Quinoxalin-2(1H)-ones have been found important applications in natural products and bioactive compounds [1-9], and their superior biological pharmacological effects and outstanding chemical properties, such as aldose reductase inhibitor [10, 11], MDR antagonist [12] and antitumor agents [13] can be significantly influenced if the C3 position of quinoxalin-2(1H)-ones is functionalized. Therefore, the synthesis of 3-substituted quinoxalin-2(1H)-ones have received much attention. Due to the advantages of high atom economy and simple experimental operation, C(sp2)-H functionalization of quinoxalin-2(1H)-ones have emerged as a powerful tool for the synthesis of 3-functionalized quinoxalin-2(1H)-ones, and various methods have been developed for the construction of C-C [14-39], C-O [40-45], C-N [46-54], C-S [55-57], C-P [58-63] and C-Si bonds [64].
As a basic chemical bond, the construction of C-C bond of quinoxalin-2(1H)-ones are particularly important, which may promote its application in new drug research and development. In 2018, Guo group reported the direct C3-alkylation of quinoxalin-2(1H)-ones with cyclobutanone oxime esters under the catalysis of Fe(acac)2 [65]. Subsequently, Li and co-workers in 2019 demonstrated a visible-light induced direct C3-alkylation of quinoxalin-2(1H)-ones by using aliphatic carboxylic acids as alkylation reagents [66]. In the same year, our group also reported the photo-promoted C3-alkylation of quinoxalin-2(1H)-ones with N-hydroxyphthalimide ester [23].
In particular, Yuan and co-workers developed a new alkylation method using isopropanol as alkylation reagent to realize hydroxyalkylation at the C3 position of quinoxalin-2(1H)-ones [67]. Although the hydroxyalkylation of quinoxalin-2(1H)-ones are rare and alcohols are easy to obtain and stable, this method cannot get rid of the waste caused by metal catalysis and additives. With the deepening of the concept of green chemistry, it is our goal to find a more mild, functional group tolerant, transition-metal free C-H hydroxyalkylation of quinoxalin-2(1H)-ones.
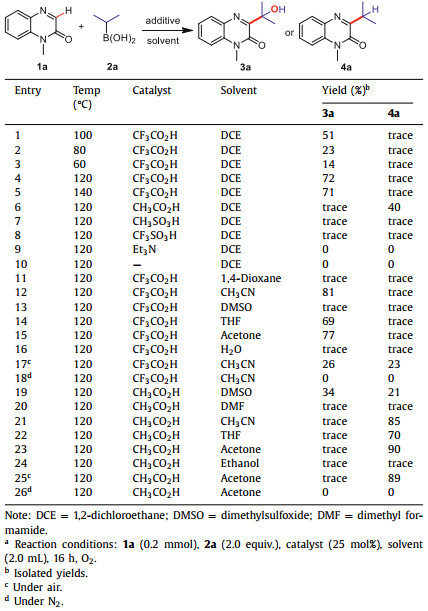
On the other hand, boric acid, as a cheap, easily handled inorganic compound is widely used in chemical and biological fields involving: asymmetric synthesis of amino acids [68], hydroxyl protection reaction [69], Suzuki/Heck coupling reaction [70] and so on. In 2007, Zhu group successfully synthesized 3-arylquinoxalin-2(1H)-ones using aryl boric acids as the arylation reagent under palladium catalyzed conditions [71]. In the same year, Alami group also reported direct C-H arylation of quinoxalin-2(1H)-ones with aryl boric acids providing aryl groups [72]. In 2021, Wang and coworkers reported an electro oxidation method to achieve C-H alkylation of quinoxalin-2(1H)-ones to obtain C-C bond by radical addition reaction of alkyl boric acid (Scheme 1a) [73]. Despite the wide range of substrates and good yields, they both inevitably needed noble metal catalysis, excessive additives and harsh reaction conditions. At the same time, we noticed that most of boric acids are used for arylation reagent, and alkyl boronic acid used as alkylation reagents are rarely reported. Since Anastas and Warner put forward 12 principles of green chemistry, using molecular oxygen as the only oxidant has become a very meaningful exploration [74-79]. In 1860, Frankland first proposed the application of alkyl borane in free radical reaction and then the R3B/O2 reaction system was often used to initiate free radicals [80]. Therefore, based on the above research and our previous works [81-87], we tried to use alkyl boric acid/O2 to construct the C-C bond of quinoxalin-2(1H)-ones. After a series of reaction studies, we were surprised to find that this reaction can not only produce 3-alkyl quinoxalone, but also further oxidize C-C bond to C-O bond under different solvent and catalyst conditions (Scheme 1b).
