b Interdisciplinary Sciences Institute, Huazhong Agricultural University, Wuhan 430070, China;
c State Key Laboratory of Agricultural Microbiology, College of Animal Sciences and Technology, Huazhong Agricultural University, Wuhan 430070, China;
d Hubei Hongshan Laboratory, Wuhan 430070, China;
e The Cooperative Innovation Center of Sustainable Pig Production, Wuhan 430070, China;
f Hubei Provincial Engineering Laboratory for Pig Precision Feeding and Feed Safety Technology, Wuhan 430070, China;
g Institute of Pharmaceutical Analysis, School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan 250012, China
Microorganisms, including bacteria, viruses, fungi and algae, live in almost every habitat on the planet, from deep-sea vents to the highest clouds to the digestive tracts of humans [1]. They are so small, diverse, highly adaptable and highly mutable, that advanced technologies are essential to identify, characterize, isolate, culture, engineer and utilize microorganisms, and to uncover complex interactions in microbial ecosystems and/or between microorganisms and their hosts [2, 3]. Droplet microfluidics, as one subcategory of microfluidics that generates and manipulates subnanoliter volume droplets inside microdevices, has become an indispensable tool changing the paradigm of experimentation in microbiology [4-6]. Droplet microfluidics provides multiple unique benefits for microbiological research. Firstly, by encapsulating microorganisms in microdroplets, a small quantity of cells, or even single cells can be confined in isolated environments [7]; interspecies competition and biases due to growth rate differences are eliminated, which facilitate recovery of rare or slow-growing microorganisms from complex ecosystems [8]; fast accumulation of metabolites in droplets enables easy activation of concentration-dependent processes such as quorum sensing [9]. Secondly, droplet microfluidics allows rapid droplet production with a speed up to 20, 000 per second and massive droplet analysis, opening up the possibility of ultrahigh-throughput identification, screening and strain improvement of microorganisms [5, 10-12]. Thirdly, in droplet microfluidics, configurable channel designs and incorporated control modules engender precise droplet manipulations, including fusion, mixing, splitting, long-term incubation and sorting [13]; these allow, for example, introducing multiple detection reagents or stimuli to microorganisms [14, 15], creating well-controlled and variable culture environments [16], or selectively collecting improved microbial strains [17].
The above features collectively make droplet microfluidics a robust and valuable platform for microbiology studies. Combining with advanced sequencing technologies [18] or dynamic fluorescence imaging [19], droplet microfluidics helps bring microbiologists closer to the ultimate goal of microbial ecology: observing and understanding who does what, when, where and why [3]. In the present review, we focus on the advances in droplet microfluidics for microbiology within the past five years. We start the review with a brief introduction of droplet generation strategies in microfluidics, followed by introducing the advances and applications of droplet microfluidics in various fields of microbiology: i) microbial cultivation, ii) microorganism detection and characterization, iii) antibiotic susceptibility testing (AST), iv) microbial interactions, v) microbial biotechnology (Fig. 1). Finally, we will discuss the challenges and future perspectives of droplet microfluidics for microbiology research, inspiring the collaborations between microbiologists and microfluidic researchers to push the boundaries of this field beyond what we now consider possible.
|
Download:
|
| Fig. 1. Applications of droplet microfluidics in various fields of microbiology. | |
Most droplet generation approaches in microfluidics could be categorized into flow-based strategy and array-based strategy. In flow-based strategy, droplets are formed through the extrusion and shearing of two mutually immiscible phases in a microchannel [4]. According to different channel configurations, the flow-based strategy can be classified as co-flowing, T-junction, flow-focusing and step emulsification methods [20, 21]. Flow-focusing is the most common geometry, where the dispersed phase (aqueous phase) and continuous phases (oil phase) flow co-axially through a contraction region and generate an elongation filament that eventually breaks into droplets [7]. Droplet volumes are controlled by the flow rate ratios and channel dimensions [22]. To realize on-demand droplet generation, active modules, such as electrical, magnetic, thermal, or mechanical controls, can be incorporated into flow-based droplet microfluidics, offering additional handles to regulate droplet volumes and generation rates [23]. In array-based strategy, aqueous droplets are spatially organized in arrays of microwells, microchambers or hydrophilic micropatterns, which are isolated from each other by the oil phase [24, 25]. Droplet volumes are mainly controlled by the dimensions of microwells, microchambers or micropatterns. Array-based strategy allows droplets to be indexed by their spatial layout, which will be beneficial to identify, monitor, and address individual droplets [26, 27]. Both droplet generation strategies have been successfully utilized in microbiology research. By using a microorganism suspension as the aqueous phase, microorganisms can be encapsulated in droplets, and the distribution of the cell number per droplet follows the Poisson distribution [28].
3. Microbial cultivationMicrobial cultivation is the foundational method in microbiology, to grow and diagnose microorganisms, to isolate and obtain pure cultures, and to investigate effects of environmental factors [29]. In traditional culture methods, such as agar plating and nutrient broth, it takes a long time for microbes to grow and multiply to be detectable, and rare or slow-growing species in the microbial community can be omitted because of interspecies competitions and growth rate differences [30]. Droplet-based microbial cultivation can resolve these issues by isolating and culturing a small quantity of cells or single cells in microdroplets, which enables real-time monitoring of microbial growth by microscopy, prevents interspecies competition, and allows precise control and flexible regulation of microbial growth conditions.
Droplet microfluidics represents one of the most powerful techniques to isolate and cultivate single microbial cells in confined environments [31]. The growth of microbes within individual droplets can be tracked by recording cell numbers under bright field or performing fluorescence-based quantification over time [26, 32]. Dynamic microbial monitoring is valuable for investigating the effects of different nutrient sources as well as stress conditions, such as acid stress [32, 33], heavy metal exposition [34, 35], antibiotics [36] or electric field [37], on microorganisms. Jusková et al. presented a microfluidic system named "Basicles", which generated giant unilamellar lipid vesicles (GUVs) for single-bacteria encapsulation and immobilized the GUVs by hydrodynamic traps for real-time monitoring of microbial growth and production [36]. Escherichia coli (E. coli) could be cultivated in GUVs for up to 15 h, and the growth of bacteria per GUV was monitored based on the increase of sfGFP (super-folder green fluorescent protein) fluorescence. A significant advantage of this system was that membrane-permeable reagents could passively diffuse across the liposomal membrane [38], achieving controlled feeding and stimulation of the encapsulated bacteria. Therefore, riboflavin biosynthesis behaviors of E. coli were assessed under different conditions with inducers or antibiotics. Compared to optical-based growth assays, molecular techniques, such as quantitative PCR (qPCR) and gene sequencing, can enable more comprehensive and systematic growth measurements of hundreds of gut bacterial species in droplets. Villa et al. developed a MicDrop platform to isolate and culture individual gut bacteria in droplets from human gut microbiota (Fig. 2a) [39]. Since a large proportion of gut microbiome are obligate anaerobes, microbial encapsulation and incubation in droplets were performed in an anaerobic chamber to ensure high cell viability. To track microbial growth, bacteria were recovered from droplets at different culture time points, followed by DNA sequencing and total quantification of the 16S rRNA gene, providing absolute levels of each sequence variant across all droplets. MicDrop enabled to cultivate 2.8 times more bacterial taxa than standard bulk culture. The high-throughput and quantitative growth measurements allowed characterizing carbohydrate utilizations of gut bacterial taxa from distinct human stool samples.
|
Download:
|
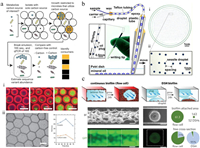
| Fig. 2. Microbial cultivation in droplets. (a) MicDrop platform for isolating and culturing gut bacteria in droplets; distinct colony morphologies could be observed across droplets of gut microbial community; scale bars: (i) 100 μm, (ii) 50 μm. Reproduced with permission [39]. Creative Commons Attribution 4.0 International License. (b) Microfluidic streak plate (MSP) for single-microbe separation and cultivation in nanoliter droplets; scale bars: (i) 1 cm, (ii) 1 mm. Reproduced with permission [40]. Copyright 2016, American Society for Microbiology. (c) Comparisons of bacterial biofilm formations in microfluidic flow cell and dynamic sessile-droplet habitats (DSHs); scale bars: 100 μm. Reproduced with permission [45]. Copyright 2018, Wiley-VCH. | |
Droplet-based microbial cultivation facilitates isolation of rare or slow-growing microorganisms from complex community. The Du group described the microfluidic streak plate (MSP) for high-throughput single-microbe separation and cultivation in nanoliter droplets (Fig. 2b) [40]. Droplets generated by a microfluidic device were streaked onto petri dishes prefilled with carrier oil, forming sessile-droplet arrays. Microbes could be cultured for up to five months at room temperature. Rapid recovery of microbes after growth could be easily achieved by using a toothpick or an automatic droplet picker [41], allowing scale-up cultivation and downstream characterization. Results from 16S rRNA sequencing demonstrated that MSP method enabled detection of the richest species diversity with better coverage of rare species compared to the agar plate method. The MSP platform provided a powerful tool in isolating the rare species from complex environmental samples, including soil [42], termite gut microbiome [43] and deep-sea sediments [41]. Watterson et al. utilized droplet microfluidics in an anaerobic chamber to isolate and cultivate gut microbiota [44]. A unique feature of this system was the incorporation of a droplet sorting unit, which allowed isolating slow growing organisms based on the optical density (OD) of droplets and further amplified the abundance of rare taxa. This system offered an increase of taxonomic richness by 15% to 410% over conventional plates depending on the culture medium, and the droplet sorting enriched six separate Alistipes populations, which were either not detected or in very low abundance in plate-based cultivations.
Apart from single microorganism culture, droplet microfluidics also enables controllable cultivation of bacterial biofilms. The Du group introduced a dynamic sessile-droplet habitat (DSH) platform, containing an array of hydrophilic patterns in different sizes at the downstream of a T-junction droplet generator (Fig. 2c) [45]. By flowing nanoliter plugs containing bacteria through the patterns, an array of discrete sessile droplets with inoculated bacteria was produced, which could form spatially confined biofilms and culture over a long term by flowing media plugs through the patterns subsequently. The DSH method could avoid channel clogging and flow disruption that always appeared in microfluidic flow cell-based method, providing more consistent hydrodynamic environments for biofilm growth and stimulation. This platform allowed lifespan monitoring of Pseudomonas aeruginosa biofilm formation by fluorescence, and the roles of flagella and pili in biofilm development were investigated.
