b Institute of Molecular Materials and Devices, Fudan University, Shanghai 200433, China;
c MIIT Key Laboratory for Low-Dimensional Quantum Structure and Devices, Beijing Institute of Technology, Beijing 100081, China
As a type of well-ordered crystalline frameworks, covalent organic frameworks (COFs) are formed by organic building blocks with strong covalent bonds [1]. The predesignable structures and controllable bandgap of these ordered crystalline materials affect their functionality, which has therefore garnered increasing attention for applications such as gas absorption [2-4], catalysis [5-8] and electronics [9, 10].
In the application of photoelectric conversion, a certain number of works have been reported employing COFs as photosensitive materials. In 2009, Jiang's group has reported the first example using COFs as photoconductive materials [11]. Later, COFs with dye groups of thienoisoindigo have been firstly synthesized in 2017, and have realized spectrally switchable photodetection [12]. In most cases, the dynamic covalent linkages in COFs are usually boroxine [13], boronate ester [14] and imine bonds [15, 16], which could form high crystallinity but lack of π-delocalization and stability owing to their highly reversible nature [17]. Thus, these COFs are always hybrid with other materials to realize high photoelectric conversion efficiency [18]. As a contrast, olefin-linked COFs (oCOFs) could realize efficient electron delocalization [19-21] and highly enhanced stability [22], which could benefit to the pristine COFs' photoelectric application.
However, such oCOFs are still in the infant stage, mostly attributed to the lack of available building blocks for reversible olefin-linked polymerization. Recently, two special monomers with active methylene group, 3, 5-dicyano-2, 4, 6-trimethylpyridine and 2, 4, 6-trimethyl-1, 3, 5-triazine (TMT), were found to be available for effective synthesis of oCOFs through facile Knoevenagel/Aldol condensation [23-25]. Though, the existing oCOF structures are still limited and the relationship between their structures and photoelectrical properties is still unclear.
Herein, a new type of oCOFs is synthesized through Knoevenagel condensation with TMT and tris(4-formylphenyl)-amine (TFPA) and its photoelectric performance is thoroughly investigated. Tertiary amine knots offered by TFPA and triazine knots in TMT could form a typical electron donor-acceptor (D-A) structure together, contributing to the charge transportation across the framework [26]. Besides, the tertiary amine knots, which has been widely used in photoelectrical investigation [27-30], favor the photoinduced electron-hole (e–h) separation with twisted intramolecular charge transfer (TICT) effect [31]. As a result, much decreased optical bandgap (1.90 eV) is measured in oCOF-TFPA, compared with other two oCOFs with similar composition. Moreover, the device constructed with oCOF-TFPA shows a photoresponsivity of 23.20 mA/W and a detectivity of 1.01 × 1010 Jones under UV illumination, which are 20–30 times of other two oCOFs' performance.
The three target oCOFs are synthesized under optimized thermodynamic reversible conditions of Knoevenagel condensation (Fig. 1a) [8, 23, 24]. In synthesis of oCOF-TFPA, monomers including TMT (0.1 mmol), TFPA (0.1 mmol) and catalyst EtONa (0.3 mmol) are all dissolved in 1 mL 1, 4-dioxane and ultrasonic mixed. After three cycles of freeze-pump-thaw and flame seal [27], all the mixtures are heated at 120 ℃ for 3 days. Then, 12 h of Soxhlet extraction with tetrahydrofuran and 24 h vacuum drying at 60 ℃ are executed. Finally, pure oCOF powder is afforded in 84%–91% yield, and the corresponding oCOF films could be obtained [32, 33] and be prepared for device construction [34, 35].
|
Download:
|
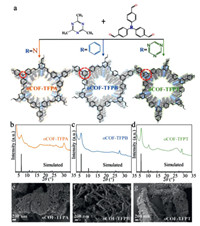
| Fig. 1. (a) Synthetic routine for three oCOFs, (b–d) PXRD patterns of three oCOFs and (e–g) their corresponded micromorphology observed in SEM images. | |
The three monomers, including TFPA, 1, 3, 5-tris(4-formylphenyl)-benzene (TFPB), and 2, 4, 6-tris(4-formylphenyl)-1, 3, 5-triazine (TFPT), have varied structures and lead to different properties in corresponding oCOFs [34]. The crystallinity of these oCOFs is confirmed by powder X-ray diffraction (PXRD) analyses (Figs. 1b–d). Highly intense peaks at 2θ = 5.81° and 5.61° for oCOF-TFPB and oCOF-TFPT are reflected by (110) planes. To the contrast, the oCOF-TFPA with tertiary amine knots exhibits a minor peak at 6.38° reflected by (010) planes, which indicates the impaired crystallinity with twisted knots. All these results match well with the simulated patterns of honeycomb hexagonal unit cells adopting AA stacking models (simulated by Vesta software) [36]. Besides, micromorphology of these oCOFs (Figs. 1e–g) varies from twisted nanofibers (oCOF-TFPB) to rod-like particles (oCOF-TFPT) and amorphous mass (oCOF-TFPA), along with decreasing interlayer interaction. Three oCOFs' transmission electron microscope (TEM) images (Fig. S1 in Supporting information) and N2 adsorption-desorption analyses (Fig. S2 in Supporting information) also show close relationship with the knot's structure, which ensure the profound influence of knot structure on oCOFs' crystallinity and interlayer interaction [37]. All oCOFs shows good chemical and thermal stability (Figs. S3 and S4 in Supporting information).
