b Department of Drug Clinical Trial Institution, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hosptial, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen 518116, China;
c Department of Urology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510220, China
miRNAs are endogenous small non-coding RNAs (20–24 nucleotides) that participate in various physiological and pathological processes through post-transcriptional regulation by regulating multiple mRNAs and miRNA levels [1]. It is demonstrated that the level of miRNAs can influence the occurrence and development of diseases and the regulation of drug resistance, so miRNAs can treat various incurable diseases such as cancer and neurodegenerative diseases as promising gene drugs. For instance, miR-34a and miR-16–5p have the potential to treat cancer because miR-34a can participate in tumor repression and autophagy regulation, and miR-16–5p can induce apoptosis and inhibit cell migration [2, 3]. At present, some miRNA drugs are undergoing clinical trials, and great progress has been made in the research and development of miRNA drug patents and miRNA therapy [4]. However, there are some barriers to the clinical application of miRNAs such as the instability in vivo and difficulty associated with crossing biological barriers.
To overcome these problems, nanoparticles have been used as drug carriers to carry genes effectively and promote the targeting of receptor cells. The miRNA nanocarriers can be inorganic materials, organic materials, and inorganic/organic materials, such as magnetic nanoparticles [5], mesoporous silica, carbon nanotubes, quantum dots [6], dendrimers [7], liposomes [8], intelligent hydrogels [9], peptides [10, 11], metal-organic frameworks (MOFs). For example, the gold nanorods with single-stranded DNA could be used to load complementary miRNAs. Interestingly, the single-stranded DNA with distinct melting temperatures enables the controlled release of miRNA by adjusting the powers of the near-infrared laser [12]. Kang and co-workers synthesized a semiconducting polymer to load miR-7, this nanocarrier could target tumor treatments by the guidance of a near-infrared (NIR) pulse laser [13]. Zhao et al. utilized ZIF-8 for the delivery of miR-34a-m through electrostatic and coordination interactions to realize synergistic therapeutic for three negative breast cancer [14]. Although these nanocarriers protect miRNA from degradation in vivo, promote cell internalization and improve the targeting performance of miRNA, these nanocarriers still have some problems just like poor biocompatibility, potential cytotoxicity, and short cycling times. More unfortunately, the difficulties of large-scale production of nanoparticles and crossing the biological barrier still limit the clinical application of nanoparticles [15, 16]. To solve these problems, some natural functional biomaterials, such as egg white [17], cancer cell-platelet-fusion-membrane vesicle and erythrocyte carriers [18], have been more and more widely used as drug carriers in the recent year. Among these, exosomes have attracted much attention as miRNA drug delivery systems.
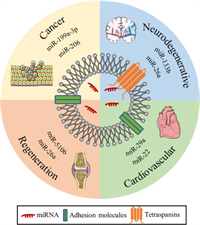
Extracellular vesicles are highly heterogeneous vesicles secreted by various types of cells, with sizes ranging from several nanometers to microns. Depending on the source, extracellular vesicles can be classified as exosomes, microvesicles, apoptotic bodies, and so on. Exosomes were found in the endosome structure of multivesicular bodies (MVBs) with sizes ranging from 30 nm to 120 nm [19]. Exosomes are involved in a variety of physiological and pathological activities, such as immune responses, cardiovascular diseases, central nervous system-related diseases, and cancer progression [20]. Exosomes carrying abundant proteins, nucleic acids, and other biological macromolecules can be endocytosed into recipient cells through multiple mechanisms, such as clathrin-mediated endocytosis, or can directly fuse with the cell membrane to send sorts to the recipient cells with the corresponding biological activity [21]. It is reported that exosomes have good biocompatibility and biodegradability, can protect miRNAs from degradation, prolong blood circulation time, and cross various physiological barriers, such as the blood-brain barrier [22] and cell membrane. In addition, exosomes can not only load miRNA drugs by traditional electroporation and freeze-thaw methods but can also enrich specific miRNA drugs by studying the endogenous cargo sorting pathway, which enables exosomes to load more miRNA drugs. With the advantages of crossing the biological barrier and endogenous loading miRNA, an increasing amount of evidence indicates that exosomes have good potential as excellent miRNA carriers for the treatment of various diseases. For instance, exosomes carrying miR-644-5p inhibited the apoptosis of ovarian granulosa cells by targeting p53, which has the potential to treat premature ovarian failure (POF) and restore ovarian function [23]; the exosome-mediated delivery of miR-155 inhibitors can prevent DSS-induced colitis [24], and exosomes carrying miR-21 can critically maintain photoreceptor activity against N-methyl-N-nitrosourea (MNU) damage by targeting programmed cell death 4 (PDCD4) and have potential therapeutic effects on photoreceptor cell apoptosis and retinal dysfunction [25]. This article mainly discusses the potential application and challenges of exosomes as miRNA drug carriers in cancer, neurodegenerative diseases, cardiovascular diseases, and regenerative medicine (Fig. 1).
|
Download:
|
| Fig. 1. Application of exosomes as miRNA drug carriers. | |
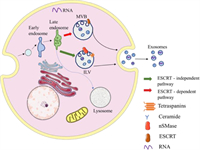
The formation of exosomes experienced continuous invagination of the intima and the formation of MVBs (Fig. 2). First, the plasma membrane buds inward so that some cell-surface proteins, extracellular metabolites, and lipids enter the cell to form the early sorting endosome (ESE). Before the formation of late sorting endosomes (LSEs), ESEs can fuse with the membrane of the trans-Golgi network and endoplasmic reticulum to enrich the proteins of ESEs. Subsequently, ESEs mature into LSEs [26]. The invagination of the LSEs leads to the generation of MVBs, which contain abundant intraluminal vesicles (future exosomes). Distinct proteins take part in the process, such as endosomal sorting complexes required for transport proteins (ESCRT), ceramide, RAB31 [27], and sphingosine-1-phosphate (S1P) [28]. Of these, ESCRT is the most perfect driving factor for the early maturation of endosomes and the formation of MVBs. First, the ESCRT-0 complex binds to ubiquitinated cargo to promote the invagination of the plasma and further recruit ESCRT-Ⅰ and ESCRT-Ⅱ, which appear to be responsible for the invagination of the membrane. Then, ESCRT-Ⅱ combines with ESCRT-Ⅰ and is activated to bind to ESCRT-Ⅲ. Thus, vesicles are separated from the cytoplasmic membrane by the ESCRT-Ⅲ complex [29, 30]. It has been reported that part of the MVBs can undergo degradation in the lysosomes and another part can move along microtubules and then fuse with the plasma membrane to release exosomes by exocytosis. In the secretion process, it has been shown that Ras-related proteins such as the Rab27A, Rab27B protein, and Rab11 protein are the key regulatory factors [31]. Rab27A and Rab27B can regulate the distribution of multivesicular endosomes (MVEs) in cells and promote the secretion of exosomes [32]. It is an approach that is considered for the control of exosome secretion.
|
Download:
|
| Fig. 2. The biogenesis of exosomes. | |
Because of the biogenesis of exosomes, exosomes contain different surface proteins, intracellular proteins, RNAs, DNAs, amino acids, and metabolites. Thus, exosomes, as carriers of various biomacromolecule drugs, have attracted much interest. The secreted exosomes can transport biological macromolecules into the cell in a variety of ways, such as endocytosis and fusion with cell membranes, to realize the regulation of life activities. By studying the biological pathway of exosomes, it is possible to realize the controllable production of exosomes and increase the drug loading rate of exosomes.