4. Microorganism detection and characterizationDetection and characterization of microorganisms are pivotal from fundamental microbiology to infectious disease diagnosis to food industry [46, 47]. Attributed to the capabilities of rapid droplet production and massive droplet analysis, droplet microfluidics paves the way to ultrahigh-throughput and highly parallelized microbial analysis. Recently, various detection techniques have been utilized to analyze microorganisms in droplets, including optical-, electrical-, mass spectrometry-, and molecular biology-based methods [10, 48-51]. In this section, we will discuss the recent advances in droplet microfluidic-based microorganism detection and characterization, mainly focusing on the optical detection and molecular biology methods including droplet digital PCR, sequencing-based characterization and Cas13a-based detection.
4.1. Optical detectionOptical detection of droplets is one of the most frequently used techniques [52], which is straightforward and enables real-time analysis. Hsieh et al. developed resazurin-amplified picoarray detection (RAPiD) for simple and precise counting of viable bacteria [53]. Using vacuum-assisted sample loading and oil-driven digitization, single bacteria were enclosed in picochambers with resazurin, an indicator dye that could be reduced by viable cells to strongly fluorescent resorufin. By counting the number of chambers exhibiting fluorescence signals, viable bacteria in E. coli and Staphylococcus aureus (S. aureus) samples could be quantified with high precision. Viable bacteria enumeration could also be achieved by using microdroplet turbidity imaging-based digital counting, which was label-free and allowed signal acquisition using a smartphone camera, benefiting point-of-care applications [54].
Despite being highly convenient for microbial analysis, fluorescent detection relies on the labeling of fluorescent tags or introduction of fluorescent substrates, which is only applicable to some specific target molecules and may affect microbial metabolism. In contrast, single-cell Raman spectrum (SCRS), as a label-free and non-invasive technique for metabolite detection in a given cell based on the vibrational signals from molecules, can provide intrinsic biochemical "fingerprints" for individual cells [55, 56]. The Xu group coupled the SCRS with droplet microfluidics for culture-free and label-free microbial characterization and screening. They developed Raman-activated gravity-driven single-cell encapsulation and sequencing (RAGE-Seq) for phenome–genome profiling of single bacterial cells [57]. Individual cells were phenotypically screened via SCRS at first, and the targeted cells were respectively encapsulated in picoliter droplets, which were then exported in a precisely indexed, "one-cell-one-tube" manner. Genomic DNA from the sorted cell was individually amplified in droplet and subjected to whole genome sequencing. The RAGE-seq system allowed to link metabolic phenotype with high-quality genotype at the one-cell resolution. However, the throughput of the RAGE-seq system was 2 cells per minute, which was relatively low. To achieve higher throughput, they further presented positive dielectrophoresis-based Raman-activated droplet sorting (pDEP-RADS), in which fast-moving single microbes were efficiently trapped by pDEP field, profiled via SCRS, encapsulated in droplets for DEP-based sorting, and collected for sequencing-based identification (Fig. 3a) [58]. The pDEP-RADS was capable of processing hundreds of cells per minute, allowing culture-free and label-free screening of enzyme functions in vivo. Two previously unknown diacylglycerol acyltransferases (DGATs) variants were discovered from an engineered yeast library, which were undetectable via conventional fluorescence-activated cell sorting.
|
Download:
|
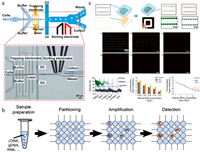
| Fig. 3. Microorganism detection and characterization in droplets. (a) Positive dielectrophoresis–based Raman-activated droplet sorting; scale bar: 200 μm. Reproduced, with permission [58]. Creative Commons Attribution 4.0 International License. (b) Schematic diagram of droplet digital PCR. Reproduced, with permission [60]. Creative Commons Attribution 4.0 International License. (c) Slip-induced self-partitioning for droplet generation and ddPCR quantification of BK virus DNA in different concentrations; scale bar: 2 mm. Reproduced with permission [80]. Copyright 2021, Elsevier. | |
Droplet digital PCR (ddPCR) is the first commercially significant application of droplet microfluidics [4]. As a powerful technology allowing absolute nucleic acid quantitation, ddPCR has been widely adopted in the detection of pathogenic microorganisms [59]. ddPCR involves partitioning PCR solution into tens of thousands of nanoliter droplets, with the requirement that each droplet contains either "one" or "zero" target nucleic acid (Fig. 3b) [60]. Droplets are then amplified individually, and the concentration of target nucleic acid can be obtained by counting the number of positive (fluorescent) droplets at the end point and calculating based on Poisson statistics [61]. ddPCR is independent of external standard calibrations and exhibits higher sensitivity and precision compared to quantitative PCR (qPCR). Recently, it has been successfully applied to quantify various bacterial species in diverse samples [62-65], such as Lactococcus lactis group during milk fermentation [66], Listeria monocytogenes recovered from biofilms [67], total coliforms and E. coli in urban water [68], and Helicobacter pylori in stomach and stool samples [69]. Besides specific bacteria detection, ddPCR could be combined with gene sequencing to provide a quantitative framework measuring absolute abundances of individual bacterial taxa in complex communities [70-72]. ddPCR was utilized to measure the total amount of 16S rRNA gene copies, which represented the total microbial load in the sample, and 16S rRNA gene sequencing offered the relative abundance of each taxon; the absolute abundances of taxa were calculated by multiplying the total load with the relative abundance. Absolute instead of relative microbial abundances could provide more accurate analyses of changes in taxa among different samples. This framework has been applied to determine the differential effects of diet on each taxon in murine lumenal and mucosal samples along the gastrointestinal (GI) tract [71]. ddPCR is also advantageous for early diagnosis of viral infection [73-77]. For example, Dong et al. reported the rapid detection of fowl adenovirus serotypes 4/10 (FAdV-4/10) [78]. Compared to conventional PCR detection, ddPCR exhibited 1000-fold higher sensitivity. This assay may serve as a tool for detection of FAdV-4 contamination in live-attenuated vaccines. Jiang et al. introduced the design and implementation of an automatic, integrated instrument with a built-in power supply for continuous-flow ddPCR, as demonstrated by absolute quantitation of hepatitis B virus (HBV) in serum samples [79]. The Shen group developed a portable self-partitioning SlipChip (sp-SlipChip) microfluidic device for quantification of BK virus (BKV) DNA [80]. A major feature of this method is a "chain-of-pearls" microchannel, with which individual droplets for PCR reactions are generated in a robust and reproducible manner through a slip-induced self-partitioning mechanism (Fig. 3c). As a result, this system allows for "sample-to-digital-result" detection, making it ideal for point-of-care analysis. Earlier, the group also utilized this droplet formation strategy for digital loop-mediated isothermal amplification (LAMP) quantification of human papillomavirus (HPV) DNA [81].
In response to the outbreak of SARS-CoV-2, real-time reverse-transcriptase PCR (RT-PCR) has been considered as the gold standard for clinical diagnosis. However, because of the low viral load collected by throat swabs, a considerable false-negative rate has been recognized as a key challenge to minimize the risk of viral transmission based on a false sense of security [82]. Thus, Suo et al. proposed an optimized ddPCR assay as a complementary to reduce the false negatives [83]. Based on the results with clinical samples from 77 patients, ddPCR exhibited not only a significantly enhancement in sensitivity, but also a much lower negative likelihood ratio. The overall accuracy was improved from 47% for RT-PCR to 95% for ddPCR. Similarly, Yu et al. parallelly applied RT-PCR and ddPCR tests to 323 samples from 76 SARS-CoV-2-infected patients [84]. ddPCR was found more accurate for low-viral-load samples. Besides, several new droplet manipulation strategies have been utilized for point-of-care diagnosis of SARS-CoV-2, including the commercialized thin-film-transistor digital-microfluidics [85]. Recently, to avoid the use of bulky lab-bound equipment and thus accelerate the workflow for SARS-CoV-2 detection, Chen et al. used a head-flattened pipette tip with an elliptical cross-section to manually produce monodispersed droplets ~150 µm to 350 µm in size for ddPCR [86]. Moreover, using a smartphone as the detector, a limit of detection (LOD) of 3.8 copies per 20 µL could be achieved, comparable to commercial PCR platforms.
4.3. Sequencing-based characterizationIn recent years, the revolutionary improvements of sequencing technology allow high-throughput microbial identification and genotyping from a large, mixed communities without prior cultivation [46, 87, 88]. Conventional sequencing techniques, such as metagenomic sequencing and 16S rRNA sequencing, require homogenization of input material, which will result in the loss of the heterogeneity at the single-cell level as well as the spatial microbiome information [89]. Combining droplet microfluidics with sequencing techniques can circumvent the above limitations, by confining and indexing individual cells or spatially organized cell clusters in thousands of independent microdroplets [90, 91]. Lan et al. described single-cell genome sequencing (SiC-seq) using droplet microfluidics (Fig. 4) [14]. The principal strategy of SiC-seq is to isolate single cells, extract and purified genomic DNA from the cellular matter, and label all DNA sequences from the same genome with a unique barcode sequence for single-cell indexing. The whole workflow was assisted by droplet microfluidics as it is capable of processing millions of compartmentalized picoliter reactions and introducing reagents via droplet merging. Single cells were first encapsulated in agarose microgels, which enabled confined genomic extraction and purification from individual cells through a series of bulk enzymatic and detergent lysis steps. By re-encapsulating the microgels into droplets containing tagmentation reagents and introducing PCR materials and barcode reagents sequentially, genomic DNAs were fragmented and attached with barcodes, which were ready for subsequent sequencing. The sequencing data were grouped according to the barcodes, providing a library of single-cell genomes for downstream analysis. The SiC-seq demonstrated an ultra-high-throughput of > 50, 000 cells per run in a few hours, which was highly promising to uncover the genetic heterogeneity in diverse cell populations. Using SiC-seq, the distributions of antibiotic-resistance genes, virulence factors, and transduction potential in microbial communities from environmental samples were characterized. Droplet-based barcoding strategy has also been utilized in single-cell RNA sequencing (scRNA-seq), which has recently become a gold standard method for molecularly defining cell states and phenotypes [92]. However, conventional scRNA-seq is optimized for mammalian cells, which is ineffective when directly applied to microbial cells that contain less mRNA and have cell walls. To resolve this issue, Liu et al. developed isogenic colony sequencing (ICO-seq), in which single yeast cells were encapsulated in microgels and cultured to yield isogenic yeast colonies of tens to hundreds of cells before subjected to RNA sequencing [93]. The culture process amplified the amount of RNA available for deep sequencing and reduced errors. Cell walls were removed via bulk enzymatic treatment of the microgels, making the mRNA more accessible. Released mRNA was captured on barcoded beads, and followed by reverse transcription, amplification and sequencing, transcriptome-wide screening of thousands of isogenic yeast colonies could be achieved in a single experiment.