To estimate the crystalline structures and interlayer stacking of three oCOFs, structure simulations have been performed with Vienna Ab-initio Simulation Package (VASP) based on density functional theory (DFT) [38]. All these simulated structures (Fig. 2) keep good correspondence with their PXRD data. The parameters of simulated cells in oCOF-TFPA are calculated to be a = b = 15.98 Å, c = 3.79 Å, while a = b = 17.56 Å, c = 3.45 Å in oCOF-TFPB and a = b = 17.76 Å, c = 3.25 Å in oCOF-TFPT (Tables S1–S3 in Supporting information). Obviously, three phenyl rings connected to the triazine knots are almost in the same plane for oCOF-TFPB and oCOF-TFPT, whereas for oCOF-TFPA the phenyl rings are twisted in a certain angle to avoid steric hindrance. Thus, a tiny in-plane twist angle could be observed in the side view of oCOF-TFPA, which lead to the increase of interlayer distance from 3.25 Å (oCOF-TFPT) to 3.79 Å. The increased interlayer distance arises from tertiary amine knots, indicating the attenuated interlayer interaction in oCOF-TFPA.

|
Download:
|
| Fig. 2. DFT calculated structures and calculated electronic band structures for (a, b) oCOF-TFPA, (c, d) oCOF-TFPB and (e, f) oCOF-TFPT. | |
Meanwhile, electronic band structures of three oCOFs are also obtained by VASP. Considering the domination of direct transition in photo-absorption process [39], the direct energy gap in three oCOFs are estimated (Fig. 2). The direct energy gap in oCOF-TFPA is calculated to be the narrowest at 1.57 eV, while the value in oCOF-TFPB and oCOF-TFPT are 2.14 eV and 2.27 eV, respectively. According to the theory of TICT effect, the tertiary amine knots could be twisted in the excitation process and form a new excited energy state, which could contribute to the decrease of direct energy gap [29]. In addition, the obtained energy gap is not a precise reference to the measured optical gap, as the former does not take into account electron-hole pairing (excitonic effect) in contrast with the later. Nevertheless, the tendency shown in the simulated results still implies the variation of three oCOFs' real transition energy gap and their photoelectric ability, provided that the variation of excitonic effect is negligible in three oCOF materials with similar atomic structures.
Further analysis of oCOF-TFPA's chemical structure and optical properties is executed (Fig. 3). The polymerization of oCOF-TFPA could be confirmed through Raman spectra (Fig. 3a). Newly formed olefin (C=C) linkage in oCOF-TFPA is manifested via disappearance of methyl C-H vibration doublet peak at 2930 cm−1 and occurrence of C=C stretching peak at 1615 cm−1, compared with spectra of monomers. Meanwhile, C-N peak at about 1400 cm−1 and vanishing C=O peak at 1688 cm−1 indicate well-reserved tertiary amine knots. Other two oCOFs' Raman spectra (Fig. S5 in Supporting information) and their Fourier transform infrared (FT-IR) spectra (Fig. S6 in Supporting information) also verify the successful polymerization of three oCOFs.