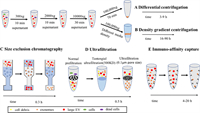
3. Isolation of exosomesRecently, there has been an increasing number of choices for isolating exosomes. Among these methods, exosomes are mainly separated according to their density, size, and immunoaffinity (Fig. 3) [33]. As we know, the membrane of exosomes has many special receptors and proteins so that exosomes can be enriched by specific antibodies based on the common markers of exosomes. Therefore, immunoaffinity capture is a promising approach for isolating exosomes selectively and can be applied to the examination and prognosis of diseases efficiently. Traditional immunoaffinity capture which can specifically isolate exosomes by co-incubating exosomes with magnetic beads with antibodies on the surface has some problems just like long process times, low purity. So microfluidic devices based on immunoaffinity-based capture have been developed for efficient exosome separation and isolation. For example, Kang et al. developed a microfluidic device with melanoma-specific antibodies, which could be used for the separation of melanoma exosomes [34]. Yang et al. reported a pH-responsive superparamagnetic nanoparticle cluster (SPMN) for the precise and mild separation of blood TfR+ exosomes [35]. However, the separation of antigen-antibody after binding is difficult, so it is possible to destroy the vesicular structure of exosomes.
|
Download:
|
| Fig. 3. The isolation of exosomes. Reproduced with permission [33]. Copyright 2020, Elsevier Ltd. | |
Based on different sizes, techniques such as size exclusion color blocking, microfluidic filtration, and field-flow fractionation [36] can be used to remove large cell fragments and protein aggregates in order to achieve the purpose of separating and purifying exosomes. Size exclusion chromatography (SEC) has a specific distribution of spherical beads, so it can separate exosomes from cell debris and soluble proteins according to the residence time. Owing to the low cost and speed of isolation, SEC may be an option for the large-scale processing of exosomes [37]. In addition to traditional SEC, a variety of microfluidic systems based on size separation have been developed to separate exosomes from large cell debris and protein aggregates. For example, Wu et al. separated exosomes with a complete structure and approximate size via acoustic waves and microfluidics [38]. Compared to ultracentrifugation (UC), the size-based separation method can maintain the integrity of exosomes and prevent protein contamination.
However, the standard method of isolating exosomes continues to be UC, including gradient centrifugation. UC separates molecules with different densities at different rates according to the density of vesicles to obtain relatively pure exosomes. First, a low centrifugal force was used to remove cellular debris and increase the centrifugal force to remove microbubbles. Finally, exosomes are obtained by UC at 10000g [39]. The exosome yield obtained from UC is among the highest, but there is a risk of contamination by soluble proteins and destruction of exosome integrity. Density gradient UC is a more stringent form of UC and is used to obtain exosomes with higher purity [36]. The limitation of this method is with respect to its throughput and the contamination of lipoproteins. All of these methods have their limitations, so there is still a long way to go to achieve the efficient separation of exosomes, which significantly limits their further application.
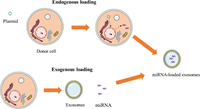
4. Methods of loading drugs in exosomesThe loading of miRNAs into exosomes is an important problem. Currently, there are mainly endogenous and exogenous loading methods. This section introduces endogenous and exogenous loading methods and discusses the researchers' attempts to increase exosome loading efficiency (Fig. 4).
|
Download:
|
| Fig. 4. The schematic diagram of exogenous and endogenous loads. | |
Endogenous loading occurs when cells secreting exosomes synthesize both exosomes and miRNAs, after which they load miRNAs into exosomes through a sorting mechanism. This method maintains the integrity of the exosome membrane. At present, transfection is the most common method of endogenous transfer. The target miRNA expression plasmid was introduced into the mother cell by transfection, and the secreted exosomes contained the target cargo. For example, Mark et al. transfected target cells with a miR-146b expression plasmid by electroporation, and they obtained exosomes containing miR-146b released by the target cells [40].
The loading of exosomes with special miRNAs can be promoted by discussing the mechanism of miRNA sorting into exosomes so that the content of miRNA in exosomes can be increased. Some studies have shown that the special binding motif of miRNA can be specifically recognized by some proteins (such as hnRNPA2B1, hnRNPA1, HuR) and selectively loaded into exosomes [41]. Carolina et al. showed that sumoylated hnRNPA2B1 can recognize the specific short motifs of miR-198 [42]. The level of miR-198 in exosomes can be increased by the overexpression of hnRNPA2B1. Therefore, specific miRNA levels can be increased by increasing the content of hnRNPA2B1 or by inserting a specific motif into the desired miRNA motif. Furthermore, researchers fused exosomal membrane protein CD9 with HuR, which is a protein that can interact with miR-155 effectively. The results showed that the presence of CD9-HuR significantly enriched miR-155 entering exosomes [43]. Similarly, Sutaria et al. used the TAT peptide/HIV-1 transactivation response (TAR) RNA-interacting peptide to enhance the loading of modified miR-199a into exosomes by 65-fold [44]. Therefore, the high efficiency of miRNA loading in exosomes can be achieved by using proteins that can specifically bind to miRNA.
Further studies on the separation mechanism of exosome cargos have the potential to increase the loading of specific miRNA drugs. However, different loading methods need to be adjusted for different cell types and miRNAs, which limits their further development.
4.2. Exogenous load strategyAt present, commonly used methods to achieve exogenous loading are electroporation, freeze-thaw, and co-incubation. Electroporation was initially used to load exogenous genes into living cells. Because of the similar membrane characteristics of exosomes and cell membranes, electroporation can also be used to load miRNAs in exosomes. Electroporation is the formation of pores in the exosome membrane under the action of an electric field, and miRNA enters the exosomes through diffusion. Exosomes were then incubated at 37 ℃ to allow the exosomes to fully recover. Liang et al. used electroporation to load miR-21i and 5-FU into exosomes to treat colon cancer [45]. However, this method can cause damage to the exosome membrane and the accumulation of miRNA [46].
The principle of the freeze-thaw cycle is that through repeated freezing and thawing, the exosome membrane will be slightly disrupted so that the cargo can diffuse into the exosomes. Won Lee et al. successfully synthesized exosomes containing miR-140 using the freeze and thaw method. Their studies confirmed that exosomes maintain the bioactivity of miR-140 and participate in cartilage healing [47].
In addition, miRNAs can be successfully loaded by simple incubation. For example, miR-150 can be loaded into exosomes by co-incubation at 37 ℃ for 1 h. Unfortunately, the loading efficiency is low because there is no external force during loading [48]. To solve the problem of the low loading efficiency of simple incubation and to effectively increase the content of nucleic acid cargo in exosomes, Jeyaram et al. generated a pH gradient across exosome membranes by the protonation of exosomes to enhance vesicle loading of miRNAs so that the load of miRNAs in this method is similar to that of electroporation and ultrasound [49].
Although the loading effects of exogenous loads on cargo are different, these techniques may lead to the aggregation of exosomes or their cargo and can change their physicochemical and morphological characteristics [50]. Therefore, it is necessary to develop more efficient and mild nucleic acid loading methods for exosomes.
5. Strategy of targeted therapyTargeting cells, tissues, or organs can achieve more precise treatments with fewer side effects, so it is a research focus of the nano-drug system. For example, the precise and controllable release of drugs can be achieved by designing a pH-sensitive, GSH-responsive, or H2O2-responsive nanoparticle system [51-53]. This paper will introduce the approaches of exosome targeting from two perspectives: passive targeting and active targeting.