|
Download:
|
| Fig. 4. Single-cell genome sequencing (SiC-seq) using droplet microfluidics; scale bar: 100 μm. Reproduced with permission [14]. | |
To connect microbial function to phylogeny in single cells from a community, the epicPCR (Emulsion, Paired Isolation and Concatenation PCR) method was developed [94]. Single cells enclosed in polyacrylamide microgels were lysed and re-encapsulated in droplets with fused primers. The key step of the epicPCR is the in-droplet fusion of functional target genes with 16S rRNA gene (Fig. 5a), which presents in almost all bacteria and contains species-specific signature sequences useful for bacteria identification. Sequencing the fused amplicons provided each read containing 16S rRNA and functional genes simultaneously, allowing phylogenetic identification of the functional taxa. The epicPCR provides a versatile technique to disclose the linked phylogenetic and functional information from millions of uncultured single cells in parallel. It has been applied to discover new putative sulfate reducers in a microbial community from a freshwater lake [94] and capture virus–host interactions [95]. To characterize spatial organization of a microbiome at micrometer-scale resolution, Sheth et al. presented the metagenomic plot sampling by sequencing (MaPS-seq) [96]. In MaPS-seq, intact microbiome samples were first immobilized in a gel matrix and cryofractured into particles. After cell lysis, the particles contained genomic DNA immobilized in the original arrangement, preserving local spatial information. Similar to the aforementioned methods, individual particles were co-encapsulated with barcoded gel beads in droplets for 16S rRNA amplification, and microbiome in the corresponding particles could be obtained by deep sequencing. MaPS-seq is a promising technique for studying microbial biogeography in complex habitats, and it has been used to survey microbiome in three regions of the mouse GI tract, revealing heterogeneous microbial distributions with positive and negative co-associations between specific taxa (Fig. 5b).

|
Download:
|
| Fig. 5. Microbial characterization in droplets based on sequencing technique. (a) epicPCR for phylogenetic identification of the functional taxa. Reproduced with permission [94]. Creative Commons Attribution 4.0 International License. (b) Metagenomic plot sampling by sequencing (MaPS-seq) for characterizing spatial organization of a microbiome at micrometer-scale resolution. Reproduced with permission [96]. Copyright 2019, Springer Nature. | |
Apart from droplet-based sequencing, droplet microfluidics can also serve as a robust platform for whole-genome amplification [97]. Multiple displacement amplification (MDA) is a widely used technique for amplification of minute amounts of DNA samples to a reasonable quantity before whole genome sequencing [98, 99]. Random bias of amplification could be reduced through compartmentation [100]. Shi et al. integrated a microbe enrichment microfluidic device with droplet-based MDA for the preparation of airway microbiome DNA from sputa for shotgun metagenomic sequencing [101]. A microfluidic device with four cascades of 20 selection units was developed to deplete the host cells and enrich particles with diameters less than 5 µm, which were suitable for most bacteria and virus. Genomic DNA was extracted and then amplified with MDA in uniformed droplets, following by the shotgun metagenome sequencing. This method presented greater microbiome complexity and more genome information at a strain level comparing with direct sequencing, with higher sensitivity for identifying prophages and DNA viruses.
4.4. Cas13a-based detectionRapid, sensitive, high-throughput and point-of-care detection of pathogenic microorganisms is critical for disease diagnosis and surveillance. CRISPR-Cas13a is a recently discovered RNA-guided RNase, which can be programmed with a guide CRISPR RNA (crRNA) to target a single strand RNA of interest [102]. Once binding to its RNA target, activated Cas13a exhibits nonspecific endonuclease activity that can cleave nearby nontargeted RNAs. This mechanism motivated the development of SHERLOCK (specific high-sensitivity enzymatic reporter unLOCKing) technique, which enables rapid detection of nucleic acids with attomolar sensitivity based on nucleic acid amplification and Cas13a-mediated collateral cleavage of a reporter RNA [103]. Combining Cas13a-based detection with droplet microfluidics opens a new possibility for pathogen detection. An outstanding work was presented by the Blainey group, which developed the CARMEN (combinatorial arrayed reactions for multiplexed evaluation of nucleic acids) platform and combined it with SHERLOCK for highly scalable, multiplexed detection of pathogens (Fig. 6) [104]. First, Cas13a-detection mixes (containing different specific crRNA) or amplified samples were emulsified by droplet microfluidics to form nanoliter droplets, and each of the individual droplets carried a color code. Droplets were then pooled together and loaded into CARMEN chip, which contained more than 150, 000 microwells and each well accommodates exactly two droplets from the pool at random. Based on the color codes of the droplets, pairwise combinations of droplets containing specific Cas13a-detection mixes and droplets containing specific samples could be determined and addressed. Detection reactions were initiated by applying an electric field to merge the droplet pairs in all the wells. When Cas13a finds a target, reporter RNA was cleaved, generating fluorescence in the corresponding droplet. The CARMEN-Cas13a system was able to test more than 4, 500 crRNA-target pairs on a single array, with the reagent cost per test decreased by more than 300-fold. It was able to simultaneously test dozens of samples for all 169 human-associated viruses, including the novel coronavirus SARS-CoV-2. This highly scalable and multiplexed assay will greatly benefit early disease diagnosis and promote comprehensive infection surveillance, helping to improve patient care and public health. Another exciting work was introduced by Tian et al., who combined droplet digital concept with Cas13a-based detection to realize single-molecule RNA diagnostics [105]. Cas13a assay mix was partitioned in more than 20, 000 picoliter droplets, and droplet involving target RNA exhibited fluorescence ater merely a 60-min isothermal incubation at 37 ℃, allowing absolute quantitation of target RNA in samples based on Poisson distribution. This method enabled ultrasensitive detection of uropathogen 16S rRNAs and diagnosis of SARS-CoV-2 infection from clinical samples with attomolar sensitivity. The unique advantage of the digital Cas13a assay is the elimination of reverse transcription and nucleic acid amplification, which avoids the introduction of errors due to sample loss, cross-contamination, and amplification bias. For further improvement of this method, more work needs to be done to achieve multiplexed target detection and increase the throughput.

|
Download:
|
| Fig. 6. CARMEN platform combined with Cas13a-based detection for highly scalable, multiplexed detection of pathogens; scale bar = 200 μm. Reproduced with permission [104]. Creative Commons Attribution 4.0 International License. | |
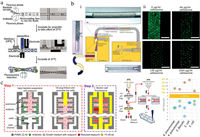
The inappropriate use and massive consumption of antibiotics have caused antibiotic resistance of pathogens, which has become a global health crisis in recent decades [106]. AST is a widely used approach for evaluating the susceptibility of microorganisms to antibiotics and identifying possible antibiotic resistance [107, 108]. It provides essential information to determine suitable antibiotics for pathogen infection treatment. Droplet microfluidics offers a powerful and promising platform for rapid, sensitive and high-throughput AST [109-112]. It enables AST down to a single-cell level, uncovering heteroresistance in an isogenic population [113]. Kaushik et al. presented dropFAST platform, which confined single bacteria in picoliter droplets with antibiotics and the viable cell indicator resazurin [114]. After 1 h incubation, fluorescence of droplets was measured for quantitation of growing bacteria and assessment of antibiotic susceptibility. Introduction of resazurin into the droplets at the very beginning may increase the background fluorescence of droplets. To increase the contrast in fluorescence between droplets containing resistant cells and susceptible cells, Lyu et al. developed a droplet AST system in which resazurin was injected into droplets after incubating the cells with antibiotics for 1 h (Fig. 7a) [115]. This system allowed to detect a resistant sub-population that comprised as low as 10−6 of the entire population. To further enhance the testing throughput, Postek et al. developed a microfluidic platform that could produce a series of emulsions each containing densely packed droplets (called as tankers) and separated from each other by a third immiscible phase (Fig. 7b) [116]. Single bacteria were encapsulated in droplets with antibiotics. Each tanker contained different concentrations of antibiotic and the same concentration of bacteria, and it was spatially addressable by its position in the train. This system enabled single-cell AST with hundreds of replications in a range of antibiotic concentrations within a single experimental run. To realize multiplexed AST, Kang et al. presented a platform consisting of four integrated microdroplet arrays, each hosting over 8000 docking sites [117]. Four combinations of antibiotics/pathogens could be screened simultaneously, providing antibiotic sensitivity assessments in 15-30 min.
|
Download:
|
| Fig. 7. Antibiotic susceptibility testing in droplets. (a) Single-cell AST in droplet microfluidics for quantitative phenotyping of heteroresistance; scale bar = 200 μm. Reproduced with permission [115]. Copyright 2018, Elsevier. (b) Generation of a series of emulsions each containing densely packed droplets for high-throughput single-cell AST; scale bar = 400 μm. Reproduced with permission [116]. Copyright 2018, The Royal Society of Chemistry. (c) Stationary nanoliter droplet arrays (SNDA) combined the resazurin assay for rapid and scalable phenotypic AST. Reproduced permission [123]. Copyright 2017, the National Academy of Sciences of the United States of America. | |
Combinatorial drug treatment is an appealing strategy to overcoming antibiotic resistance [118]. However, the discovery of new combinations has been slowed due to the high complexity, cost, and compound consumption of conventional screening methods [119]. Recently, the Blainey group introduced a high-throughput combinatorial drug screening platform that automatically constructs drug combinations from nanoliter-scale droplets [120]. The design and working principle of this platform is the same as the aforementioned CARMEN chip. Pools of droplets containing bacteria with different drugs were loaded into the microwell array, with each microwell capturing two droplets at random. The pairwise droplets were also indexed by the color codes, and after merging, bacteria could be incubated with differently drug combinations and their growth were assayed by constitutive GFP fluorescence. This system realized the prediction of synergy between more than 4, 000 investigational and approved drugs and a panel of 10 antibiotics against E. coli, and discovered a range of drugs not previously indicated for infectious disease that synergized with antibiotics.