|
Download:
|
| Scheme 1. The C–H alkylation of quinoxalin-2(1H)-ones using alkylboronic acids as alkylation reagent. | |
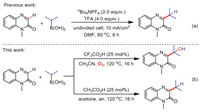
In the beginning, we chose quinoxalin-2(1H)-ones (1a) and isopropyl boronic acid (2a) as starting substrates, CF3CO2H as Catalyst, 1,2-dichloroethane (DCE) as solvent, the yield of 3a was 51% under oxygen atmosphere and 100 ℃ (Table 1, entry 1). When the temperature dropped to 80 ℃ or 60 ℃, the yields immediately drop to 23% and 14% (Table 1, entries 2 and 3). Conversely, when the reaction temperature was increased to 120 ℃, the yield increased to 72%, while a further increase in temperature did not significantly increase the yield (Table 1, entries 4 and 5). Subsequently, when the catalyst was CH3CO2H, CH3SO3H, CF3SO3H or Et3N, the reaction effect was not as good as CF3CO2H, but unexpectedly, the reaction could give product 4a in 40% yield under CH3CO2H condition (Table 1, entries 6–10). After screening the reaction solvent, we found that CH3CN was the most suitable solvent for this reaction, compared with DCE, 1,4-dioxane, dimethylsulfoxide (DMSO), THF, acetone and H2O (Table 1, entries 11–16). Finally, when the gas atmosphere was air, 26% of 3a and 23% of 4a were obtained (Table 1, entry 17). The reaction hardly proceeded under N2 atmosphere (Table 1, entry 18). This shows that O2 was essential for the transformations. Combining entry 6, we believed that under the condition of using CH3CO2H as a catalyst, 120 ℃ and an oxygen atmosphere, by changing the reaction solvent, the most favorable reaction condition for the formation of 4a may be found. By screening the solvent, acetone came to the fore, it can increase the yield of product 4a to 90% without the formation of 3a (Table 1, entries 19–24). Similarly, in order to determine the most suitable gas atmosphere for 4a, we tried to change the oxygen atmosphere to air or nitrogen. It can be seen from Table 1, entries 25 and 26 that when the reaction atmosphere was changed to air, the yield of 4a was not affected and the nitrogen atmosphere greatly hinders the production of 4a. Therefore, the CH3CO2H/acetone system only needs air atmosphere, which relatively reduces the experimental operation steps.
|
|
Table 1 Optimization of reaction conditions.a |
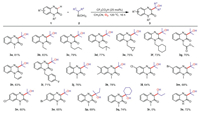
Under the optimal conditions, we next investigated the effect of different structures of quinoxalin-2(1H)-one derivatives and alkyl boronic acids under the conditions of CF3CO2H as catalyst, CH3CN as solvent and oxygen atmosphere (Scheme 2). The experimental results proved that the reaction of quinoxalin-2(1H)-ones was good regardless of whether it has electron-donating substituents or electron-withdrawing substituents, and the corresponding products (3a-3o) can be produced in moderate-to-good yields. Among them, quinoxalin-2(1H)-ones exhibit good functional group tolerance whether they have cyclopropylmethyl, cyclohexylmethyl, propenyl, benzyl group at the N-1 position or methyl, halogen groups at the 6, 7 positions. In addition, when 2-butylboronic acid and cyclohexyl boronic acid were substituted for isopropyl boronic acid, the target product yields were 69% and 74%, respectively (3p, 3q). However, when primary alkyl boronic acid such as n-propyl boronic acid, the corresponding product (3r) cannot be obtained through this reaction. It is worth mentioning that the N-unprotected quinoxalin-2(1H)-one (3s) also gives 72% of the desired product in this reaction.