5.1. Passive targetingPassive targeting is a method of targeting without changing the genome of exosome-producing cells. Selecting the appropriate exosome-producing cells according to the target cells was used to achieve the target. For example, Peter et al. found that bone marrow mesenchymal stem cells (MSC-Exo) can also be driven by inflammation, and specifically target the brain lesions in mice [54]. The exosomes derived from the RAW264.7 macrophage cell line not only secrete anti-inflammatory mediators but also have inflammation targeting properties. He et al. produced curcumin-containing exosomes (Ex-cur) by incubating curcumin and macrophage RAW264.7. This work demonstrated that Ex-cur can target ischemic brain tissue and accumulate in the ischemic region; therefore, Ex-cur can effectively reduce the accumulation of ROS and treat ischemia-reperfusion injury [55].
Another passive targeting method is exosome engineering, which involves embedding homing peptides or specific antibodies on the surface of exosomes, which can target specific cells [56]. Yan et al. modified the folic acid polyethylene glycol cholesterol complex (FPC) on macrophage-derived exosomes. In inflammatory tissue, a large number of activated macrophages express folic acid receptors (FRs), and thus the accumulation of exosomes can be promoted by folic acid. In addition, polyethylene glycol can improve the stability of the drug delivery system and can fix the FPC into the exosome membrane with cholesterol. The results show that the modified exosomes have a more obvious accumulation in the inflammatory site [57]. Further, Royo et al. exhibited that the removal of sialic acid residues on the surface of exosomes allows more exosomes to reach the lung and accumulate in the lung, which confirms that the glycosylation of extracellular vesicles can change their biodistribution in vivo [58]. Therefore, the surface modification of exosomes by glycosylation is another method for exosome engineering to achieve exosome targeting [59]. In addition to the above two methods, the exosome surface can be modified by click chemistry and non-covalent modification. For example, Tamura et al. modified cationic amylopectin on the surface of exosomes by electrostatic interactions, which can target the damaged liver [60].
Magnetic nanoparticles have a good response to an external magnetic field, which can achieve local aggregation under the action of an external magnetic field [61]. Therefore, the introduction of magnetic nanoparticles into exosomes is a potential way of achieving targeting. Li et al. magnetically introduced Fe3O4 particles into mesenchymal stem cells, and the secreted exosomes were modified with magnetic nanoparticles on the surface, which can be targeted under the action of an external magnetic field [62]. Liu et al. modified the surface of magnetic nanoparticles with antibodies targeting CD63 on the surface of exosomes, which can be connected to exosomes expressing CD63 protein and achieve aggregation at the infarct site under magnetic guidance. Interestingly, another antibody (anti-myosin light chain) is modified on the surface of the magnetic nanoparticles, which can target damaged cardiomyocytes and achieve dual targeting. Compared with pure magnetic targeting, it has a better targeting effect [63].
It can be seen that the exosomes have better targeting ability through the engineering of exosomes, and dual targeting is a way of further improving the targeting ability. Inspired by the virus targeting multiple receptors on the cell surface simultaneously to achieve enhanced adhesion, Wang et al. modified exosomes with two different ligands to improve their targeting effect, which can effectively target cancer cells [64]. In addition to functionalizing exosomes using traditional physicochemical methods, exosomes can also achieve targets by modifying the genome of exosome-producing cells.
5.2. Active targetingActive targeting can express a special ligand to achieve targeting by changing the transcriptome to produce exosomes. To achieve the target, one strategy is to combine the gene of the exosome membrane protein and the target protein to transfect the donor cell. Among them, membrane proteins act as anchors to anchor the targeted peptides on the surface of exosomes, and Lamp2b is currently the most common membrane protein (Fig. 5A) [28]. For example, the Lamp2b gene was fused with the gene of the ischemic myocardium-targeting peptide, and the complete gene sequence was cloned into the plasmid. The plasmid was then transfected into mesenchymal stem cells. Therefore, the exosomes secreted by the transfected cells contained the fusion protein and could target ischemic cardiomyocytes, promote the weakening of inflammation and apoptosis, reduce fibrosis, and enhance angiogenesis and cardiac function [65]. Lydia et al. engineered self-derived dendritic cells to express Lamp2b fused to the neuron-specific RVG peptide to target neurons, microglia, and oligodendrocytes. Thus, RVG-targeted exosomes can effectively treat Alzheimer's disease [66].

|
Download:
|
| Fig. 5. Examples of the different active targeting approaches used by the studies included. (A) Targeted proteins fused with trans member proteins; (B) Targeted proteincontaining transmembrane domain. (C) Overexpression of homing protein. Reproduced with permission [28]. Copyright 2019, Elsevier B.V. | |
In addition to targeting peptides directly binding to the transmembrane protein, targeted peptides containing the transmembrane domain can also be constructed to achieve targeting (Fig. 5B). Ohno et al. produced exosomes that can express the GE11 peptide that can target the epidermal growth factor receptor (EGFR) fused to the transmembrane domain of the platelet-derived growth factor. Exosomes can target tumor tissues expressing the EGFR receptor without any major organ damage [67]. Because EGFR is upregulated in several types of cancer cells, it has the potential for overall cancer treatment. In addition to the above two methods, exosomes can be targeted by inducing overexpression of targeted proteins in donor cells [68]. The exosomes produced by rat choroidal epithelial cells that overexpress folate receptor-α can target brain parenchyma, which opens up a new way of achieving brain drug targeting (Fig. 5C) [69].
6. Application of exosomes as drug deliverymiRNA is a small non-coding nucleotide sequence that can target multiple genes and mediate different cell pathways; therefore, it is crucial in the occurrence and progression of a variety of diseases. Therefore, miRNAs can regulate physiological activities and treat various diseases, enabling the overcoming of complicated diseases. Despite the rapid development of disease diagnosis and treatment in recent years, cancer, neurodegenerative disease, cardiovascular disease, tissue regeneration, and chronic inflammation are still incurable diseases, which will affect the quality of patients' lives and even cause death. As a drug to treat these diseases, miRNA can prolong life and improve the quality of life, which is still a hot research topic. However, the delivery of miRNAs remains a challenge owing to the instability of miRNAs.
Compared with other drug carriers, exosomes have the advantages of being nontoxic, non-immunogenic, and have excellent biocompatibility, long cycle time, and can cross a variety of physiological barriers. In addition, exosomes can not only load miRNAs through conventional external methods, such as electroporation and freeze-thaw but can also load miRNAs through endogenous pathways. The loading rate of miRNAs can be improved by studying the regulation of endogenous pathways. Therefore, exosomes have the potential to carry more miRNAs. Studies have shown that exosomes inherit the unique characteristics of mother cells and have homing characteristics, which can target specific tissues in vivo [70]. For instance, the exosomes secreted by MSCs and macrophages can target inflammatory tissues [54, 55]. Finally, exosomes can be absorbed by cells through almost all major uptake pathways, effectively carrying cargo into cells. It has been reported that many pharmaceutical companies have focused on the development of therapeutic exosomes as vectors for gene and RNA therapy [71]. Therefore, exosomes have good potential in the treatment of various diseases with miRNA drug carriers. By exploring the literature of exosome loaded miRNA, it is found that exosome carrying miRNA is mainly used in cancer treatment, nerve regeneration, cardiovascular diseases, and regenerative medicine, so this chapter mainly introduces the research progress of exosomes as miRNA vectors when treating cancer, neurodegenerative diseases, and so on.