To shorten the turnaround time for AST, several rapid and portable AST systems have been developed [121, 122]. Avesar et al. proposed a system that combined the resazurin assay with stationary nanoliter droplet arrays (SNDA) containing lyophilized antibiotics within each well, allowing rapid and scalable phenotypic AST within less than 5.5 h (Fig. 7c) [123]. Sample loading and bacteria confinement were achieved simply by pipette injection of bacterial suspension plug and oil plug sequentially, increasing its range of applications, such as for resource-poor settings. Yi et al. described an integrated microfluidic SlipChip device for direct AST of bloodstream pathogens without the need of overnight subculture to obtain isolated colonies [124]. Bacteria were extracted and enriched from positive blood cultures by dielectrophoresis, and SlipChip strategy enabled parallel inoculation of the extracted bacteria into nanoliter-scale broth droplets to perform multiplexed ASTs simultaneously. Entropy-based image analysis was used for the characterization of bacterial susceptibility patterns, eliminating the requirement for single-cell morphological analysis and fluorescence labeling. The whole on-chip AST process took 3-8 h, and it has been applied to evaluate the susceptibility patterns of E. coli and S. aureus against several broad-spectrum antibiotics.
Droplet microfluidics have also been utilized to evaluate antiviral agents. Mashaghi et al. proposed a viral fusion assay for screening of candidate compounds [125]. By using highly quantitative fluorescent probes, the fusion kinetics of influenza A virions with target liposomes in microdroplets were obtained with sub-second temporal resolution. Drug candidates on viral fusion dynamics could be characterized for information on the mechanism of action. Since viral fusion is an essential step for enveloped viruses to enter their host cells, insights into this process should facilitate the development of antiviral therapeutics. Furthermore, to determine the impact of latency reversing agents (LRAs) on HIV reactivation, a microfluidic device was developed for high-throughput encapsulation and direct characterization of HIV infected cells [126]. HIV transcriptionally reactivated cells in response to LRAs, which produce unspliced RNA and multiply spliced RNA, were identified through intra-droplet PCR amplification and then sorted out for downstream quantification of genomic DNA or mRNA. Further analysis of single CD4+ T cells obtained from individuals receiving antiretroviral therapy found that LRAs enhance transcription from active cells in some cases while increase the number of transcriptionally active cells in others, demonstrating the importance of single-cell analysis for deeper mechanistic understanding of HIV reactivation strategies. Liu et al. characterized molecular inhibitors of poliovirus by tracking thousands of individual virus–cell interactions in microdroplets [127, 128]. Single-cell analysis has the ability to reveal inhibitor-specific signatures of antiviral action.
6. Microbial interactionsMicrobial interactions are the major drivers of microbial community dynamics and functions [129-131]. These interactions occur by the environmental recognition followed by transference of molecular and genetic information. Interactions patterns could be positive, negative, or negligible [129]. Detecting and investigating various types of interactions in microbial ecosystems remain challenging due to the complex, highly diverse and highly dynamic nature of these communities [132]. Droplet microfluidics facilitates overcoming this challenge by encapsulating and co-culturing mixtures of microbes in droplets, which allow high-throughput characterization of interactions in highly controlled environments and conditions [133-135]. Tan et al. co-cultivated sub-communities from a fecal sample in droplets, supporting ecological interactions between a reduced number of cells [136]. After a 1-week coculture, 22 individual droplets with strong bacterial co-growth were isolated for whole genome sequencing to characterize the bacterial sub-community. Mixed cultures of microalgae in millions of parallel droplets enabled investigations into the interactions that might affect the biomass accumulation, offering a high-throughput approach to detect transgressive overyielding, which occurs when the biomass production of a community of algae species exceeds that of the most productive constitutive species [137].
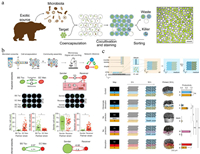
Droplet-based high-throughput isolation allows screening of probiotics with antimicrobial activity. Terekhov et al. developed an ultrahigh-throughput microfluidic platform, which co-encapsulated microbiota with GFP-labeled target pathogen in tens of thousands water-in-oil-in-water double emulsions (Fig. 8a) [138, 139]. After co-cultivation, calcein violet AM was added to MDE for viable bacteria indication. Unlike water-in-oil droplets whose external phase is oil, double emulsions are in aqueous carrier fluid, making them compatible with the flow cytometry. Droplets demonstrating anti-pathogen features (high initial target load, low GFP level, and high metabolic activity) were isolated by fluorescence-activated cell sorting and characterized by sequencing and liquid chromatography-mass spectrometry, providing genotype, phenotype and secretome descriptions of the encapsulated microorganisms. This platform has been successfully utilized to profile the anti-pathogen properties of an entire oral microbial community of the Siberian bear [139]. An antibiotic amicoumacin A produced by a Bacillus pumilus strain was identified, which was responsible for growth inhibiting of S. aureus.
|
Download:
|
| Fig. 8. Microbial interactions in droplets. (a) Ultrahigh-throughput microfluidic platform for droplet-based screening and isolation of probiotics with antimicrobial activity; scale bar = 50 μm. Reproduced with permission [139]. Creative Commons Attribution 4.0 International License. (b) Microbial interaction network inference in microdroplets (MINI-Drop) to identify the interactions within microbial communities across different environments; scale bar = 40 μm. Reproduced with permission [145]. Copyright 2019, Elsevier. (c) Droplet printing enabling to position interacting microbes in specific patterns in sub-millimetre arrays; scale bar = 200 μm. Reproduced with permission [148]. Creative Commons Attribution 4.0 International License. | |
Gene transformation is the genetic alteration of a recipient bacterium by directly uptake of exogenous genetic material released from donor bacteria [140, 141]. It is one important pathway leading to antibiotic resistance of pathogens [142]. Lam et al. developed a droplet microfluidic system for encapsulation of individual pneumococcal cell pairs in femtoliter droplets [143, 144]. Gene transformation between cell combinations was characterized using whole-genome sequencing, and the results indicated that both multiple coincident transfers and transfers of large blocks or tracts were displayed after short-term cell–cell competence interactions.
Besides pairwise microbial interactions, higher-order interactions, which are challenging to be controlled and analyzed by conventional methods, can be deciphered using microfluidic droplets in a high-throughput manner. Hsu et al. presented microbial interaction network inference in microdroplets (MINI-Drop) to identify the interactions within microbial communities across different environments (Fig. 8b) [145]. Two or three bacterial strains were mixed and randomly encapsulated in droplets, enabling parallel culturing of many sub-communities. The highlight of this work was coupling fluorescence microscopy to an automated computational method, which allowed rapid determination of the absolute abundance of each strain in hundreds to thousands of droplets per condition, inferring the type and strength of microbial interactions after co-cultivation. The MINI-Drop could accurately depict pairwise and higher-order interactions in synthetic consortia with an inferred interaction network. The complex web of interactions linking antibiotics and different species was thus elucidated in a synthetic consortium. The Blainey group introduced the kChip, a platform similar to their CARMAN chip, performing rapid, massively parallel, bottom-up construction and screening of synthetic microbial communities [146]. Different strains (in different environmental conditions) were distributed into nanoliter droplets and randomly self-assembled in kChip. The kChip contained a high-density array of microwells, each of which was designed to precisely group k droplets (k = 1, 2, …, 7, 19). The grouped droplets were subsequently merged, allowing for rapid functional profiling of fluorescently labeled and unlabeled strains across libraries of conditions (e.g., antibiotics, natural products, carbon sources) with flexible temporal resolution. The kChip was successfully applied to screen ~100, 000 multispecies communities comprising up to 19 soil isolates, and specific compositions of bacteria that promoted the growth of a model plant symbiont Herbaspirillum frisingense were identified. It is a promising technique for the discovery of microbial consorftia with functional properties, such as pathogen suppression and recalcitrant substrate degradation.
In microbial community, the spatial arrangement of strains and species is considered critical for their ecology [147]. Kumar et al. presented a high-resolution droplet printing method that enabled interacting microbes to be positioned in specific patterns in sub-millimetre arrays (Fig. 8c) [148]. Using piezo droplet printers, droplets containing bacteria and agarose were deposited into 2D patterns by line-by-line printing in an oil/phospholipid solution. Stabilized structures were formed after gelation and then transferred to medium for long-term cultivation. Results revealed that spatial structure impacted the outcome of both interference and exploitative competition. By printing different potential barriers between a toxin-producing strain and a susceptible strain, the ecological refuge effect was investigated, demonstrating that cells closest to the toxin producer mopped up the toxin and protected their clonemates. This method realizes precisely patterning and manipulating bacterial interactions at the relevant spatial scales, which is highly valuable for establishing a causal link between the spatial organization of bacterial strains and ecological outcomes.