|
Download:
|
| Scheme 2. Substrate scope for the hydroxyalkylation of quinoxalin-2(1H)-ones with alkylboronic acids. Reaction conditions: 1 (0.2 mmol), 2 (2.0 equiv.), CF3CO2H (25 mol%), CH3CN (2.0 mL), O2, 120 ℃, 16 h, isolated yields. | |
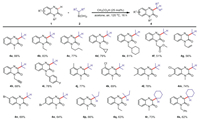
Next, we discussed the influence of the structure of different quinoxalin-2(1H)-one derivatives and alkyl boronic acids on the reaction results with CH3CO2H as a catalyst, acetone as a solvent, and air atmosphere conditions (Scheme 3). It can be seen from the table that quinoxalin-2(1H)-ones with different saturated alkyl carbon chains (methyl, ethyl, n-butyl, cyclopropylmethyl, cyclohexylmethyl) and fluorobenzyl at the N-1 position performed well with yields of 77%−88% (4a-4e, 4i). However, when the quinoxalin-2(1H)-one has an unsaturated carbon chain such as ester group, allyl, or alkynyl at the N-1 position, the corresponding product can be obtained in moderate yield (4f-4h). In addition, when the quinoxalin-2(1H)-one benzene ring has methyl or halogen group at C5, C6 and C7 positions, respectively, the corresponding products (4j-4o) were obtained in higher yields of 64%−78%. The corresponding products (4p-4s) were also prepared in good yields when 2-butylboronic acid and cyclohexylboronic acid were substituted for isopropylboronic acid, respectively. To extend the substrate scope, in addition to quinoxalin-2(1H)-ones, we also tested other substrates such as dihydroquinoline (1aa), quinoline (1ab), isoquinoline (1ac) and quinoxaline (1ad) in these two catalytic systems, however, no corresponding product was obtained (see Supporting information).
|
Download:
|
| Scheme 3. Substrate scope for the alkylation of quinoxalin-2(1H)-ones with alkylboronic acids. Reaction conditions: 1 (0.2 mmol), 2 (2.0 equiv.), CH3CO2H (25 mol%), acetone (2.0 mL), air, 120 ℃, 16 h, isolated yields. | |
Furthermore, to display the value of these two methods, gram-scale synthesis of 1-methylquinoxalin-2(1H)-one (1a, 8.0 mmol) was carried out. The target products 3a (72%, 1.27 g) (Scheme 4a) and 4a (76%, 1.23 g) (Scheme 4b) were obtained in good yields, respectively. In addition, in order to expand the applicable substrates of the CF3CO2H/CH3CN system, two arylboronic acids, phenyl boronic acid and 4-methoxyphenyl boronic acid, were used as raw materials for the reaction (Scheme 4c). The corresponding products (6a and 6b) were obtained in 54% and 51% yields. However, the 4-nitrophenylboronic acid was incompatible probably due to the strong electron-withdrawing effect.
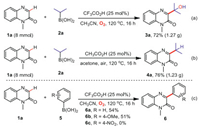
|
Download:
|
| Scheme 4. Gram-scale synthesis and study of aromatic boric acid. | |
Subsequently, in order to clarify the plausible mechanism by which the reaction occurs, we performed free radical trapping experiments. Under the same reaction conditions, we added 2 equiv. of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) (Schemes 5a and b), 1,1-diphenylethene (DPE) (Scheme S1 in Supporting information) or butylated hydroxytoluene (BHT) (Scheme S2 in Supporting information) radical inhibitors to the CF3CO2H/CH3CN and CH3CO2H/acetone reaction systems, respectively, the transformations were obviously inhibited, and the adducts of TEMPO and DPE with isopropanol radicals in both reaction systems were detected by high resolution mass spectrometer (HRMS). Although the ring-open experiment of phenylcyclopropane did not give the corresponding product 10 (Scheme S3 in Supporting information), the cascade reaction of diethyl 2,2-diallylmalonate with 1a and 2a provided the product 11 (Scheme S4 in Supporting information). It was clear that both reaction systems involved isopropyl radicals, and the CF3CO2H/CH3CN system probably involved further oxidation of isopropyl to form isopropanol radicals process. To verify the oxygen source of product 3a in the CF3CO2H/CH3CN system, we used the 18O2 instead of normal oxygen for the 18O-labeling experiments (Scheme 5c). After the reaction, HRMS successfully captured a large amount of product, which contained 18O atoms, confirming that the oxygen source of the reaction came from the oxygen in the system. Besides, we tried to purify the alkylated product 4a and put it into the CH3CN as raw material (Scheme 5d). In the presence of alkyl boronic acid (2a), 3a can be obtained in 85% yield, but in the absence of 2a, 3a cannot be obtained. The results show that 4a can be oxidized to 3a by oxygen under the condition of alkyl boric acid. Furthermore, when the reaction was conducted in the presence of DPE or BHT respectively, it was completely inhibited, and adducts 12 and 13 were detected, implying a radical pathway was involved (Scheme S5 in Supporting information).