6.1. Exosomes in cancer therapyCancer is one of the most threatening diseases to human life. At present, the treatment of cancer is still based on traditional chemotherapy, radiotherapy, and surgical resection, but these treatment methods have high recurrence frequency, great damage to the body, and may produce drug resistance. Therefore, there is an urgent need for new therapies to prolong the life and improve the quality of life of cancer patients. In recent years, immunotherapy and gene therapy which can effectively control tumor growth and inhibit tumor metastasis have attracted people's attention as new cancer treatments. For example, editing oncogenes or tumor suppressor genes by CRISPR/Cas9 technique [72] or designing nanoparticles loaded with mRNA and immunosuppressant R848 as a cancer vaccine to stimulate the immune system could both inhibit tumor growth effectively [73]. Among these, therapeutic miRNAs may be expected to enter the clinic as the next generation of therapeutic drugs.
In the development of tumors, tumor-derived exosomes can act as messengers to mediate intercellular communication, regulate cancer cells and their microenvironment, promote tumor growth and metastasis, and tumor immune escape [74]. In this process, miRNAs in exosomes play an important role, such as miR-19b-3p, miR-17–5p, miR-30a-5p, and miR-106a-5p are involved in the pathogenesis of gastric cancer. Let-7b-5p, miR-224–5p, miR-122–5p, miR-615–3p, and miR5787 can be downregulated in exosomes secreted by gastric cancer cells [75]. miR-122 can inhibit the migration and invasion of hepatoma cells [76]. Therefore, specific miRNAs in exosomes can be used as the basis of tumor diagnosis [77, 78] and development stage, and exosomes loaded with miRNAs that can inhibit tumors are also widely used in tumor therapy.
6.1.1. Exosomes in glioblastomaGlioblastoma multiforme (GBM) is the most common and incurable kind of primary brain tumor, with high invasiveness and specificity. Conventional surgical resection cannot completely remove tumor cells in the brain, resulting in a high mortality rate of glioblastoma, with an average overall survival rate of 15 months [22]. Recent studies have shown that miRNAs can regulate the microenvironment of glioma, and play an important role in tumor proliferation, invasion, angiogenesis, and immune escape. For example, Bronisz et al. explored the role of miR-1 in glioblastoma multiforme and confirmed that miR-1 could reduce the adhesion of glioblastoma multiforme cells and inhibit the growth, invasion, and angiogenesis of glioblastoma multiforme by targeting ANXA2, which is the main component of the cancer signaling network [79]. miR-7 targeting the XIAP gene could enhance the sensitivity of glioma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and promote the apoptosis of cancer cells [80]. Studies have shown that miR-146b could bind to EGFR mRNA to reduce the expression of the EGFR gene in malignant glioma and reduce glioma cell motility and invasion. Therefore, miRNAs are considered as promising therapeutic drugs.
Exosomes are excellent carriers for carrying miRNA to treat glioblastoma because of their ability to cross the blood-brain barrier. Mark et al. used exosomes derived from bone marrow mesenchymal stem cells to load miR-146b, which could effectively transfer miR-146b to tumor cells and inhibit the growth of tumor cells without affecting normal astrocytes. In vivo experiments showed that the intratumoral injection of M146-Exo significantly reduced the growth of glioma in the rat brain [40]. And Mohamed et al. prepared primary glioma cells that could stably express and secrete miR-302–367 exosomes. The orthotopic xenograft of miR-302–367-expressing cells could change the proliferation and tumorigenicity of adjacent glioblastoma cells in a paracrine-dependent manner, which could effectively treat malignant glioma [81]. It is reported that miR-124a rich exosomes obtained by transfecting bone marrow mesenchymal stem cells could target Forkhead box (FOX) A2 to interfere with the lipid metabolism of tumor cells and promote the apoptosis of glioma cells [82]. Yu et al. found that miR-199a not only inhibits glioma cells by inhibiting the expression of arf GTPase-activating protein-2 (AGAP2), which is found to be over-expressed in glioma to mediate endosomal trafficking. More importantly, miR-199a could improve the therapeutic efficacy of glioma by enhancing the sensitivity of temozolomide to chemotherapy, which could provide a new strategy for the treatment of glioma in combination with chemotherapy. As shown in Figs. 6A and B, miR-199a-containing exosomes secreted by transfected human bone marrow mesenchymal stem cells could more effectively inhibit the invasion and migration of gliomas than transfected human bone marrow stem cells [83].
|
Download:
|
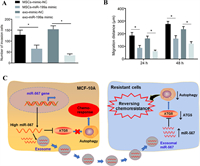
| Fig. 6. Exosomes carrying miRNAs can target tumor tissues and inhibit cancer migration. (A and B) Exosomes carrying miR-199a can inhibit the invasion and migration of glioma. Copied with permission [83]. Copyright 2019, Yu et al. (C) The mechanism that exosomes loaded miR-567 improve the chemosensitivity of breast cancer. Copied with permission [88]. Copyright 2020, Han et al. | |
It can be seen that miRNA can effectively treat gliomas by inhibiting tumor growth and migration, and by improving the sensitivity of glioma cells to chemotherapy. A large number of studies have shown that exosomes are excellent carriers that can protect miRNAs in vivo, successfully transport them to glioma cells, and maintain their original activity. However, the use of exosomes as a carrier to treat gliomas clinically still has a long way to go, for example, to further confirm whether exosomes carrying miRNA can pass through the blood-brain barrier.
6.1.2. Exosomes in breast cancerIn addition to the treatment of gliomas, miRNA-loaded exosomes can also treat a variety of common tumors, similar to breast cancer. Breast cancer is still cancer with the highest morbidity and mortality among women worldwide. According to GLOBOCAN data, the incidence of breast cancer in 2020 accounted for 11.7% of the total incidence rate, and the mortality rate accounted for 15.5% of the total female mortality rate [84]. At present, the main obstacles to the treatment of breast cancer are drug resistance and distant metastasis, including bone metastasis, lung metastasis, and brain metastasis. Many studies have shown that miRNAs can regulate the microenvironment of breast cancer, and can play an important role in the drug resistance and metastasis of breast cancer cells; similar to miR-381 [85], miR-190 can suppress breast cancer metastasis [86] and miR-22 can induce tamoxifen resistance [87]. Therefore, it is a potential drug and is a target for the treatment of breast cancer.
Exosomes loaded with miRNAs that regulate drug resistance can be used to improve the therapeutic effects of breast cancer. For example, the overexpression of miR-567 blocked the formation of autophagy, which could effectively reverse the resistance of breast cancer cells to trastuzumab. The drug resistance of different breast cancer cells could be eliminated by miR-567 in exosomes, which could improve the chemosensitivity of breast cancer (Fig. 6C) [88].
Moreover, exosome-loaded miRNAs could inhibit tumor migration by regulating the content of corresponding proteins. Exosomes loaded with miR-381 could promote apoptosis, migration, and invasion of breast cancer cells by significantly downregulating the expression of EMT-related genes and proteins [89]. Park et al. confirmed that a variety of miRNAs could reduce the expression of aquaporin 5 to inhibit the migration of breast cancer cells. For example, miR-19b-3p and miR-19a-3p could directly bind to AQP5 mRNA to regulate AQP5 protein translation, while miR1226–3p regulated aquaporin 5 by degrading the AQP5 gene. They used exosomes that express IL-4R to load miR-19b-3p by taking advantage of the high expression of IL-4R on the surface of breast cancer cells. They also significantly improved the efficiency of exosomes loaded with miRNA into MDA-MB-231 cells, and targeted the treatment of breast cancer cells. The loaded miR-19b-3p entered breast cancer cells and significantly inhibited the migration of breast cancer [90]. To deliver miRNA to breast cancer cells more effectively, Ohno et al. used exosomes that express the transmembrane region of the PDGF receptor with GE11 peptide to load let-7a. Let-7a was successfully delivered to breast cancer tissues expressing EGFR by intravenous injection of exosomes, which confirmed the potential for the systemic application of exosome-loaded miRNA in the treatment of breast cancer [67]. Interestingly, exosome-loaded miRNA can also act on immune cells to inhibit tumor progression, besides directly acting on tumor cells. Exosomes loaded with miR-130 or miR-33 increased the expression levels of miR-130 and miR-33 in macrophages. Then, macrophage polarization could be changed from M2 to M1 phenotype, which decreased the migration and invasion ability of cancer cells [91].