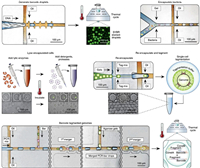
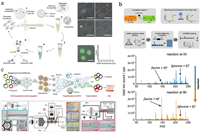
7. Microbial biotechnologyMicrobial biotechnology deals with the manipulation of genetic engineering of microorganisms or their components to produce useful products for the human welfare [149]. It is critical for advances in food industry, agriculture and pharmaceuticals [150-152]. Droplet microfluidics provides an efficient platform for strain improvement, metabolite screening and biosynthetic gene cluster discovery in microbial biotechnology [17, 153, 154]. Jian et al. reported a microbial microdroplet culture system (MMC), which performed automated microbial cultivation, growth monitoring and adaptive evolution in up to 200 replicate droplets [155]. An 18-day process of adaptive evolution of methanol-essential E. coli strain was conducted in MMC, obtaining two strains exhibiting higher growth rates compared with the parent strain. By co-encapsulating screening microbes with a sensor strain or metabolite capturing particles, the microbial production of metabolites can be evaluated in droplets for strain improvement. Hernandez-Valdes et al. developed a growth-based sensor strain to detect essential amino acids [156]. Encapsulating potential amino acid producing strains with the sensor strain in droplets enabled to identify strains that naturally secreted amino acids. This method isolated three mutated Lactococcus lactis IPLA838 strains with 5~10-fold increased amino acid-secretion compared to the wild type. Similarly, Mahler et al. implemented a high-throughput screening of antibiotic producers from natural microbial communities by pico-injecting bacterial reporter strains (E. coli and Bacillus subtilis, as representatives of Gram-negative and Gram-positive bacteria) into all droplets [157]. Napiorkowska et al. co-encapsulated single microbes with protein-capturing microparticles in agarose gel droplets (Fig. 9a) [158]. During microbial cultivation, the secreted proteins with hexahistidine sequences (His6-tag) could be continuously captured by the microparticles. The agarose gel beads were then recovered into an aqueous buffer, treated for protein crosslinking and labeling, and sorted by FACS. The highly productive variants could be obtained by isolating the most fluorescent fraction of agarose gel beads. This system permitted the analysis of > 106 genotypes per day and was applied to optimize cutinase secretion in Komagataella phaffii, isolating a strain with 5.7-fold improvement.
|
Download:
|
| Fig. 9. Microbial biotechnology in droplets. (a) High-throughput optimization of recombinant protein production in microfluidic gel droplets; scale bar = 50 μm. Reproduced with permission [158]. Creative Commons Attribution 4.0 International License. (b) Droplet-based ESI-MS for microbial metabolite characterization. Reproduced with permission [159]. Copyright 2020, American Chemical Society. (c) Microfluidic automated plasmid library enrichment for biosynthetic gene cluster discovery; scale bar = 100 μm. Reproduced with permission [164]. Creative Commons Attribution 4.0 International License. | |
Combing droplet microfluidics with mass spectrometry allows label-free characterization of microbial products. Mahler et al. integrated droplet microfluidic device with electrospray mass spectrometry (ESI-MS) (Fig. 9b) [159, 160]. A few of microbes were cultivated in droplets, which were then electrosprayed from an integrated steel emitter and analyzed by MS for the detection of accumulated metabolites. This system enabled analysis of the conversion of d-glucose to l-lysine by Corynebacterium glutamicum. Currently, this method only provided qualitative results of lysine production. Limited by the attenuated detection sensitivity due to the ion suppression by buffer salt, it encapsulated multiple microbes in each droplet for metabolite accumulation. Future works need to be done to realize metabolite quantitation and improve sensitivity, allowing us to disclose metabolism heterogeneity of single microbes in a complex community.
Microbial communities provide a rich source of bioactive molecules such as enzymes, but their experimental identification is challenging. Coupling metagenomic library construction with droplet-based screening can promote the discovery of biosynthetic gene clusters from nature [159-161]. Ma et al. constructed a metagenomic library hosted in E. coli from domestic running water samples [162]. Single E. coli cells were then encapsulated in water-in-oil-in-water double emulsion droplets, along with the enzyme substrate. After cell lysis, the released enzyme could catalyze the substrate to generate a fluorescent product, and the droplets were sorted by FACS to enrich the positive clones. This platform enabled to identify a novel esterase with high catalytic efficiency and distinct evolutionary origin from other lipolytic enzymes. Similar system was also presented by Tauzin et al., who constructed a metagenomic library from mucus-associated human gut microbiome and screened for host-glycan degrading enzymes [163]. Results revealed novel pathways of metabolization of human glycans by gut bacteria, in particular pathobionts. To offer a more generalizable way to identify sequences of interest, Xu et al. developed a screening system which merged the colony droplets with PCR reagent droplets (Fig. 9c) [164]. After PCR, positive droplets were sorted directly on chip by dielectrophoretic force. DNA was then recovered from droplets for downstream sequencing to identify the target gene clusters. This system was successfully applied to isolate and sequencing type I polyketide synthase gene clusters from an Antarctic soil metagenome.
8. Challenges and future perspectivesDroplet microfluidics, as a versatile and powerful technique that allows high-throughput and precise manipulation of microorganisms, has expanded the scope and context of microbiology. The technical innovations of droplet microfluidics have opened new possibilities to various fields of microbiology, from defining single-cell phenotypic and genetic heterogeneity to investigating spatiotemporal dynamics of microbial communities, from precise quantitation of microbiota to systematic decipherment of microbial interactions, from isolating rare and uncultured microbes to improving genetic engineered strains. Attributed to its unique advantages, droplet microfluidics is being expected to serve as basic tools for microbiologists to explore the world of microorganisms. However, there are still challenges encountered in droplet microfluidics before its extensive adoption in mainstream microbiology.
There are several issues related to the inherent properties of droplets. Firstly, droplet stability during droplet manipulation and microbial cultivation is highly important to maintain the isolation of microorganisms; despite having a wide variety of surfactants [165, 166], it is not easy to balance the high droplet stability with low microbial cytotoxicity for microbial culture. The second issue is the limited capability of substance exchange in droplets, which throws up difficulties in long-term cultivation, especially for metabolically active microorganisms. Encapsulating microbes in giant unilamellar lipid vesicles [36, 38] or hydrogel microcapsules [167-169] can solve this issue to some extent, but these methods will lead to crosstalk among compartments, and individually regulating compositions of each droplet is still hard to achieve. Thirdly, for microorganisms, the oxygen requirement varies greatly among species. Currently, the growth of aerobic microbes in droplets are mainly supported by the dissolved oxygen in oil phase (fluorinated oil such as FC-40 and HFE-7500), while anaerobic microbes in droplets require to be generated, manipulated and cultured in anaerobic chamber. Precise control and flexible regulation of oxygen level in droplets is still challenging to realize in droplet microfluidic systems. Fourthly, recent studies revealed that small molecules, particularly lipophilic molecules, can partly dissolve in oil phase and exchange among droplets [170]; although some research takes advantage of this phenomenon for investigation of microbial interactions among droplets [171], it may often lead to undesirable cross-contamination, affecting the outcomes of droplet-based microbial study. Finally, but not least, the liquid environment of droplets may be not suitable for some microorganisms that need to grow on solid medium or require air-water interface [172].
There are also some challenges toward the widespread application of microfluidics in microbiology. The first is the lack of standardized devices and procedures. A variety of droplet microfluidic platforms have been developed for microbiological studies in various fields. Owing to the complexity of experimental conditions, comparisons among systems could be rather difficult, inevitably limiting the adoption of microfluidic methods by a broader community. Currently, ddPCR has made a good example of standardization and practical transformation of novel microfluidic technologies, which will inspire further development of the microfluidic industry. Secondly, most of current droplet microfluidic systems require tedious device fabrication as well as complex fluid operations, which are challenging for non-experts to learn and use. To address this issue, several off-chip droplet systems have been recently developed, such as cross-interface emulsification (XiE) [173, 174], spinning-based emulsification [175-177], vertical step emulsification [178, 179] and vibrating sharp-tip capillary [180], which use commercially available "off-the-shelf" components for system setup and rely less on complicated chip manufacture and experienced operators. This issue can also be solved by exploring further automation of liquid handling protocols, improving the accessibility of droplet microfluidics to microbiologists.
Recently, droplet microfluidic technology has become one of the most powerful and promising tools for microbiological studies. It has been extensively utilized in various fields and is still in rapid progression. There are fascinating opportunities for droplet microfluidics in exploration of novel or uncultured microbial resources, diagnosis and surveillance of pathogen infections, discovery of antimicrobial drugs or probiotics, elucidation of complex microbial interactions, and directed evolution of microbial communities. For further development, one important direction is the automation and simplification of the whole droplet microfluidic system, providing standardized equipment and routine protocols for diverse aspects of microbiological applications. Another direction is the improvements of downstream analysis techniques. Currently, molecular techniques, such as PCR, sequencing and CRISPR-Cas13, have been commendably integrated with droplet microfluidics to acquire genome or transcriptome information of microorganisms in droplets. However, proteome or metabolome information of microorganisms in droplets is less documented. Label-free techniques, including single-cell Raman spectrum and mass spectrometry, are highly promising for microbial proteomic and metabolomic analysis; but their integration with droplet microfluidics still needs further optimization, to improve the detection sensitivity as well as the processing throughput. We believe that in-depth collaborations between multidisciplinary researchers (microbiologists, chemists, engineers and clinicians) will promote further development and maturation of droplet microfluidics, continually introduce new paradigms for microbiology, and contribute to frontier microbial ecology.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 82173774, 31925037, 22104041), the Fundamental Research Funds for the Central Universities (Nos. 2662021DKQD001, 2662021JC001), and the Cheeloo Scholar Program of Shandong University (to W. Liu).
| [1] |
Nat. Rev. Microbiol. 7 (2009) 174. DOI:10.1038/nrmicro2106 |
| [2] |
A.K. Wessel, L. Hmelo, M.R. Parsek, M. Whiteley, Nat. Rev. Microbiol. 11 (2013) 337-348. DOI:10.1038/nrmicro3010 |
| [3] |
F.L. Hellweger, R.J. Clegg, J.R. Clark, C.M. Plugge, J.U. Kreft, Nat. Rev. Microbiol. 14 (2016) 461-471. DOI:10.1038/nrmicro.2016.62 |
| [4] |
Y. Ding, P.D. Howes, A.J. deMello, Anal. Chem. 92 (2020) 132-149. DOI:10.1021/acs.analchem.9b05047 |
| [5] |
T.S. Kaminski, O. Scheler, P. Garstecki, Lab Chip 16 (2016) 2168-2187. DOI:10.1039/C6LC00367B |
| [6] |
A. Suea-Ngam, P.D. Howes, M. Srisa-Art, A.J. deMello, Chem. Commun. 55 (2019) 9895-9903. DOI:10.1039/c9cc04750f |
| [7] |
K. Matula, F. Rivello, W.T.S. Huck, Adv. Biosyst. 4 (2020) e1900188. DOI:10.1002/adbi.201900188 |
| [8] |
G.R. Huys, J. Raes, Curr. Opin. Microbiol. 44 (2018) 1-8. DOI:10.1016/j.mib.2018.05.002 |
| [9] |
E.C. Carnes, D.M. Lopez, N.P. Donegan, et al., Nat. Chem. Biol. 6 (2010) 41-45. DOI:10.1038/nchembio.264 |
| [10] |
S. Hengoju, M. Tovar, D.K.W. Man, S. Buchheim, M.A. Rosenbaum, Droplet microfluidics for microbial biotechnology, in: Adv. Biochem. Eng. /Biotechnol., Springer, Berlin, Heidelberg, 2020, pp. 1-29.