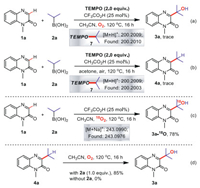
|
Download:
|
| Scheme 5. Control experiments. | |
Based on the above results and literature reports [88-91], a possible mechanism was proposed (Scheme 6). Firstly, the alkyl boric acid 2a produced isopropyl radical B and boric acid radical D under the condition of heating oxygen atmosphere. Then, in under acidic condition, quinoxalin-2(1H)-one 1a was converted into corresponding salt A, which was attacked by isopropyl radical B at the C3 position of quinoxalin-2(1H)-one to form intermediate C, followed by the loss of one electron and one proton to give intermediate E. Product 4a was obtained through a deprotonation process. For the formation of product 3a, initially, with the assistance of isopropyl radical B, 4a was transformed into intermediate F, which was captured by oxygen molecule to give peroxy radical G. Then, the radical coupling of F and G provided peroxides H, which was converted into intermediate I by the homolysis of O-O bond. The final product 3a was obtained through a hydrogen atom transfer process of intermediate I with 4a.
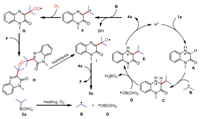
|
Download:
|
| Scheme 6. Plausible mechanism. | |
In conclusion, we have achieved a chemselective method for hydroxyalkylation and alkylation of quinoxalin-2(1H)-ones with alkylboronic acids. Such transformations were performed under metal-free conditions, various quinoxalin-2(1)-ones and alkylboronic acids were compatible, providing the corresponding products in moderate-to-good yields.
Declaration of competing interestThe authors declare that they have no conflict of interest.
AcknowledgementWe thank the Natural Science Foundation of Zhejiang Province (No. LY21B060009) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.069.
| [1] |
K. Makino, G. Sakata, K. Morimoto, Y. Ochiai, Heterocycles 23 (1985) 2025-2034. DOI:10.3987/R-1985-08-2025 |
| [2] |
A. Monge, F.J. Martinez-Crespo, A.L. Cerai, et al., J. Med. Chem. 38 (1995) 4488 4494. |
| [3] |
M.M. Badran, K.A.M. Abouzid, M.H.M. Hussein, Arch. Pharma-cal Res 26 (2003) 107-113. |
| [4] |
H.M. Refaat, A.A. Moneer, O.M. Khalil, Arch. Pharmacal Res. 27 (2004) 1093-1098. |
| [5] |
A. Carta, S. Piras, G. Loriga, G. Paglietti, Mini-Rev. Med. Chem. 6 (2006) 1179-1200. DOI:10.2174/138955706778742713 |
| [6] |
X. Li, K. Yang, W. Li, Xu W, Drugs Future 31 (2006) 979-989. DOI:10.1358/dof.2006.031.11.1037128 |
| [7] |
M. Weïwer, J. Spoonamore, J. Wei, et al., ACS Med. Chem. Lett. 3 (2012) 1034-1038. DOI:10.1021/ml300246r |
| [8] |
W. Wei, L.L. Wang, H.L. Yue, et al., ACS Sustainable Chem. Eng. 6 (2018) 17252-17257. DOI:10.1021/acssuschemeng.8b04652 |
| [9] |
J.W. Yuan, J.H. Fu, S.N. Liu, et al., Org. Biomol. Chem. 16 (2018) 3203-3213. |
| [10] |
B. Wu, Y. Yang, X. Qin, et al., ChemMedChem 8 (2013) 1913-1917. DOI:10.1002/cmdc.201300324 |