Therefore, exosomes loaded with miRNA can not only specifically target breast cancer cells, regulate the physiological activities of breast cancer cells, improve the chemosensitivity of breast cancer, inhibit the metastasis of breast cancer, and promote the elimination of cancer cells by acting on immune cells. It provides a ray of light for the treatment of metastatic breast cancer.
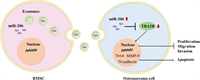
6.1.3. Exosomes in other cancersExosomes carrying miRNA can be used not only in glioma and breast cancer but also in other common cancers. For instance, the results showed that the miR-193a loaded exosomes could inhibit the resistance of cisplatin and the migration and invasion of tumor cells, promote the apoptosis of tumor cells and inhibit the tumor volume and weight [92]. Bone marrow mesenchymal stem cell-derived exosomal miR-206 could be transferred into osteosarcoma cells and target TRA2B for osteosarcoma treatment (Fig. 7) [93]. The expression of miR-139–5p was decreased in bladder cancer cells. Exosomes overexpressing miR-139–5p could target PRC1 and showed an inhibitory effect on bladder cancer in vitro and in vivo [94].
|
Download:
|
| Fig. 7. A schematic drawing of BMSC-derived exosomal miR-206 entering osteosarcoma cells. Reproduced with permission [93]. Copyright 2020, Elsevier B.V. | |
In addition to increasing the sensitivity of cells to chemotherapeutic drugs or targeting corresponding genes to promote tumor cell apoptosis, exosomes carrying miRNA could also inhibit tumor migration and metastasis to inhibit the further deterioration of cancer. For example, human umbilical cord mesenchymal stem cell-derived exosomal miR-375 could inhibit the proliferation, invasion, and migration of esophageal squamous cell carcinoma cells, the formation of tumor ball, and tumor growth [95]. Therefore, the exosomes containing miRNA are a potential drug for curing esophageal squamous cell carcinoma. Chen et al. found that the exosomes loaded with miR-6785–5p inhibited angiogenesis and metastasis in gastric cancer by targeting inhibin subunit beta A (INHBA) [96]. The exosomes loaded with miR-199a-3p by electroporation could inhibit the expression of c-Met-to inhibit the proliferation and invasion of tumor cells. It was showed that the exosomes were the excellent drug delivery system of miR-199a-3p to effectively prolong the circulation time of miR-199a-3p in vivo and inhibit the peritoneal diffusion of the ovarian cancer mouse model [97]. The hypoxic environment of colorectal cancer can lead to epithelial-mesenchymal transition (EMT) to promote cancer metastasis. Zhang et al. reported that miR-1255b-5p inhibits EMT by targeting human telomerase reverse transcriptase (hTERT). The overexpression of miR-1255b-5p hypoxic exosomes reduced EMT, tumor progression, and liver metastasis to treat colorectal cancer efficiently [98].
Taken together, exosomes can carry exogenous and endogenous miRNAs, target specific genes, and have shown promising results for the treatment of various common cancers, such as breast cancer and bladder cancer. Therefore, intensive studies on exosomes play a key role in increasing the efficacy of cancer therapy. At present, the screening of different miRNA target genes and the study of their mechanism of action on cancer can provide an idea for the research of new cancer-targeting drugs.
6.2. Exosomes in neurodegenerative diseasesGenerally, neurodegenerative disease is a serious disease that may lead to inflammation and apoptosis of nerve cells. It is a kind of special debilitating disease that may cause disability and serious death. Currently, neurodegenerative diseases can be classified as acute and chronic neurodegenerative diseases. We will introduce the application of exosomes as a good vector of miRNAs in neurodegenerative diseases from these two perspectives.
6.2.1. Exosomes in acute neurodegenerative diseasesAcute neurodegenerative diseases include stroke and brain injury. Stroke remains a major health problem in today's world to cause more and more people to pass away. The majority of strokes are caused by cerebral aorta occlusion, resulting in ischemic neuron death and blood-brain barrier damage, which can lead to death and disability [99]. Clinically, intravenous thrombolysis and other means can restore the blood supply to the brain, saving the lives of patients, but cerebral ischemia-reperfusion affects nerve cells and their subsequent recovery. An increasing body of evidence has shown that miRNAs can help the recovery of nerve injury and can be used in the treatment of stroke. Because exosomes can cross the blood-brain barrier, many researchers have tried to use exosome-loaded miRNAs to treat stroke.
At present, the ability of exosomes secreted by cells to replace cells in the treatment of stroke recovery has attracted the attention of researchers. A large number of studies have shown that exosomes secreted by cells carrying miRNA can promote stroke recovery. For instance, exosomes secreted by mesenchymal stem cells contain miR-133b, miR-184, and so on, to promote nerve and vascular regeneration, resulting in the reduction of the ischemic border area in the brain and the improvement of functional recovery, which was a potential drug for the treatment of stroke [100, 101]. Moreover, neurons and microglia in the brain may also secrete exosomes to protect the damaged nerves. Huang et al. found that miR-124–3p carried by microglia exosomes could target PDE4B to inhibit the activity of mTOR signaling, thus inhibiting neuronal inflammation [102]. Further, M2 microglia-derived exosomes, including miR-214, reduced ischemic brain injury and promoted neuronal survival, and effectively treated ischemic strokes [103]. It has been demonstrated that neurons might release exosomes containing miR-98, which could be delivered to microglia to target platelet-activating factor receptors (PAFRs), inhibit the phagocytosis of microglia to damaged and salvageable neurons, reduce inflammatory damage, and facilitate the recovery of damaged nerves [104].
Exosomes rich in certain miRNAs can be obtained by stimulating the mother cells or transfecting the corresponding miRNAs. Exosomes rich in miR-17–92 clusters could be obtained by transfection, which could improve neurological function and promote functional recovery after stroke [105]. Interestingly, Haupt et al. stimulated bone marrow mesenchymal stem cells with lithium to increase the level of miR-1906 in exosomes. By reducing the TLR4 abundance and inhibiting the activation of the B nuclear factor kappa light chain enhancer signaling pathway, they reduced the inflammatory response at the infarct site and promoted nerve recovery [106].
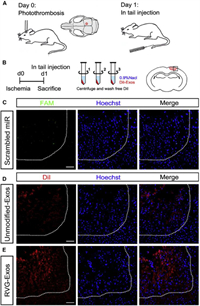
Further studies have confirmed that engineered exosomes can achieve targeted therapy. Yang et al. used RVG-Lamp2b-modified exosomes to load miR-124, which could promote neurogenesis to achieve neuroprotection and nerve remodeling to achieve neuron-specific targeting. Fig. 8 showed that there were almost no fluorescent-labeled miRNAs in the ischemic part of the brain. The miRNA encapsulated by unmodified exosomes could partially enter the ischemic part, while the exosomes modified with RVG-Lamp2b could enter the core of the ischemic region, confirming that the engineered exosomes had good targeting ability [107].
|
Download:
|
| Fig. 8. The distribution of miRNA, unmodified-Exos, and RVG-Exos in the ischemic region. (A) The model was established and exosomes were injected into the tail vein one day later. (B) The mice were centrifuged with 0.9% NaCl and washed three times to remove the free dye. Two hours after injection, the mice were killed. The square represents the selected area of the coronal section of the brain. (C-E) Representative immunofluorescence images of ischemic region from mice receiving free FAM-labeled scrambled miRNAs (C), DiIlabeled unmodified-Exos (D), and DiIlabeled RVG-Exos (E). Copied with permission [107]. Copyright 2017, The Authors. | |
Chronic neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) are commonly generated by the accumulation of misfolded/aggregated mutant proteins, leading to irreversible brain tissue damage [108]. Among these neurodegenerative diseases, AD is caused by hyperphosphorylated microtubule-associated protein tau and amyloid–β (Aβ) peptide [109, 110] resulting in the deposition of neuronal tangles, which can cause cognitive impairment, affect daily life, and even completely lose self-care ability. The ability to achieve early diagnosis and treatment of AD is still a research hotspot. Here, we mainly discuss the therapeutic potential of exosome-loaded miRNAs in AD.