|
| [11] |
E.K. Bowman, H.S. Alper, Trends. Biotechnol. 38 (2020) 701-714. DOI:10.1016/j.tibtech.2019.11.002 |
| [12] |
Y. Vervoort, A.G. Linares, M. Roncoroni, et al., Curr. Opin. Biotechnol. 46 (2017) 120-125. DOI:10.1016/j.copbio.2017.02.011 |
| [13] |
S. Sohrabi, N. kassir, M.K. Moraveji, RSC Adv. 10 (2020) 27560-27574. DOI:10.1039/d0ra04566g |
| [14] |
F. Lan, B. Demaree, N. Ahmed, A.R. Abate, Nat. Biotechnol. 35 (2017) 640-646. DOI:10.1038/nbt.3880 |
| [15] |
X. Jian, X. Guo, J. Wang, et al., Biotechnol. Bioeng. 117 (2020) 1724-1737. DOI:10.1002/bit.27327 |
| [16] |
K. Churski, T.S. Kaminski, S. Jakiela, et al., Lab Chip 12 (2012) 1629-1637. DOI:10.1039/c2lc21284f |
| [17] |
J. Chen, M. Vestergaard, J. Shen, et al., FEMS Microbiol. Lett. 365 (2018) fny258. |
| [18] |
S. Goodwin, J.D. McPherson, W.R. McCombie, Nat. Rev. Genet. 17 (2016) 333-351. DOI:10.1038/nrg.2016.49 |
| [19] |
E.A. Specht, E. Braselmann, A.E. Palmer, Annu. Rev. Physiol. 79 (2017) 93-117. DOI:10.1146/annurev-physiol-022516-034055 |
| [20] |
L. Shang, Y. Cheng, Y. Zhao, Chem. Rev. 117 (2017) 7964-8040. DOI:10.1021/acs.chemrev.6b00848 |
| [21] |
R. Dangla, S.C. Kayi, C.N. Baroud, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 853-858. DOI:10.1073/pnas.1209186110 |
| [22] |
A. Lashkaripour, C. Rodriguez, L. Ortiz, D. Densmore, Lab Chip 19 (2019) 1041-1053. DOI:10.1039/c8lc01253a |
| [23] |
Z.Z. Chong, S.H. Tan, A.M. Ganan-Calvo, et al., Lab Chip 16 (2016) 35-58. DOI:10.1039/C5LC01012H |
| [24] |
R.R. Pompano, W. Liu, W. Du, R.F. Ismagilov, Anal. Chem. 4 (2011) 59-81. DOI:10.1146/annurev.anchem.012809.102303 |
| [25] |
L. Cao, X. Cui, J. Hu, et al., Biosens. Bioelectron. 90 (2017) 459-474. DOI:10.1016/j.bios.2016.09.082 |
| [26] |
H. Wu, X. Chen, X. Gao, et al., Anal. Chem. 90 (2018) 4303-4309. DOI:10.1021/acs.analchem.8b00048 |
| [27] |
M. Sun, S.S. Bithi, S.A. Vanapalli, Lab Chip 11 (2011) 3949-3952. DOI:10.1039/c1lc20709a |
| [28] |
L. Mazutis, J. Gilbert, W.L. Ung, et al., Nat. Protoc. 8 (2013) 870-891. DOI:10.1038/nprot.2013.046 |
| [29] |
J. Overmann, B. Abt, J. Sikorski, Annu. Rev. Microbiol. 71 (2017) 711-730. DOI:10.1146/annurev-micro-090816-093449 |
| [30] |
L. Boitard, D. Cottinet, N. Bremond, J. Baudry, J. Bibette, Eng. Life Sci. 15 (2015) 318-326. DOI:10.1002/elsc.201400089 |
| [31] |
B. Hu, P. Xu, L. Ma, et al., Mar. Life Sci. Technol. 3 (2021) 169-188. DOI:10.1007/s42995-020-00082-8 |
| [32] |
H. Liu, X. Xu, K. Peng, et al., Biotechnol. Bioeng. 118 (2021) 647-658. DOI:10.1002/bit.27591 |
| [33] |
M. Tovar, L. Mahler, S. Buchheim, M. Roth, M.A. Rosenbaum, Microb. Cell Fact. 19 (2020) 16. DOI:10.1186/s12934-020-1282-y |
| [34] |
J. Cao, F. Kalensee, P.M. Günther, J.M. Köhler, Int. J. Environ. Sci. Technol. (Tehran) 17 (2020) 1-16. DOI:10.1007/s13762-019-02424-1 |
| [35] |
J. Cao, L. Hafermann, J.M. Köhler, Eng. Life Sci. 17 (2017) 792-800. DOI:10.1002/elsc.201600264 |
| [36] |
P. Juskova, Y.R.F. Schmid, A. Stucki, et al., ACS Appl. Mater. Interfaces 11 (2019) 34698-34706. DOI:10.1021/acsami.9b12169 |
| [37] |
C.M.B. Ho, Q. Sun, A.J.T. Teo, et al., ACS Biomater. Sci. Eng. 6 (2020) 3630-3637. DOI:10.1021/acsbiomaterials.0c00292 |
| [38] |
S. Deshpande, Y. Caspi, A.E. Meijering, C. Dekker, Nat. Commun. 7 (2016) 10447. DOI:10.1038/ncomms10447 |
| [39] |
M.M. Villa, R.J. Bloom, J.D. Silverman, et al., mSystems. |
| [40] |
C.Y. Jiang, L. Dong, J.K. Zhao, et al., Appl. Environ. Microbiol. 82 (2016) 2210-2218. DOI:10.1128/AEM.03588-15 |
| [41] |
B. Hu, B. Xu, J. Yun, et al., Lab Chip 20 (2020) 363-372. DOI:10.1039/c9lc00761j |
| [42] |
D. Chen, S.J. Liu, W. Du, J. Hazard. Mater. 366 (2019) 512-519. DOI:10.1016/j.jhazmat.2018.12.029 |
| [43] |
N. Zhou, Y.T. Sun, D.W. Chen, et al., Microbiologyopen 8 (2019) e00654. DOI:10.1002/mbo3.654 |
| [44] |
W.J. Watterson, M. Tanyeri, A.R. Watson, et al., eLife 9 (2020) e56998. DOI:10.7554/eLife.56998 |
| [45] |
Z. Jin, M. Nie, R. Hu, et al., Small 14 (2018) 1800658. DOI:10.1002/smll.201800658 |
| [46] |
L. Varadi, J.L. Luo, D.E. Hibbs, et al., Chem. Soc. Rev. 46 (2017) 4818-4832. DOI:10.1039/C6CS00693K |
| [47] |
M. Ferone, A. Gowen, S. Fanning, A.G.M. Scannell, Compr. Rev. Food. Sci. Food Saf. 19 (2020) 3106-3129. DOI:10.1111/1541-4337.12618 |
| [48] |
K. Wink, L. Mahler, J.R. Beulig, et al., Anal. Bioanal. Chem. 410 (2018) 7679-7687. DOI:10.1007/s00216-018-1383-1 |
| [49] |
L.C. Duarte, F. Figueredo, L.E.B. Ribeiro, E. Corton, W.K.T. Coltro, Anal. Chim. Acta 1071 (2019) 36-43. DOI:10.1016/j.aca.2019.04.045 |
| [50] |
M. Kogawa, M. Hosokawa, Y. Nishikawa, K. Mori, H. Takeyama, Sci. Rep. 8 (2018) 2059. DOI:10.1038/s41598-018-20384-3 |
| [51] |
J.B. Harmon, H.K. Gray, C.C. Young, K.J. Schwab, PLoS One 15 (2020) e0233239. DOI:10.1371/journal.pone.0233239 |
| [52] |
H. Gai, Y. Li, E.S. Yeung, Top. Curr. Chem. 304 (2011) 171-201. DOI:10.1007/128_2011_144 |
| [53] |
K. Hsieh, H.C. Zec, L. Chen, et al., Anal. Chem. 90 (2018) 9449-9456. DOI:10.1021/acs.analchem.8b02096 |
| [54] |
X. Cui, L. Ren, Y. Shan, et al., Analyst 143 (2018) 3309-3316. DOI:10.1039/c8an00456k |
| [55] |
M. Li, J. Xu, M. Romero-Gonzalez, S.A. Banwart, W.E. Huang, Curr. Opin. Biotechnol. 23 (2012) 56-63. DOI:10.1016/j.copbio.2011.11.019 |
| [56] |
Y. He, X. Wang, B. Ma, J. Xu, Biotechnol. Adv. 37 (2019) 107388. DOI:10.1016/j.biotechadv.2019.04.010 |
| [57] |
T. Xu, Y. Gong, X. Su, et al., Small 16 (2020) 2001172. DOI:10.1002/smll.202001172 |
| [58] |
X. Wang, Y. Xin, L. Ren, et al., Sci. Adv. 6 (2020) eabb3521. DOI:10.1126/sciadv.abb3521 |
| [59] |
B. Chen, Y. Jiang, X. Cao, et al., Clin. Chim. Acta 517 (2021) 156-161. DOI:10.1016/j.cca.2021.02.008 |
| [60] |
P.L. Quan, M. Sauzade, E. Brouzes, Sensors 18 (2018) 1271. DOI:10.3390/s18041271 |
| [61] |
L. Cao, X. Cui, J. Hu, et al., Biosens. Bioelectron. 90 (2017) 459-474. DOI:10.1016/j.bios.2016.09.082 |
| [62] |
X. Huang, X. Lin, K. Urmann, et al., Environ. Sci. Technol. 52 (2018) 6399-6407. DOI:10.1021/acs.est.8b00241 |
| [63] |
G. Galazzo, N. van Best, B.J. Benedikter, et al., Front. Cell. Infect. Microbiol. 10 (2020) 403. DOI:10.3389/fcimb.2020.00403 |
| [64] |
H. Pan, K. Dong, L. Rao, et al., Food Control 112 (2020) 107140. DOI:10.1016/j.foodcont.2020.107140 |
| [65] |
K. Zhu, B. Suttner, A. Pickering, K.T. Konstantinidis, J. Brown, Water Res. 183 (2020) 116085. DOI:10.1016/j.watres.2020.116085 |
| [66] |
M.A. Caillaud, M. Abeilhou, I. Gonzalez, et al., Front. Microbiol. 11 (2020) 1906. DOI:10.3389/fmicb.2020.01906 |
| [67] |
