselective Friedel-Crafts reaction of pyrazole-4, 5-diones with β-naphthol was developed, which provided a useful strategy for the asymmetric synthesis of pyrazolone derivatives. The reaction can be carried out under the mild condition to afford the desired products with high yields (up to 85%) and excellent enantioselectivities (up to 99%). Moreover, a gram scale of the Friedel-Crafts reaction was carried out successfully, delivering the desired product with good yield and excellent ee value (95%). More applications and further mechanism investigation are ongoing in our group.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe are grateful for the financial support from the Natural Science Foundation of China (No. 21772185) and National Natural Science Foundation of China (No. 22001241). This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.038.
| [1] |
M.S. Chande, P.A. Barve, V. Suryanarayan, J. Heterocycl. Chem. 44 (2007) 49-53. DOI:10.1002/jhet.5570440108 |
| [2] |
Y. Kakiuchi, N. Sasaki, M.S. Masuoka, H. Murofushi, K.M. Murofushi, Biochem. Biophys. Res. Commun. 320 (2004) 1351-1358. DOI:10.1016/j.bbrc.2004.06.094 |
| [3] |
M.K. Purohit, I. Scovell, A. Neschadim, et al.
, Bioorg. Med. Chem. Lett. 23 (2013) 2324-2327. DOI:10.1016/j.bmcl.2013.02.064 |
| [4] |
C.W. Lindsley, D.D. Wisnoski, W.H. Leister, et al.
, J. Med. Chem. 47 (2004) 5825-5828. DOI:10.1021/jm049400d |
| [5] |
T.D. Penning, J.J. Talley, S.R. Bertenshaw, et al.
, J. Med. Chem. 40 (1997) 1347-1365. DOI:10.1021/jm960803q |
| [6] |
L.F.V. Gaal, A.M. Rissanen, A.J. Scheen, et al.
, Lancet 365 (2005) 1389-1397. DOI:10.1016/S0140-6736(05)66374-X |
| [7] |
R.N. Jadeja, K.M. Vyas, K.K. Upadhyayc, et al.
, RSC Adv. 7 (2017) 17107. DOI:10.1039/C7RA01025G |
| [8] |
V. Hadi, Y.H. Koh, T.W. Sanchez, et al.
, Bioorg. Med. Chem. Lett. 20 (2010) 6854-6857. DOI:10.1016/j.bmcl.2010.08.057 |
| [9] |
R. Pettinari, C. Pettinari, F. Marchetti, et al.
, J. Med. Chem. 57 (2014) 4532-4542. DOI:10.1021/jm500458c |
| [10] |
X.Z. Kou, Q.H. Shao, C.H. Ye, et al.
, J. Am. Chem. Soc. 140 (2018) 7587-7597. DOI:10.1021/jacs.8b02865 |
| [11] |
H.J. Yuan, Y. Li, H.H. Zhao, et al.
, Chem. Commun. 55 (2019) 12715-12718. DOI:10.1039/C9CC06360A |
| [12] |
J. Xie, X.Y. Xing, F. Sha, et al.
, Org. Biomol. Chem. 14 (2016) 8346-8355. DOI:10.1039/C6OB01256F |
| [13] |
H.C. Lin, P.S. Wang, Z.L. Tao, et al.
, J. Am. Chem. Soc. 138 (2016) 14354-14361. DOI:10.1021/jacs.6b08236 |
| [14] |
S.L. Wang, C.R. Escrich, M.A. Pericas, Org. Lett. 18 (2016) 556-559. DOI:10.1021/acs.orglett.5b03575 |
| [15] |
S. Gogoi, C.G. Zhao, D.R. Ding, Org. Lett. 11 (2009) 2249-2252. DOI:10.1021/ol900538q |
| [16] |
Q.H. Nguyen, S.M. Guo, T. Royal, et al.
, J. Am. Chem. Soc. 142 (2020) 2161-2167. DOI:10.1021/jacs.9b12299 |
| [17] |
P. Chauhan, S. Mahajan, D. Enders, Chem. Commun. 51 (2015) 12890-12907. DOI:10.1039/C5CC04930J |
| [18] |
P. Chauhan, S. Mahajan, U. Kaya, et al.
, J. Org. Chem. 82 (2017) 7050-7058. DOI:10.1021/acs.joc.7b01113 |
| [19] |
S. Mahajan, P. Chauhan, U. Kaya, et al.
, Chem. Commun. 53 (2017) 6633-6636. DOI:10.1039/C7CC02874A |
| [20] |
Z.G. Yang, Z. Wang, S. Bai, et al.
, Org. Lett. 13 (2011) 596-599. DOI:10.1021/ol102804p |
| [21] |
S. Fustero, M.S. Rosello, P. Barrio, et al.
, Chem. Rev. 111 (2011) 6984-7034. DOI:10.1021/cr2000459 |
| [22] |
T. Okitsu, K. Sato, A. Wada, Org. Lett. 12 (2010) 3506-3509. DOI:10.1021/ol101365x |
| [23] |
J. Zhang, Y. Shao, H.X. Wang, et al.
, Org. Lett. 16 (2014) 3312-3315. DOI:10.1021/ol501312s |
| [24] |
D.C. Schmitt, A.P. Taylor, A.C. Flick, et al.