It has been demonstrated that miRNAs have excellent potential for the early diagnosis and treatment of AD owing to their involvement in the development of AD. Cao et al. found that miR-195 could downregulate synaptojanin 1 (synj1) by binding to the miRNA synj1. Therefore, Aβ was dumped through the lysosomal degradation pathway, and the hyperphosphorylation of tau was improved to improve cognitive impairment and amyloid plaque load [111]. The expression of amyloid-β precursor protein (APP) and β-site APP-cleaving enzyme 1 (BACE1) could be reduced by miR-298 to reduce the content of Aβ so that miR-298 might be a suitable drug for AD therapy [112]. Similarly, miR-31 could also mediate the modulation of APP and BACE1 to treat AD [113]. In conclusion, miRNA can reduce the expression of Aβ protein in a variety of ways, which shows the good potential of miRNA in the treatment of AD.
Exosomes are suitable carriers for carrying miRNA drugs for neurodegenerative diseases because of their ability to cross the blood-brain barrier and their low immunogenicity. For instance, Jahangard et al. confirmed that miR-29 in exosomes had a possible therapeutic effect in reducing the pathological effects of Aβ peptide, making it a promising drug for the treatment of AD [114]. Recent studies have shown that AD may lead to brain hypoxia, nerve injury, and Aβ accumulation. Mesenchymal exosomes containing miR-223 could protect neurons at the cellular level via the PTEN-PI3K/Akt pathway [115]. Therefore, exosomes carrying miRNA could improve the clinical symptoms of AD by downregulating the content of Aβ in the brain and protecting neurons.
Exosomal miRNAs regulate the activity of multiple physiological pathways in neurodegenerative diseases. Therefore, miRNAs can be treated not only as diagnostic and prognostic criteria for neurodegenerative diseases but also as a potential drug system for the treatment of neurodegenerative diseases. Currently, there is still much work to be done to realize the treatment of neurodegenerative diseases. Research on the early diagnosis of neurodegenerative diseases and the exploration of exosomes carrying miRNA drugs may be an avenue for the prevention or treatment of neurodegenerative diseases in the future.
6.3. Exosomes in cardiovascular diseasesThe morbidity and mortality of cardiovascular diseases remain high worldwide despite dramatic diagnostic and therapeutic advances. Myocardial infarction is the leading cause of death involving cardiovascular disease, which leads to hypoxia, lack of nutrients, and irreversible cardiac damage. Therefore, the prevention, treatment, and prognosis of myocardial infarction are particularly important.
It has been demonstrated that miRNA is involved in the occurrence and development of myocardial infarctions, so it plays an important role in the prevention and treatment of myocardial infarction [116]. For instance, miR-30e and miR-92a could regulate lipid metabolism to prevent atherosclerosis, which might lead to vascular stenosis; thus, it was an important cause of myocardial infarction [117]. Therefore, miR-92a and miR-30e might prevent the occurrence of myocardial infarction to some extent. miRNAs could promote the survival of myocardial cells and maintain the metabolic balance in cells by regulating autophagy by miRNAs. miR-210 could regulate the vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) to the proliferation and migration of endothelial cells, stimulate the formation of capillary-like structures, promote the formation of cardiac blood vessels, and improve ventricular remodeling [118]. Overall, miRNA can prevent and treat myocardial infarction, but its degradation in vivo limits its further application in cardiovascular disease.
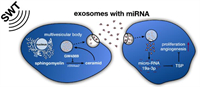
Exosomes can protect miRNAs from degradation and act as postmen to facilitate long-distance communication of cells. Studies have shown that the exosomes secreted by the damaged heart can protect miRNAs from degradation in the blood, and can stimulate the bone marrow to secrete exosomes to realize the repair of myocardial damaged cells [119, 120]. Therefore, exosomes loaded with miRNAs as medicine are the future direction of cardiovascular disease. Xu et al. confirmed that melatonin could promote the upregulation of miR-201/miR-211 in exosomes secreted by vascular smooth muscle cells, directly target BMP2 (a growth factor that induces bone formation), and reduce the osteogenic differentiation and aging of vascular smooth muscle cells to reduce vascular calcification and aging [121]; it might be used for the prevention of myocardial infarction in the future. One of the treatment directions of myocardial infarction is to protect the damaged myocardial cells and reduce the apoptosis of myocardial cells. Exosomes from young bone marrow mesenchymal stem cells contained miR-136, which could downregulate the apoptotic peptidase activating factor (Apaf1), improve the activity of elderly bone marrow mesenchymal stem cells, improve the survival rate of cardiomyocytes, and enhance their myocardial repair function [122]. Later studies have shown that exosomes containing miR-21 [123] or miR-144 [124] could also prevent cardiomyocyte apoptosis and effectively improve ischemic cardiovascular disease. It is another feasible method of promoting cardiac angiogenesis and restoring the blood supply of the ischemic myocardium. Exosomes secreted by ischemia were rich in miR-222 and miR-143, which could promote the proliferation and migration of endothelial cells and promote angiogenesis [125]. Besides, exosomes released by human umbilical vein endothelial cells (HUVECs) by the stimulation of shock wave therapy (SWT) could improve angiogenesis and reduce myocardial fibrosis (Fig. 9). Further studies showed that the therapeutic effect of exosomes in receptor cells was mainly achieved through miR-19a-3p [126]. Youn et al. prepared exosomes of cardiac progenitor cells (CPC) rich in miR-322 by electroporation. It was confirmed that CPCexo-322 could promote the increase of the NOX2 gene to produce more reactive oxygen species, thereby increasing endothelial cell migration and capillary formation and promoting angiogenesis to reduce infarct size. The results showed that CPCexo-322 could play a good role in heart protection [127]. Furthermore, it was demonstrated that cardiomyocytes have the potential for endogenous proliferation, and could be reactivated to promote the proliferation of adult heart tissue, which provided new possibilities for new therapeutic strategies [128]. Although the ability of self-renewal of adult cardiomyocytes is very rare and limited, Wang et al. found that an injectable hydrogel containing miR-302 could promote the proliferation of cardiac myocytes within a certain range [129]. Khan et al. found that exosomes derived from embryonic stem cells carried miR-294 to deliver stimulation in the heart, promote angiogenesis, and enhance endogenous myocardial repair based on CPC and cardiomyocyte proliferation after myocardial infarction [130]. Therefore, exosomes may be combined with tissue engineering to promote angiogenesis and cardiomyocyte regeneration.
|
Download:
|
| Fig. 9. The mechanism of SWT-induced angiogenic effects. SWT stimulates the secretion of exosomes and transports the products into receptor cells through membrane fusion or endocytosis. miR-19a-3p contained in the cargo can be used as an inhibitor of TSP-1 to promote angiogenesis and proliferation. Copied with permission [126]. Copyright 2019, The Authors. | |
Overall, miRNA-rich exosomes can be obtained by stimulating specific cells, such as mesenchymal stem cells and embryonic stem cells. Exosomes rich in miRNA are excellent drugs to alleviate atherosclerosis and vascular calcification, and they play a role in the early prevention of myocardial infarction. After myocardial infarction, exosomes loaded with miRNA can reduce myocardial cell apoptosis and promote angiogenesis to achieve cardiac protection. Therefore, exosomes are excellent vectors loaded with miRNAs and are potential drugs for the treatment of myocardial infarction.