K. Grudlewska-Buda, K. Skowron, E. Gospodarek-Komkowska, AMB Express 10 (2020) 75. DOI:10.1186/s13568-020-01007-5 |
| [68] |
A. Bivins, S. Lowry, H.M. Murphy, et al., npj Clean Water 3 (2020) 35. DOI:10.1038/s41545-020-00081-3 |
| [69] |
L. Sun, S. Talarico, L. Yao, et al., J. Clin. Microbiol.. |
| [70] |
C. Manzari, A. Oranger, B. Fosso, et al., Microb. Genom. 6 (2020) mgen000417. |
| [71] |
J.T. Barlow, S.R. Bogatyrev, R.F. Ismagilov, Nat. Commun. 11 (2020) 2590. DOI:10.1038/s41467-020-16224-6 |
| [72] |
B. Brooks, M.R. Olm, B.A. Firek, et al., Microbiome 6 (2018) 112. DOI:10.1186/s40168-018-0493-5 |
| [73] |
A.A. Kojabad, M. Farzanehpour, H.E.G. Galeh, et al., J. Med. Virol. 93 (2021) 4182-4197. DOI:10.1002/jmv.26846 |
| [74] |
A. Christensen-Quick, M. Massanella, A. Frick, et al., J. Virol.. |
| [75] |
S.A. Yukl, P. Kaiser, P. Kim, et al., Sci. Transl. Med. 10 (2018) eaap9927. DOI:10.1126/scitranslmed.aap9927 |
| [76] |
M. Pichon, A. Gaymard, L. Josset, et al., Antiviral Res. 145 (2017) 160-167. DOI:10.1016/j.antiviral.2017.07.021 |
| [77] |
A.C. Bateman, A.L. Greninger, E.E. Atienza, et al., Clin. Chem. 63 (2017) 761-769. DOI:10.1373/clinchem.2016.265512 |
| [78] |
G. Dong, F. Meng, Y. Zhang, et al., J. Virol. Methods 265 (2019) 59-65. DOI:10.1016/j.jviromet.2018.09.005 |
| [79] |
Y. Jiang, A. Manz, W. Wu, Anal. Chim. Acta 1125 (2020) 50-56. DOI:10.3390/biomedicines8030050 |
| [80] |
L. Xu, H. Qu, D.G. Alonso, et al., Biosens. Bioelectron. 175 (2021) 112908. DOI:10.1016/j.bios.2020.112908 |
| [81] |
Z. Yu, W. Lyu, M. Yu, et al., Biosens. Bioelectron. 155 (2020) 112107. DOI:10.1016/j.bios.2020.112107 |
| [82] |
C.P. West, V.M. Montori, P. Sampathkumar, Mayo Clin. Proc. 95 (2020) 1127-1129. DOI:10.1016/j.mayocp.2020.04.004 |
| [83] |
T. Suo, X. Liu, J. Feng, et al., Emerg. Microbes Infect. 9 (2020) 1259-1268. DOI:10.1080/22221751.2020.1772678 |
| [84] |
F. Yu, L. Yan, N. Wang, et al., Clin. Infect. Dis. 71 (2020) 793-798. DOI:10.1093/cid/ciaa345 |
| [85] |
S. Anderson, B. Hadwen, C. Brown, Lab Chip 21 (2021) 962-975. DOI:10.1039/d0lc01143f |
| [86] |
L. Chen, V. Yadav, C. Zhang, et al., Anal. Chem. 93 (2021) 6456-6462. DOI:10.1021/acs.analchem.1c00192 |
| [87] |
N.J. Loman, M.J. Pallen, Nat. Rev. Microbiol. 13 (2015) 787-794. DOI:10.1038/nrmicro3565 |
| [88] |
J. Jo, J. Oh, C. Park, J. Microbiol. 58 (2020) 176-192. DOI:10.1007/s12275-020-9525-5 |
| [89] |
L.W. Hugerth, A.F. Andersson, Front. Microbiol. 8 (2017) 1561. DOI:10.3389/fmicb.2017.01561 |
| [90] |
R. Salomon, D. Kaczorowski, F. Valdes-Mora, et al., Lab Chip 19 (2019) 1706-1727. DOI:10.1039/c8lc01239c |
| [91] |
K. Matuła, F. Rivello, W.T.S. Huck, Adv. Biosyst. 4 (2020) 1900188. DOI:10.1002/adbi.201900188 |
| [92] |
E.Z. Macosko, A. Basu, R. Satija, et al., Cell 161 (2015) 1202-1214. DOI:10.1016/j.cell.2015.05.002 |
| [93] |
L. Liu, C.K. Dalal, B.M. Heineike, A.R. Abate, Lab Chip 19 (2019) 1838-1849. DOI:10.1039/c9lc00084d |
| [94] |
S.J. Spencer, M.V. Tamminen, S.P. Preheim, et al., ISME J. 10 (2016) 427-436. DOI:10.1038/ismej.2015.124 |
| [95] |
E.G. Sakowski, K. Arora-Williams, F. Tian, et al., Nat. Microbiol. 6 (2021) 630-642. DOI:10.1038/s41564-021-00873-4 |
| [96] |
R.U. Sheth, M. Li, W. Jiang, et al., Nat. Biotechnol. 37 (2019) 877-883. DOI:10.1038/s41587-019-0183-2 |
| [97] |
M. Hosokawa, Y. Nishikawa, M. Kogawa, H. Takeyama, Sci. Rep. 7 (2017) 5199. DOI:10.1038/s41598-017-05436-4 |
| [98] |
N. Long, Y. Qiao, Z. Xu, J. Tu, Z. Lu, Quant. Biol. 8 (2020) 279-294. DOI:10.1007/s40484-020-0217-2 |
| [99] |
E.K. Binga, R.S. Lasken, J.D. Neufeld, ISME J 2 (2008) 233-241. DOI:10.1038/ismej.2008.10 |
| [100] |
A.M. Sidore, F. Lan, S.W. Lim, A.R. Abate, Nucleic Acids Res. 44 (2016) e66. DOI:10.1093/nar/gkv1493 |
| [101] |
X. Shi, C. Shao, C. Luo, et al., mSystems 4 (2019) e00198-19. DOI:10.1128/mSystems.00198-19 |
| [102] |
M.P. Terns, Mol. Cell 72 (2018) 404-412. DOI:10.1016/j.molcel.2018.09.018 |
| [103] |
J.S. Gootenberg, O.O. Abudayyeh, J.W. Lee, et al., Science 356 (2017) 438. DOI:10.1126/science.aam9321 |
| [104] |
C.M. Ackerman, C. Myhrvold, S.G. Thakku, et al., Nature 582 (2020) 277-282. DOI:10.1038/s41586-020-2279-8 |
| [105] |
T. Tian, B. Shu, Y. Jiang, et al., ACS Nano 15 (2021) 1167-1178. DOI:10.1021/acsnano.0c08165 |
| [106] |
C. Nathan, Nat. Rev. Microbiol. 18 (2020) 259-260. DOI:10.1038/s41579-020-0348-5 |
| [107] |
A. van Belkum, C.A.D. Burnham, J.W.A. Rossen, et al., Nat. Rev. Microbiol. 18 (2020) 299-311. DOI:10.1038/s41579-020-0327-x |
| [108] |
A. van Belkum, T.T. Bachmann, G. Lüdke, et al., Nat. Rev. Microbiol. 17 (2019) 51-62. DOI:10.1038/s41579-018-0098-9 |
| [109] |
N. Qin, P. Zhao, E.A. Ho, G. Xin, C.L. Ren, ACS Sens. 6 (2021) 3-21. DOI:10.1021/acssensors.0c02175 |
| [110] |
S. Hassan, X. Zhang, Curr. Anal. Chem. 16 (2020) 41-51. DOI:10.2174/1573411015666181224145845 |
| [111] |
C.M. Svensson, O. Shvydkiv, S. Dietrich, et al., Small 15 (2019) 1970021. DOI:10.1002/smll.201970021 |
| [112] |
T.J. Abram, H. Cherukury, C.Y. Ou, et al., Lab Chip 20 (2020) 477-489. DOI:10.1039/c9lc01212e |
| [113] |
K. Zhang, S. Qin, S. Wu, Y. Liang, J. Li, Chem. Sci. 11 (2020) 6352-6361. DOI:10.1039/d0sc01353f |
| [114] |
A.M. Kaushik, K. Hsieh, L. Chen, et al., Biosens. Bioelectron. 97 (2017) 260-266. DOI:10.1016/j.bios.2017.06.006 |
| [115] |
F. Lyu, M. Pan, S. Patil, et al., Sens. Actuators B: Chem. 270 (2018) 396-404. DOI:10.1016/j.snb.2018.05.047 |
| [116] |
W. Postek, P. Gargulinski, O. Scheler, T.S. Kaminski, P. Garstecki, Lab Chip 18 (2018) 3668-3677. DOI:10.1039/c8lc00916c |
| [117] |
W. Kang, S. Sarkar, Z.S. Lin, S. McKenney, T. Konry, Anal. Chem. 91 (2019) 6242-6249. DOI:10.1021/acs.analchem.9b00939 |
| [118] |
T. Pemovska, J.W. Bigenzahn, G. Superti-Furga, Curr. Opin. Pharmacol. 42 (2018) 102-110. DOI:10.1016/j.coph.2018.07.008 |
| [119] |
M. Tyers, G.D. Wright, Nat. Rev. Microbiol. 17 (2019) 141-155. DOI:10.1038/s41579-018-0141-x |
| [120] |
A. Kulesa, J. Kehe, J.E. Hurtado, P. Tawde, P.C. Blainey, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 6685-6690. DOI:10.1073/pnas.1802233115 |
| [121] |
A. Mepham, J.D. Besant, A.W. Weinstein, et al., Lab Chip 17 (2017) 1505-1514. DOI:10.1039/C7LC00199A |
| [122] |
M. Azizi, M. Zaferani, B. Dogan, et al., Anal. Chem. 90 (2018) 14137-14144. DOI:10.1021/acs.analchem.8b03817 |
| [123] |
J. Avesar, D. Rosenfeld, M. Truman-Rosentsvit, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) E5787-E5795. |
| [124] |
Q. Yi, D. Cai, M. Xiao, et al., Biosens. Bioelectron. 135 (2019) 200-207. DOI:10.1016/j.bios.2019.04.003 |
| [125] |