| [11] |
X. Qin, X. Hao, H. Han, et al., J. Med. Chem. 58 (2015) 1254-1267. DOI:10.1021/jm501484b |
| [12] |
D.S. Lawrence, J.E. Copper, C.D. Smith, J. Med. Chem. 44 (2001) 594-601. |
| [13] |
S.A.M. El-Hawash, N.S. Habib, M.A. Kassem, Arch. Pharm. 339 (2006) 564-571. DOI:10.1002/ardp.200600061 |
| [14] |
K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533. DOI:10.1021/acs.orglett.7b00310 |
| [15] |
J. Yuan, S. Liu, L. Qu, Adv. Synth. Catal. 359 (2017) 4197-4207. DOI:10.1002/adsc.201701058 |
| [16] |
W. Wei, L. Wang, H. Yue, et al., ACS Sustainable Chem. Eng. 6 (2018) 17252-17257. DOI:10.1021/acssuschemeng.8b04652 |
| [17] |
S. Liu, Y. Huang, F.L. Qing, X.H. Xu, Org. Lett. 20 (2018) 5497-5501. DOI:10.1021/acs.orglett.8b02451 |
| [18] |
J. Xu, L. He, C. Liang, et al., ACS Sustainable Chem. Eng. 40 (2021) 13663-13671. DOI:10.1021/acssuschemeng.1c05237 |
| [19] |
J. Yuan, J. Fu, J. Yin, et al., Qu, Org. Chem. Front 5 (2018) 2820-2828. DOI:10.1039/c8qo00731d |
| [20] |
K. Yin, R. Zhang, Synlett 29 (2018) 597-602. |
| [21] |
B. Ramesh, C.R. Reddy, G.R. Kumar, B.V.S. Reddy, Tetrahedron Lett. 59 (2018) 628-631. |
| [22] |
L. Wang, Y. Zhang, F. Li, et al., Adv. Synth. Catal. 360 (2018) 3969-3977. DOI:10.1002/adsc.201800863 |
| [23] |
H. Zhang, J. Xu, M. Zhou, et al., Org. Biomol. Chem. 17 (2019) 10201-10208. DOI:10.1039/c9ob02203a |
| [24] |
L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464. DOI:10.1007/s11426-018-9446-1 |
| [25] |
L.X. Liu, N. Pan, W. Sheng, et al., Adv. Synth. Catal. 361 (2019) 4126-4132. DOI:10.1002/adsc.201900572 |
| [26] |
L.Y. Xie, L.L. Jiang, J.X. Tan, et al., ACS Sustainable. Chem. Eng. 7 (2019) 14153-14160. DOI:10.1021/acssuschemeng.9b02822 |
| [27] |
G. Hong, J. Yuan, J. Fu, et al., Org. Chem. Front. 6 (2019) 1173-1182. DOI:10.1039/c9qo00105k |
| [28] |
J. Shen, J. Xu, L. He, C. Liang, X. Zhuang, B. Sun, W. Li, Chin. Chem. Lett. (2021) 10.1016/j.cclet.2021.09.005. DOI:10.1016/j.cclet.2021.09.005 |
| [29] |
J. Wang, B. Sun, L. Zhang, et al., Asian J. Org. Chem. 8 (2019) 1942-1946. DOI:10.1002/ajoc.201900414 |
| [30] |
L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725. DOI:10.1039/c9gc03899j |
| [31] |
P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498. DOI:10.1039/c9qo01334b |
| [32] |
J. Wang, B. Sun, L. Zhang, et al., Org. Chem. Front. 7 (2020) 113-118. |
| [33] |
J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042. DOI:10.1039/d0qo00872a |
| [34] |
J. Shen, J. Xu, L. Huang, Q. Zhu, P. Zhang, Adv. Synth. Catal. 362 (2020) 230-241. DOI:10.1002/adsc.201901314 |
| [35] |
Y.F. Si, X.L.Chen Sun, Org. Lett. 22 (2020) 6960-6965. DOI:10.1021/acs.orglett.0c02518 |
| [36] |
J. Xu, H. Yang, L. He, et al., Org. Lett. 23 (2021) 195-201. |
| [37] |
J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 2123-2129. DOI:10.1039/d0gc04235h |