6.4. Exosomes in bone regenerativemiRNAs are important regulators of gene expression in various physiological and pathological processes. They can promote tissue repair and regeneration, and has potential applications in regenerative medicine. Presently, there have been many studies on the use of miRNAs as genetic drugs to promote tissue regeneration, and most of the studies have focused on bone regeneration. For example, Zhang et al. found that miR-26a resulted in the upregulation of osteogenic factors, such as runt-related transcription factor 2 (Runx2) and osteocalcin (OCN), to induce osteogenic differentiation by targeting Gsk-3b. Further studies have shown that long-term miR-26a could promote the repair of bone defects and overcome the damaged bone healing in osteoporotic mice [131]. In addition, miRNAs play a crucial role in osteogenic differentiation to promote bone formation and regeneration [132].
Exosomes with the advantages of non-immunogenicity and excellent biocompatibility have been proposed as powerful tools for the treatment of bone defects. Xiong et al. demonstrated that M2 macrophage-derived exosomes containing numerous miR-5106 could accelerate healing in vivo because miR-5106 could target SIK2 and SIK3 to promote osteoblast differentiation (Fig. 10) [132]. miR-140 was loaded into exosomes by the freeze-thaw method, which could promote the differentiation of bone marrow mesenchymal stem cells into chondrocytes and promote cartilage healing [47].
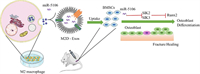
|
Download:
|
| Fig. 10. Schematic diagram showing proposed mechanisms by which exosomal miR-5106 derived from M2 macrophages induce the BMSCs towards osteoblastic differentiation. miR-5106, which is rich in Med exos, can inhibit the expression of SIK2 and SIK3, thus inducing BMSCs differentiation and accelerating bone remodeling. Reproduced with permission [132]. Copyright 2020, The Authors. | |
Researchers have embedded exosomes in hydrogels or loaded them onto scaffolds to optimize the application of exosomes in vivo, achieve long-term miRNA release, provide sites for enriching stem cells and support bone, and have great potential in bone tissue engineering. Exosomes containing miR-375, which could target insulin-like growth factor 3 (IGFBP3) to exert osteogenic effects, were embedded in a commercial hydrogel. In vivo experiments showed that exosomes effectively treated skull defects with a slow and controlled release [133]. Hu et al. prepared an injectable and UV-cross-linked Gelma/nanoclay hydrogel loaded with exosomes containing miR-23a-3p to realize the complete filling of the defect and the controlled sustained release of exosomes. Their study confirmed that the hydrogel could promote the proliferation, migration, and differentiation of chondrocytes and hBMSCs, which provided a novel promising therapeutic method for cartilage defect therapy [134]. Titanium scaffolds could support the attachment of stem cells and the mineralized bone matrix. Exosomes derived from bone marrow mesenchymal stem cells rich in osteogenic miRNA onto titanium scaffolds could effectively induce and promote new bone regeneration [135].
The traditional treatment of bone injury is mainly bone transplantation, including autologous transplantation, allogeneic transplantation, and xenotransplantation. However, autologous transplantation is limited by bone supply, and allogeneic transplantation and xenotransplantation may increase the risk of disease transmission and immune rejection. In recent years, bone tissue engineering based on biomaterials to repair bone defects has attracted extensive attention. The combination of bone tissue engineering and nano drug delivery system to improve bone regeneration microenvironment and promote osteoblast differentiation is a development direction of bone defect repair in the future [136-138]. Bone tissue engineering scaffolds, such as tofu-based scaffold [139], combined with exosomes loaded with miRNA, can regulate bone tissue microenvironment and promote bone repair. Therefore, exosomes loaded with miRNA to promote bone regeneration as a cell-free therapy have broad application prospects.
7. Conclusion and future worksmiRNA is a noncoding RNA and a promising gene drug. Recently, exosomes have attracted much attention as promising carriers of miRNA drugs. Compared with traditional miRNA drug carriers, exosomes have the following advantages: (1) Exosomes are natural carriers of cell production and have good biocompatibility. (2) The surface of exosomes is rich in proteins, such as CD47, to prevent the phagocytosis of macrophages and exhibit the potential for a long cycle time. (3) Exosomes can cross a variety of biological barriers and enter cells in various ways, such as endocytosis. (4) Exosomes can be loaded with miRNA drugs in an endogenous manner. Therefore, exosomes have the potential to load more miRNA drugs by exploring the endogenous loading mode. (5) Exosomes have homing abilities, which can be targeted by active and passive methods. Exosomes have good targeting ability and can transport goods to target cells more effectively [51, 53].
However, the application of exosomes as miRNA carriers still has some difficulties to overcome. Firstly, the large-scale secretion of exosomes is still a serious problem, which leads to a low yield [140]. Therefore, it is necessary to develop a simpler and more efficient method to realize the mass production of exosomes. Secondly, the external storage methods for exosomes need to be improved because existing storage methods such as lyophilization and spray drying are unable to meet the demand of long-term storage and the maintenance of biological activity [141]. Finally, exosomes are highly heterogeneous, which makes exosomes loaded with different miRNAs have different effects on physiological and pathological activities. Therefore, the efficient and rapid separation of exosomes that suit the purpose is still a major factor restricting them from entering the clinic. Although there is still a long way to go for exosomes as miRNA carriers in clinical practice, exosomes show excellent therapeutic effects in cancer treatment, neurodegenerative diseases, cardiovascular diseases, and regenerative medicine owing to their excellent biocompatibility and other characteristics. In conclusion, exosomes will have broad application prospects in many diseases as an excellent carrier of miRNA drugs in the future.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis project was supported by the National Natural Science Foundation of China (No. 51973243), International Cooperation and Exchange of the National Natural Science Foundation of China (No. 51820105004), Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06S029), the Science and Technology Planning Project of Shenzhen (No. JCY20190807155801657), the Fundamental Research Funds for the Central Universities (No. 191gzd35). Guangdong Basic and Applied Basic Research Fund (No. 2019A1515110903). We sincerely acknowledge the funding and generous support from these foundations.