, Org. Lett. 17 (2015) 1405-1408. DOI:10.1021/acs.orglett.5b00266 |
| [25] |
Y. Ding, T. Zhang, Q.Y. Chen, et al.
, Org. Lett. 18 (2016) 4206-4209. DOI:10.1021/acs.orglett.6b01867 |
| [26] |
Y.J. Zhong, T. Qi, Y.L. Ji, et al.
, J. Org. Chem. 86 (2021) 2582-2592. DOI:10.1021/acs.joc.0c02674 |
| [27] |
N. Kumarswamyreddy, V. Kesavan, Org. Lett. 18 (2016) 1354-1357. DOI:10.1021/acs.orglett.6b00287 |
| [28] |
U. Kaya, P. Chauhan, S. Mahajan, et al.
, Angew. Chem. Int. Ed. 56 (2017) 15358-15362. DOI:10.1002/anie.201709224 |
| [29] |
J. Lu, L.S. Luo, F. Sha, et al.
, Chem. Commun. 55 (2019) 11603-11606. DOI:10.1039/C9CC05252F |
| [30] |
F.X. Xu, D. Huang, C. Han, et al.
, J. Org. Chem. 75 (2010) 8677-8680. DOI:10.1021/jo101640z |
| [31] |
D. Zhou, Z. Huang, X.T. Yu, et al.
, Org. Lett. 17 (2015) 5554-5557. DOI:10.1021/acs.orglett.5b02668 |
| [32] |
T.B. Poulsen, K.A. Jørgensen, Chem. Rev. 108 (2008) 2903-2915. DOI:10.1021/cr078372e |
| [33] |
M. Hatano, K. Toh, K. Ishihara, Org. Lett. 22 (2020) 9614-9620. DOI:10.1021/acs.orglett.0c03662 |
| [34] |
W.-.J. Zhu, J.-.F. Gong, M.-.P. Song, J. Org. Chem. 85 (2020) 9525-9537. DOI:10.1021/acs.joc.0c00336 |
| [35] |
F. Vetica, P. Chauhan, S. Mahajan, et al.
, Synthesis (Mass) 50 (2018) 1039-1046. DOI:10.1055/s-0036-1591860 |
| [36] |
P.K. Warghude, A.S. Sabale, R.G. Bhat, Org. Biomol. Chem. 18 (2020) 1794-1799. DOI:10.1039/D0OB00007H |
| [37] |
Q.D. Zhang, B.L. Zhao, B.Y. Li, et al.
, Org. Biomol. Chem. 17 (2019) 7182-7191. DOI:10.1039/C9OB01350D |
| [38] |
S. Mahajan, P. Chauhan, U. Kaya, et al.
, Chem. Commun. 53 (2017) 6633-6636. DOI:10.1039/C7CC02874A |
| [39] |
B. Ray, S. Mukherjee, J. Org. Chem. 83 (2018) 10871-10880. DOI:10.1021/acs.joc.8b01566 |
| [40] |
Y. Zhou, Y. You, Z.H. Wang, et al.
, Eur. J. Org. Chem. (2019) 3112-3116. |
| [41] |
R.H. Wang, Y.L. Li, H.J. He, et al.
, Chem. Eur. J. 27 (2021) 4302-4306. |
| [42] |
J.P. Lan, Y.N. Lu, K.W. Chen, et al.
, Synthesis (Mass) 52 (2020) 3684-3692. DOI:10.1055/s-0040-1707237 |
| [43] |
P. Chauhan, S.S. Chimni, Eur. J. Org. Chem. (2011) 1636-1640. |
| [44] |
C. Parida, R. Maity, S.C. Sahoo, et al.
, Org. Lett. 21 (2019) 6700-6704. |
| [45] |
M.M. Magraner, R. Canton, C. Vila, et al.
, RSC Adv. 5 (2015) 60101-60105. |
| [46] |
W.J. Lu, J.D. Li, Y.M. Lu, et al.
, ChemistrySelect 6 (2021) 410-414. |
| [47] |
X.P. Liang, Y. Gui, K.L. Li, et al.
, Chem. Commun. 56 (2020) 11118-11121. |
| [48] |
J.D. Li, Y.N. Li, J.N. Sun, et al.
, Chem. Commun. 55 (2019) 6309-6312. |
| [49] |
J.N. Sun, Y. Gui, Y.K. Huang, et al.
, ACS Omega 5 (2020) 11962-11970. |
| [50] |
K.L. Li, X. Sun, L.G. Li, et al.
, Chem. Eur. J. 27 (2021) 581-584. |
| [51] |
N. Tabata, H. Tomoda, S.O. Erabulenols, J. Antibiot. 51 (1998) 624-628. |
| [52] |
M. Itoigawa, C. Ito, H.T.W. Tan, et al.
, Cancer Lett. 174 (2001) 135-139. |
| [53] |
M.F. Elsebai, S. Kehraus, U. Lindequist, et al.
, Org. Biomol. Chem. 9 (2011) 802-808. |
 2022, Vol. 33
2022, Vol. 33