|
Download:
|
| Fig. 3. Optical characteristics of oCOF-TFPA: (a) Raman spectra, (b) powder-state fluorescent spectra, (c) fluorescent emission in different solvents, (d) UV-vis diffuse reflective spectra and (e) Kubelka-Munk transformed plot. | |
In the powder-state fluorescent measurement of oCOF-TFPA (Fig. 3b), an excitation peak at 530 nm accompanied with several emission peaks in the range of 600–800 nm could be observed, which suggests the excited state generated with TICT effect. To the contrast, oCOF-TFPB and oCOF-TFPB both exhibit simple emission spectra (Fig. S5). As the TICT effect suggest, the twisted structure of tertiary amine knots could facilitate the e–h separation and strengthen the materials' fluorescence [28]. Weak interlayer π-interaction in oCOF-TFPA also reduces possibility of aggregation-caused quenching (ACQ) effect [40], which is also conducive for fluorescent enhancement. As a result, the oCOF-TFPA emits intensive orange fluorescence (Fig. 3d inset), while the other two oCOFs show much depressed fluorescence (Figs. S7–S9 in Supporting information). As polar solvent could facilitate the charge transfer process [31], the emission peak of oCOF-TFPA shows obvious red shift of 65.5 nm along with the polarity increasing (Fig. 3c): 547.5 nm (n-hexane), 610 nm (ethanol) and 613 nm (DMSO). The observed red shift and the gradually obvious emission peak at about 720 nm both verify the TICT effect in oCOF-TFPA.
Optical bandgap of three oCOFs could be evaluated through UV-vis diffuse reflective spectrum (Fig. 3d). The absorption edge of oCOF-TFPA shows a typical red shift at about 600 nm, while the absorption edge in the other two oCOFs are settled at 470–500 nm (Fig. S10 in Supporting information). Accordingly, the optical bandgap of oCOF-TFPA is calculated to be 1.90 eV (Fig. 3e) using Kubelka-Munk function [41], which is obviously narrower than those of oCOF-TFPB (2.32 eV) and oCOF-TFPT (2.65 eV). The decrease of optical bandgap could be attributed to excited state generated with TICT effect and is well consistent with tendency of DFT calculated energy gap (Fig. 2). Additionally, the related characteristics of amorphous polymer with the same structure of oCOF-TFPA (noted as oPOP-TFPA) have been also measured, proving that poor crystallinity leads to larger optical bandgap and lower conductivity (Figs. S11 and S12 in Supporting information).
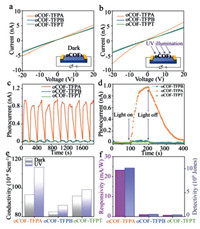
Considering the similarities of electron excitation in fluorescence and photoelectric conversion, oCOF-TFPA might also have advantages in the later field. Thus, electrical measurements of all these oCOFs devices are executed. The I-V curves of three oCOFs in dark and UV-exposed environment are shown in Figs. 4a and b. As shown in these linear I-V curves, conductivity (σ) could be evaluated based on Ohm's law: σ = (U·L)/(I·S), where the letter U, I, L and S refer to voltage, current, device's equivalent channel length and equivalent cross-sectional area, respectively. Film thickness is confirmed via atom force microscope (AFM) for S calculation (Figs. S13 and S14 in Supporting information). In summary, the conductivity of oCOF-TFPA is higher than the other two oCOFs no matter in dark or UV-exposed environment, which might ascribe to the D-A structure formed with tertiary amine and triazine knots [28]. Under UV irradiation (365 nm), the current of oCOF-TFPA device shows an obvious increase comparing with its performance in dark (Figs. 4b and e), which is consistent with expect.
|
Download:
|
| Fig. 4. (a) I-V curves in dark environment and (b) UV exposure. (c, d) The dynamic photoelectric response of three oCOFs. (e) Conductivity comparisons and (f) photoelectrical properties of three oCOFs. | |
Then, we investigated the photoelectric response of these oCOFs under variating illumination of 80 µW/cm2 UV light (Fig. 4c). Surprisingly, we find that the device constructed with oCOF-TFPA exhibit a distinct signal corresponded with UV status, while the other two oCOF devices show only slight signal. No obvious decrease of photoresponse have been seen after 10 on-off cycles, indicating the sensor's good reliability. A typical photoconductive relaxation process has been observed in oCOF-TFPA device during single on-off process (Fig. 4d). Consequently, the photoelectric characteristics including photoresponsivity (R) and detectivity (D*) could be calculated via R = Iph/(PlightA) and 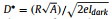
In summary, we have synthesized a new type of oCOFs containing twisted tertiary amine knots, and achieve enhanced fluorescent activity and photoelectric performance. Although twisted configuration of oCOF-TFPA leads to some coplanarity sacrifice, oCOF-TFPA's conductivity is slightly higher than the other two oCOFs with fine coplanarity, owing to its D-A structure. Furthermore, the fluorescence and photoelectric performances of oCOF-TFPA are significantly enhanced with TICT effect. As far as we know, it is the first practice to contain tertiary amine knots in oCOFs' structural design and to investigate the relationship of oCOFs' structure and their photoelectric performance. We believe this new oCOFs with twisted tertiary amine knots could provide a promising solution to the structural design for pristine oCOFs' photoelectric applications, and could avail to the further investigation for oCOFs' structural design and functionalization. Thus, this work is of great significance to the COFs' exploration in UV detection, photoelectric conversion, organic optoelectronics and integrated organic circuits.