| [38] |
L. Huang, J. Xu, L. He, et al., Chin. Chem. Lett. 32 (2021) 3627-3631. |
| [39] |
J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 6632-6638. DOI:10.1039/d1gc01899j |
| [40] |
J. Xu, H. Yang, H. Cai, et al., Org. Lett. 21 (2019) 4698-4702. DOI:10.1021/acs.orglett.9b01578 |
| [41] |
J. Zhou, P. Zhou, T. Zhao, Q. Ren, J. Li, Adv. Synth. Catal. 361 (2019) 5371-5382. DOI:10.1002/adsc.201901008 |
| [42] |
S. Peng, D. Hu, J.L. Hu, et al., Adv. Synth. Catal. 361 (2019) 5721-5726. DOI:10.1002/adsc.201901163 |
| [43] |
L. Zhao, L. Wang, Y. Gao, Z. Wang, P. Li, Adv. Synth. Catal. 361 (2019) 5363-5370. DOI:10.1002/adsc.201900732 |
| [44] |
Q. Yang, X. Han, J. Zhao, H.Y. Zhang, Y. Zhang, J. Org. Chem. 84 (2019) 11417-11424. DOI:10.1021/acs.joc.9b01181 |
| [45] |
J.Z. Jin, J.Y. Tong, W.B. Yu, J. Qiao, C. Shen, Catal. Commun. 141 (2020) 106008. |
| [46] |
A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792. DOI:10.1021/acs.joc.7b00464 |
| [47] |
T.T. Hoang, T.A. To, V.T.T. Cao, et al., Catal. Commun. 101 (2017) 20-25. |
| [48] |
Q. Yang, Y. Zhang, Q. Sun, et al., Adv. Synth. Catal. 360 (2018) 4509-4514. DOI:10.1002/adsc.201801076 |
| [49] |
W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130. DOI:10.1021/acs.orglett.8b03079 |
| [50] |
L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustainable Chem. Eng. 7 (2019) 19993-19999. DOI:10.1021/acssuschemeng.9b05715 |
| [51] |
J.W. Yuan, J.L. Zhu, B. Li, et al., Org. Biomol. Chem. 17 (2019) 10178-10187. DOI:10.1039/c9ob02157d |
| [52] |
Q. Yang, Z. Yang, Y. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667. DOI:10.1002/adsc.201801661 |
| [53] |
W. Wimonsong, S. Yotphan, Tetrahedron 81 (2021) 131919. |
| [54] |
K. Niu, L. Ding, P. Zhou, et al., Green Chem. 23 (2021) 3246-3249. DOI:10.1039/d1gc00861g |
| [55] |
L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955. DOI:10.1039/c9qo01240k |
| [56] |
Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245. DOI:10.1039/c9gc03045j |
| [57] |
K. Sun, F. Xiao, B. Yu, W.M. He, Chin. J. Catal. 42 (2021) 1921-1943. |
| [58] |
M. Gao, Y. Li, L. Xie, R. Chauvin, X. Cui, Chem. Commun. 52 (2016) 2846-2849. DOI:10.1039/C5CC08049E |
| [59] |
J. Wang, J. Li, Y. Wei, J. Yang, C. Huo, Org. Chem. Front. 5 (2018) 3534-3537. DOI:10.1039/c8qo01049h |
| [60] |
Y. Kim, D.Y. Kim, Tetrahedron Lett. 59 (2018) 2443-2446. |
| [61] |
K.J. Li, Y.Y. Jiang, K. Xu, C.C. Zeng, B.G. Sun, Green Chem. 21 (2019) 4412-4421. |
| [62] |
W.P. Mai, J.W. Yuan, J.L. Zhu, et al., ChemistrySelect 4 (2019) 11066-11070. DOI:10.1002/slct.201903478 |
| [63] |
D. Rawat, R. Kumar, A. Subbarayappa, Green Chem. 22 (2020) 6170-6175. DOI:10.1039/d0gc02168g |
| [64] |
C. Dai, Y. Zhan, P. Liu, P. Sun, Green Chem. 23 (2021) 314-319. DOI:10.1039/d0gc03697h |
| [65] |