S. Mashaghi, A.M. van Oijen, Biomicrofluidics 10 (2016) 024102. DOI:10.1063/1.4943126 |
| [126] |
R.W. Yucha, K.S. Hobbs, E. Hanhauser, et al., EBioMedicine 20 (2017) 217-229. DOI:10.1016/j.ebiom.2017.05.006 |
| [127] |
W. Liu, M.U. Caglar, Z. Mao, et al., Sci. Adv. 5 (2019) eaax4761. DOI:10.1126/sciadv.aax4761 |
| [128] |
W. Liu, H. He, S.Y. Zheng, Trends Biotechnol. 38 (2020) 1360-1372. DOI:10.1016/j.tibtech.2020.04.010 |
| [129] |
K. Faust, J. Raes, Nat. Rev. Microbiol. 10 (2012) 538-550. DOI:10.1038/nrmicro2832 |
| [130] |
T.S. Tshikantwa, M.W. Ullah, F. He, G. Yang, Front. Microbiol. 9 (2018) 1156. DOI:10.3389/fmicb.2018.01156 |
| [131] |
R.M. Braga, M.N. Dourado, W.L. Araújo, Braz. J. Microbiol. 47 (2016) 86-98. DOI:10.1016/j.bjm.2016.10.005 |
| [132] |
L. Tang, Nat. Methods 16 (2019) 19. |
| [133] |
A.X. Lu, H. Oh, J.L. Terrell, W.E. Bentley, S.R. Raghavan, Chem. Sci. 8 (2017) 6893-6903. DOI:10.1039/C7SC01335C |
| [134] |
M. Tovar, S. Hengoju, T. Weber, et al., Anal. Chem. 91 (2019) 3055-3061. DOI:10.1021/acs.analchem.8b05451 |
| [135] |
X. Guo, K.P.T. Silva, J.Q. Boedicker, Phys. Biol. 16 (2019) 036001. DOI:10.1088/1478-3975/ab005f |
| [136] |
J.Y. Tan, S. Wang, G.J. Dick, et al., Integr. Biol. 12 (2020) 263-274. DOI:10.1093/intbio/zyaa021 |
| [137] |
D.N. Carruthers, C.K. Byun, B.J. Cardinale, X.N. Lin, Integr. Biol. 9 (2017) 687-694. DOI:10.1039/C6IB00241B |
| [138] |
S.S. Terekhov, I.V. Smirnov, A.V. Stepanova, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 2550-2555. DOI:10.1073/pnas.1621226114 |
| [139] |
S.S. Terekhov, I.V. Smirnov, M.V. Malakhova, et al., Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 9551-9556. DOI:10.1073/pnas.1811250115 |
| [140] |
I.L. Brito, Nat. Rev. Microbiol. 19 (2021) 442-453. DOI:10.1038/s41579-021-00534-7 |
| [141] |
I. Chen, D. Dubnau, Nat. Rev. Microbiol. 2 (2004) 241-249. DOI:10.1038/nrmicro844 |
| [142] |
C.J.H. von Wintersdorff, J. Penders, J.M. van Niekerk, et al., Front. Microbiol. 7 (2016) 173. |
| [143] |
T. Lam, M. Maienschein-Cline, D.T. Eddington, D.A. Morrison, Integr. Biol. 11 (2019) 415-424. DOI:10.1093/intbio/zyz036 |
| [144] |
T. Lam, M.D. Brennan, D.A. Morrison, D.T. Eddington, Lab Chip 19 (2019) 682-692. DOI:10.1039/c8lc01367e |
| [145] |
R.H. Hsu, R.L. Clark, J.W. Tan, et al., Cell Syst. 9 (2019) 229-242. DOI:10.1016/j.cels.2019.06.008 |
| [146] |
J. Kehe, A. Kulesa, A. Ortiz, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 12804. DOI:10.1073/pnas.1900102116 |
| [147] |
J. Ladau, E.A. Eloe-Fadrosh, Trends Microbiol 27 (2019) 662-669. DOI:10.1016/j.tim.2019.03.003 |
| [148] |
R.K. Kumar, T.A. Meiller-Legrand, A. Alcinesio, et al., Nat. Commun. 12 (2021) 857. DOI:10.1007/978-3-030-70296-0_65 |
| [149] |
D.K. Maurya, A. Kumar, U. Chaurasiya, T. Hussain, S.K. Singh, 11-Modern era of microbial biotechnology: opportunities and future prospects, in: M.K. Solanki, P.L. Kashyap, R.A. Ansari, B. Kumari (Eds. ), Microbiomes and Plant Health, Academic Press, 2021, pp. 317-343.
|
| [150] |
L. Dufossé, M. Fouillaud, Front. Microbiol. 10 (2019) 2843. DOI:10.3389/fmicb.2019.02843 |
| [151] |
S. Sudheer, R.G. Bai, Z. Usmani, M. Sharma, Curr. Genomics 21 (2020) 321-333. DOI:10.2174/1389202921999200603165934 |
| [152] |
J.V. Pham, M.A. Yilma, A. Feliz, et al., Front. Microbiol. 10 (2019) 1404. DOI:10.3389/fmicb.2019.01404 |
| [153] |
Y. Vervoort, A.G. Linares, M. Roncoroni, et al., Curr. Opin. Biotechnol. 46 (2017) 120-125. DOI:10.1016/j.copbio.2017.02.011 |
| [154] |
E.K. Bowman, H.S. Alper, Trends Biotechnol. 38 (2020) 701-714. DOI:10.1016/j.tibtech.2019.11.002 |
| [155] |
X. Jian, X. Guo, J. Wang, et al., Biotechnol. Bioeng. 117 (2020) 1724-1737. DOI:10.1002/bit.27327 |
| [156] |
J.A. Hernandez-Valdes, M. aan de Stegge, J. Hermans, et al., Metab. Eng. Commun. 11 (2020) e00133. DOI:10.1016/j.mec.2020.e00133 |
| [157] |
L. Mahler, S.P. Niehs, K. Martin, et al., eLife 10 (2021) e64774. DOI:10.7554/eLife.64774 |
| [158] |
M. Napiorkowska, L. Pestalozzi, S. Panke, M. Held, S. Schmitt, Small 17 (2021) 2005523. DOI:10.1002/smll.202005523 |
| [159] |
M. Schirmer, K. Wink, S. Ohla, et al., Anal. Chem. 92 (2020) 10700-10708. DOI:10.1021/acs.analchem.0c01839 |
| [160] |
T. Beneyton, S. Thomas, A.D. Griffiths, et al., Microb. Cell Fact. 16 (2017) 18. DOI:10.1186/s12934-017-0629-5 |
| [161] |
L. Guo, W. Zeng, S. Xu, J. Zhou, Bioresour. Technol. 318 (2020) 124258. DOI:10.1016/j.biortech.2020.124258 |
| [162] |
F. Ma, T. Guo, Y. Zhang, et al., Environ. Microbiol. 23 (2021) 996-1008. DOI:10.1111/1462-2920.15257 |
| [163] |
A.S. Tauzin, M.R. Pereira, L.D. Van Vliet, et al., Microbiome 8 (2020) 141. DOI:10.1186/s40168-020-00911-z |
| [164] |
P. Xu, C. Modavi, B. Demaree, et al., Nucleic Acids Res. 48 (2020) e48. DOI:10.1093/nar/gkaa131 |
| [165] |
J.C. Baret, Lab Chip 12 (2012) 422-433. DOI:10.1039/C1LC20582J |
| [166] |
N.M. Kovalchuk, E. Roumpea, E. Nowak, et al., Chem. Eng. Sci. 176 (2018) 139-152. DOI:10.1016/j.ces.2017.10.026 |
| [167] |
T.C. Tang, E. Tham, X. Liu, et al., Nat. Chem. Biol. 17 (2021) 724-731. DOI:10.1038/s41589-021-00779-6 |
| [168] |
S.D. Girolamo, C. Puorger, G. Lipps, Microb. Cell Fact. 19 (2020) 170. DOI:10.1186/s12934-020-01427-9 |
| [169] |
Z. Xu, S. Wang, C. Zhao, et al., Nat. Commun. 11 (2020) 5985. DOI:10.1038/s41467-020-19823-5 |
| [170] |
P. Gruner, B. Riechers, B. Semin, et al., Nat. Commun. 7 (2016) 10392. DOI:10.1038/ncomms10392 |
| [171] |
M. Weitz, A. Mückl, K. Kapsner, et al., J. Am. Chem. Soc. 136 (2014) 72-75. DOI:10.1021/ja411132w |
| [172] |
S. Bianchi, F. Saglimbeni, G. Frangipane, D. Dell'Arciprete, R. Di Leonardo, Soft Matter 15 (2019) 3397-3406. DOI:10.1039/c9sm00077a |
| [173] |
P. Xu, X. Zheng, Y. Tao, W. Du, Anal. Chem. 88 (2016) 3171-3177. DOI:10.1021/acs.analchem.5b04510 |
| [174] |
F. Huang, Z. Zhu, Y. Niu, et al., Lab Chip 20 (2020) 1249-1258. DOI:10.1039/d0lc00111b |
| [175] |
S.Y. Tang, K. Wang, K. Fan, et al., Anal. Chem. 91 (2019) 3725-3732. DOI:10.1021/acs.analchem.9b00093 |
| [176] |
Z. Chen, P. Liao, F. Zhang, et al., Lab Chip 17 (2017) 235-240. DOI:10.1039/C6LC01305H |
| [177] |
Y. Zhang, Q. Zhao, D. Yuan, et al., Lab Chip 20 (2020) 4592-4599. DOI:10.1039/d0lc00939c |
| [178] |
B. Shi, D. Wu, Y. Jiang, J. An, W. Wu, Adv. Mater. Interfaces 7 (2020) 2001074. DOI:10.1002/admi.202001074 |
| [179] |
L. Mei, M. Jin, S. Xie, et al., Lab Chip 18 (2018) 2806-2815. DOI:10.1039/C8LC00479J |
| [180] |
Z. He, J. Wang, B.J. Fike, et al., Biosens. Bioelectron. 191 (2021) 113458. DOI:10.1016/j.bios.2021.113458 |
 2022, Vol. 33
2022, Vol. 33 