Declaration of competing interestThe authors of the manuscript entitled "Olefin-linked covalent organic frameworks with twisted tertiary amine knots for enhanced ultraviolet detection" declare that the authors have no competing interests.
AcknowledgmentsThis work was financially supported by the National Key R & D Program of China (Nos. 2021YFE0201400, 2018YFA0703200, 2020YFA0308800), National Natural Science Foundation of China (Nos. 51773041, 61890940, 21603038, 11974045), Shanghai Committee of Science and Technology in China (No. 18ZR1404900).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.082.
| [1] |
A.P. Cǒté, A.I. Benin, N.W. Ockwig, et al., Science 310 (2005) 1166-1170. DOI:10.1126/science.1120411 |
| [2] |
L.J. Zhu, Y.B. Zhang, Molecules 22 (2017) 1149-1177. DOI:10.3390/molecules22071149 |
| [3] |
M.M. Tong, Q.Y. Yang, Q.T. Ma, D.H. Liu, C.L. Zhong, J. Mater. Chem. A 4 (2016) 124-131. DOI:10.1039/C5TA06707C |
| [4] |
M.X. Wu, Y.W. Yang, Chin. Chem. Lett. 28 (2017) 1135-1143. DOI:10.1016/j.cclet.2017.03.026 |
| [5] |
Y.T. Xiao, G.H. Tian, W. Li, et al., J. Am. Chem. Soc. 141 (2019) 2508-2515. DOI:10.1021/jacs.8b12428 |
| [6] |
Y.C. Wang, H. Liu, Q.Y. Pan, et al., J. Am. Chem. Soc. 142 (2020) 5958-5963. DOI:10.1021/jacs.0c00923 |
| [7] |
S.Y. Ding, J. Gao, Q. Wang, et al., J. Am. Chem. Soc. 133 (2011) 19816-19822. DOI:10.1021/ja206846p |
| [8] |
Y.L. Yang, H.Y. Niu, L. Xu, H. Zhang, Y.Q. Cai, Appl. Catal. B 269 (2020) 118799. DOI:10.1016/j.apcatb.2020.118799 |
| [9] |
X. Feng, L. Chen, Y. Honsho, et al., Adv. Mater. 24 (2012) 3026-3031. DOI:10.1002/adma.201201185 |
| [10] |
L. Yang, D.C. Wei, Chin. Chem. Lett. 27 (2016) 1395-1404. DOI:10.1016/j.cclet.2016.07.010 |
| [11] |
S. Wan, J. Guo, J. Kim, H. Ihee, D.L. Jiang, Angew. Chem. Int. Ed. 48 (2009) 5439-5442. DOI:10.1002/anie.200900881 |
| [12] |
D. Bessinger, L. Ascherl, F. Auras, T. Bein, J. Am. Chem. Soc. 139 (2017) 12035-12042. DOI:10.1021/jacs.7b06599 |
| [13] |
A.M. Evans, I. Castano, A. Brumberg, et al., J. Am. Chem. Soc. 141 (2019) 19728-19735. DOI:10.1021/jacs.9b08815 |
| [14] |
S. Park, Z.Q. Liao, B. Ibarlucea, et al., Angew. Chem. Int. Ed. 59 (2020) 8218-8224. DOI:10.1002/anie.201916595 |
| [15] |
C. Krishnaraj, A.M. Kaczmarek, H.S. Jena, et al., ACS Appl. Mater. Interfaces 11 (2019) 27343-27352. DOI:10.1021/acsami.9b07779 |
| [16] |
F.J. Uribe-Romo, J.R. Hunt, H. Furukawa, et al., J. Am. Chem. Soc. 131 (2009) 4570-4571. DOI:10.1021/ja8096256 |
| [17] |
X. Feng, X.S. Ding, D.L. Jiang, Chem. Soc. Rev. 41 (2012) 6010-6022. DOI:10.1039/c2cs35157a |
| [18] |
Y.F. Xiong, Q.B. Liao, Z.P. Huang, et al., Adv. Mater. 32 (2020) 1907242. DOI:10.1002/adma.201907242 |
| [19] |