L. Yang, P. Gao, X.H. Duan, Y.R. Gu, L.N. Guo, Org. Lett. 20 (2018) 1034-1037. DOI:10.1021/acs.orglett.7b03984 |
| [66] |
W. Zhang, Y.L. Pan, C. Yang, et al., J. Org. Chem. 84 (2019) 7786-7795. DOI:10.1021/acs.joc.9b00657 |
| [67] |
J. Fu, J. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390. DOI:10.1039/c8qo00979a |
| [68] |
N.A. Petasis, I.A. Zavialov, J. Am. Chem. Soc. 119 (1997) 445-446. |
| [69] |
D.S. Matteson, H.W. Man, J. Org. Chem. 61 (1996) 6047-6051. |
| [70] |
T. Ishiyama, M. Murata, N. Miyaura, Org. Chem. 60 (1995) 7508-7510. DOI:10.1021/jo00128a024 |
| [71] |
I.B. Seiple, S. Su, R.A. Rodriguez, et al., J. Am. Chem. Soc. 132 (2010) 13194-13196. DOI:10.1021/ja1066459 |
| [72] |
S. Castro, J.J. Fernández, F.J. Fañanás, R. Vicente, F. Rodríguez, Chem. Eur. J. 22 (2016) 9068-9071. DOI:10.1002/chem.201601482 |
| [73] |
K. Niu, Y. Hao, L. Song, Y. Liu, Q. Wang, Green Chem. 23 (2021) 302-306. DOI:10.1039/d0gc03892j |
| [74] |
J. Jiang, F. Xiao, W.M. He, L. Wang, Chin. Chem. Lett. 32 (2021) 1637-1644. |
| [75] |
C. Miao, B. Yu, L. He, Green Chem. 13 (2011) 541-544. DOI:10.1039/c0gc00676a |
| [76] |
A. Gonzalez-de-Castro, J. Xiao, J. Am. Chem. Soc. 137 (2015) 8206-8218. DOI:10.1021/jacs.5b03956 |
| [77] |
G. Urgoitia, R. SanMartin, M.T. Herrero, E. Domínguez, Adv. Synth. Catal. 358 (2016) 1150-1156. DOI:10.1002/adsc.201500944 |
| [78] |
S. Yang, P. Li, Z. Wang, L. Wang, Org. Lett. 19 (2017) 3386-3389. DOI:10.1021/acs.orglett.7b01230 |
| [79] |
J. Xu, Y. Zhang, X. Yue, et al., Green Chem. 23 (2021) 5549-5555. DOI:10.1039/d1gc01364e |
| [80] |
E. Frankland, B.F. Duppa, Liebigs Ann. Chem. 115 (1860) 319-322. DOI:10.1002/jlac.18601150324 |
| [81] |
H. Shen, C. Shen, C. Chen, A. Wang, P. Zhang, Catal. Sci. Technol. 5 (2015) 2065-2071. DOI:10.1039/C5CY00013K |
| [82] |
C. Xia, Z. Wei, C. Shen, et al., RSC Adv. 5 (2015) 52588-52594. DOI:10.1039/C5RA06474K |
| [83] |
J. Xu, K. Du, J. Shen, et al., ChemCatChem 10 (2018) 3675-3679. DOI:10.1002/cctc.201800328 |
| [84] |
J. Xu, K. Cheng, C. Shen, et al., ChemCatChem 10 (2018) 965-970. DOI:10.1002/cctc.201701596 |
| [85] |
W. Yu, L. Jiang, C. Shen, W. Xu, P. Zhang, Catal. Commun. 79 (2016) 11-16. |
| [86] |
B. Ying, J. Xu, X. Zhu, C. Shen, P. Zhang, ChemCatChem 8 (2016) 2604-2608. DOI:10.1002/cctc.201600555 |
| [87] |
C. Shen, M. Yang, J. Xu, et al., RSC Adv. 7 (2017) 49436-49439. DOI:10.1039/C7RA09053F |
| [88] |
A. Guo, J.B. Han, X.Y. Tang, Org. Lett. 20 (2018) 2351-2355. DOI:10.1021/acs.orglett.8b00642 |
| [89] |
T. Liu, Y.W. Li, L.F. Lai, et al., Org. Lett. 20 (2018) 3605-3608. DOI:10.1021/acs.orglett.8b01385 |
| [90] |
X. Zhu, Y. Liu, C. Liu, H. Yang, H. Fu, Green Chem. 22 (2020) 4357-4363. DOI:10.1039/d0gc00383b |
| [91] |
B. Sun, R. Zhu, X.H. Zhuang, et al., Org. Lett. 23 (2021) 617-622. DOI:10.1021/acs.orglett.0c04216 |
 2022, Vol. 33
2022, Vol. 33