M.C. Wang, M. Wang, H.H. Lin, et al., J. Am. Chem. Soc. 142 (2020) 21622-21627. DOI:10.1021/jacs.0c10482 |
| [20] |
E.Q. Jin, M. Asada, Q. Xu, et al., Science 357 (2017) 673-676. DOI:10.1126/science.aan0202 |
| [21] |
X.D. Zhuang, W.X. Zhao, F. Zhang, et al., Polym. Chem. 7 (2016) 4176-4181. DOI:10.1039/C6PY00561F |
| [22] |
E.Q. Jin, J. Li, K.Y. Geng, et al., Nat. Commun. 9 (2018) 4143. DOI:10.1038/s41467-018-06719-8 |
| [23] |
A. Acharjya, P. Pachfule, J. Roeser, F.J. Schmitt, A. Thomas, Angew. Chem. Int. Ed. 58 (2019) 14865-14870. DOI:10.1002/anie.201905886 |
| [24] |
F. Zhang, S.C. Wei, W.W. Wei, et al., Sci. Bull. 65 (2020) 1659-1666. DOI:10.1016/j.scib.2020.05.033 |
| [25] |
H. Lyu, C.S. Diercks, C.H. Zhu, O.M. Yaghi, J. Am. Chem. Soc. 141 (2019) 6848-6852. DOI:10.1021/jacs.9b02848 |
| [26] |
T. Li, X.D. Yan, W.D. Zhang, et al., Chem. Commun. 56 (2020) 14187-14190. DOI:10.1039/d0cc04109b |
| [27] |
Y.J. Zhang, K. Wang, G.L. Zhuang, et al., Chem. Eur. J. 21 (2015) 2474-2479. DOI:10.1002/chem.201405348 |
| [28] |
W.J. Li, D.D. Liu, F.Z. Shen, et al., Adv. Funct. Mater. 22 (2012) 2797-2803. DOI:10.1002/adfm.201200116 |
| [29] |
K.F. Chen, C.W. Chang, J.L. Lin, et al., Chem. Eur. J. 16 (2010) 12873-12882. DOI:10.1002/chem.201001294 |
| [30] |
A.M. El-Zohry, M. Karlsson, J. Phys. Chem. C 122 (2018) 23998-24003. DOI:10.1021/acs.jpcc.8b08326 |
| [31] |
W. Rettig, Angew. Chem. Int. Ed. 25 (1986) 971-988. DOI:10.1002/anie.198609711 |
| [32] |
Y.S. Li, Q. Chen, T.T. Xu, J. Am. Chem. Soc. 141 (2019) 13822-13828. DOI:10.1021/jacs.9b03463 |
| [33] |
L. Yang, Q.Y. Guo, H. Kang, et al., Chem. Mater. 32 (2020) 5634-5640. DOI:10.1021/acs.chemmater.0c01140 |
| [34] |
M.N. He, Y. Zhao, Y.Q. Liu, D.C. Wei, Chin. Chem. Lett. 31 (2020) 826-830. DOI:10.1016/j.cclet.2019.06.003 |
| [35] |
M.N. He, X.S. Chen, D.H. Liu, D.C. Wei, Chin. Chem. Lett. 30 (2019) 961-965. DOI:10.1016/j.cclet.2019.01.008 |
| [36] |
K. Momma, F. Izumi, J. Appl. Crystallogr. 41 (2008) 653-658. DOI:10.1107/S0021889808012016 |
| [37] |
H.J. Da, C.X. Yang, H.L. Qian, X.P. Yan, J. Mater. Chem. A 8 (2020) 12657-12664. DOI:10.1039/d0ta01037e |
| [38] |
G. Kresse, J. Furthmüller, Phys. Rev. B 54 (1996) 11169-11186. DOI:10.1103/PhysRevB.54.11169 |
| [39] |
N.W. Ashcroft, N.D. Mermin, Solid State Physics, Holt, New York, 1976
|
| [40] |
W.Z. Yuan, P. Lu, S.M. Chen, et al., Adv. Mater. 22 (2010) 2159-2163. DOI:10.1002/adma.200904056 |
| [41] |
M. Pal, U. Pal, J.M.G.Y. Jimenez, F. Perez-Rodriguez, Nanoscale Res. Lett. 7 (2012) 1. DOI:10.1186/1556-276X-7-1 |
| [42] |
Z. Cai, M. Cao, Z.P. Jin, et al., Npj 2D Mater. Appl. 2 (2018) 21. DOI:10.1038/s41699-018-0066-2 |
 2022, Vol. 33
2022, Vol. 33 