| [1] |
S. Paul, L.A. Bravo Vazquez, S. Perez Uribe, et al., Cells 9 (2020) 1698. DOI:10.3390/cells9071698 |
| [2] |
Y. Xu, L. Shen, F. Li, et al., J. Cell Physiol. 234 (2019) 21380-21394. DOI:10.1002/jcp.28747 |
| [3] |
P. Sharma, I. Dando, R. Strippoli, et al., Front. Pharmacol. 11 (2020) 1141. DOI:10.3389/fphar.2020.01141 |
| [4] |
C. Chakraborty, A.R. Sharma, G. Sharma, et al., J. Adv. Res. 28 (2021) 127-138. DOI:10.1016/j.jare.2020.08.012 |
| [5] |
Q. Bi, X. Song, A. Hu, et al., Chin. Chem. Lett. 31 (2020) 3041-3046. DOI:10.1016/j.cclet.2020.07.030 |
| [6] |
S. Raj, S. Khurana, R. Choudhari, et al., Semin. Cancer Biol. 69 (2021) 166-177. DOI:10.1016/j.semcancer.2019.11.002 |
| [7] |
F. Abedi-Gaballu, G. Dehghan, M. Ghaffari, et al., Appl. Mater. Today 12 (2018) 177-190. DOI:10.1016/j.apmt.2018.05.002 |
| [8] |
H. Zhang, X. You, X. Wang, et al., PNAS 118 (2021) e2005191118. DOI:10.1073/pnas.2005191118 |
| [9] |
C. Xian, Q. Yuan, Z. Bao, et al., Chin. Chem. Lett. 31 (2020) 19-27. DOI:10.1016/j.cclet.2019.03.052 |
| [10] |
Q. Xiong, G.Y. Lee, J. Ding, et al., Nano Res. 11 (2018) 5281-5309. DOI:10.1007/s12274-018-2146-1 |
| [11] |
Y. Liu, D. Li, J. Ding, et al., Chin. Chem. Lett. 31 (2020) 3001-3014. DOI:10.1016/j.cclet.2020.04.029 |
| [12] |
M.M. Lino, S. Simoes, A. Vilaca, et al., ACS Nano 12 (2018) 5207-5220. DOI:10.1021/acsnano.7b07538 |
| [13] |
T. Kang, J.S. Ni, T. Li, et al., Biomaterials 275 (2021) 120907. DOI:10.1016/j.biomaterials.2021.120907 |
| [14] |
H. Zhao, T. Li, C. Yao, et al., ACS Appl. Mater. Interfaces 13 (2021) 6034-6042. DOI:10.1021/acsami.0c21006 |
| [15] |
C. Zheng, M. Li, J. Ding, BIO Integration 2 (2021) 57-60. DOI:10.15212/bioi-2021-0016 |
| [16] |
L. Wei, J. Chen, J. Ding, Nanomedicine 16 (2021) 261-264. DOI:10.2217/nnm-2021-0019 |
| [17] |
J. Huang, X. You, P. Xin, et al., Chin. Chem. Lett. 32 (2021) 1737-1742. DOI:10.1016/j.cclet.2020.12.006 |
| [18] |
Y. Mao, C. Zou, Y. Jiang, et al., Chin. Chem. Lett. 32 (2021) 990-998. DOI:10.1016/j.cclet.2020.08.048 |
| [19] |
B. Zhou, K. Xu, X. Zheng, et al., Signal Transduct. Target. Ther. 5 (2020) 144. DOI:10.1038/s41392-020-00258-9 |
| [20] |
K. Jiang, Y. Wu, J. Chen, et al., Chin. Chem. Lett. 32 (2021) 1827-1830. DOI:10.1016/j.cclet.2020.11.031 |
| [21] |
X. Xia, Y. Wang, Y. Huang, et al., Prog. Neurobiol. 183 (2019) 101694. DOI:10.1016/j.pneurobio.2019.101694 |
| [22] |
J. Cheng, J. Meng, L. Zhu, et al., Mol. Cancer. 19 (2020) 66. DOI:10.1186/s12943-020-01189-3 |
| [23] |
B. Sun, Y. Ma, F. Wang, et al., Stem Cell Res. Ther. 10 (2019) 360. DOI:10.1186/s13287-019-1442-3 |
| [24] |
M. Wei, X. Gao, L. Liu, et al., ACS Nano 14 (2020) 5099-5110. DOI:10.1021/acsnano.0c01860 |
| [25] |
C.L. Deng, C.B. Hu, S.T. Ling, et al., Cell Death Differ. 28 (2020) 1041. |
| [26] |
R. Kalluri, V.S. LeBleu, Science 367 (2020) eaau6977. DOI:10.1126/science.aau6977 |
| [27] |
D. Wei, W. Zhan, Y. Gao, et al., Cell Res. 31 (2020) 157. |
| [28] |
J.M. Gudbergsson, K. Jønsson, J.B. Simonsen, et al., J. Control. Release 306 (2019) 108-120. DOI:10.1016/j.jconrel.2019.06.006 |
| [29] |
J. Dai, Y. Su, S. Zhong, et al., Signal Transduct. Tar. 5 (2020) 145. DOI:10.1038/s41392-020-00261-0 |
| [30] |
H. Wang, Z. Lu, X. Zhao, J. Hematol. Oncol. 12 (2019) 133. DOI:10.1186/s13045-019-0806-6 |
| [31] |
F. Xie, X. Zhou, M. Fang, et al., Adv. Sci. 6 (2019) 1901779. DOI:10.1002/advs.201901779 |
| [32] |
M. Ostrowski, N.B. Carmo, S. Krumeich, et al., Nat. Cell Biol. 12 (2010) 19-30. DOI:10.1038/ncb2000 |
| [33] |
P. Wu, B. Zhang, D.K.W. Ocansey, et al., Biomaterials 269 (2020) 120467. |
| [34] |
Y.T. Kang, T. Hadlock, T.W. Lo, et al., Adv. Sci. (Weinh) 7 (2020) 2001581. DOI:10.1002/advs.202001581 |
| [35] |
L. Yang, D. Han, Q. Zhan, et al., Theranostics 9 (2019) 7680-7696. DOI:10.7150/thno.37220 |
| [36] |
H. Shao, H. Im, C.M. Castro, et al., Chem. Rev. 118 (2018) 1917-1950. DOI:10.1021/acs.chemrev.7b00534 |
| [37] |
J. Wang, D. Chen, E.A. Ho, J. Control. Release 329 (2020) 894-906. DOI:10.3390/agronomy10060894 |
| [38] |
M. Wu, Y. Ouyang, Z. Wang, et al., PNAS 114 (2017) 10584-10589. DOI:10.1073/pnas.1709210114 |
| [39] |
X. Li, C. Li, L. Zhang, et al., Mol. Cancer 19 (2020) 1. DOI:10.1186/s12943-019-1085-0 |
| [40] |
M. Katakowski, B. Buller, X. Zheng, et al., Cancer Lett. 335 (2013) 201-204. DOI:10.1016/j.canlet.2013.02.019 |
| [41] |
X. Yu, M. Odenthal, J.W. Fries, Int. J. Mol. Sci. 17 (2016) 2028. DOI:10.3390/ijms17122028 |
| [42] |
C. Villarroya-Beltri, C. Gutierrez-Vazquez, F. Sanchez-Cabo, et al., Nat. Commun. 4 (2013) 2980. DOI:10.1038/ncomms3980 |
| [43] |
Z. Li, X. Zhou, M. Wei, et al., Nano Lett. 19 (2019) 19-28. DOI:10.1021/acs.nanolett.8b02689 |
| [44] |
D.S. Sutaria, J. Jiang, O.A. Elgamal, et al., J. Extracell. Vesicles 6 (2017) 1333882. DOI:10.1080/20013078.2017.1333882 |
| [45] |
G. Liang, Y. Zhu, D.J. Ali, et al., J. Nanobiotechnology 18 (2020) 10. DOI:10.1186/s12951-019-0563-2 |
| [46] |
M. Piffoux, A. Nicolas-Boluda, V. Mulens-Arias, et al., Adv. Drug Deliv. Rev. 138 (2019) 247-258. DOI:10.1016/j.addr.2018.12.009 |
| [47] |
G. Won Lee, M. Thangavelu, M. Joung Choi, et al., J. Cell Biochem. 121 (2020) 3642-3652. DOI:10.1002/jcb.29657 |
| [48] |
K. Bryniarski, W. Ptak, A. Jayakumar, et al., J. Allergy Clin. Immunol. 132 (2013) 170-181. DOI:10.1016/j.jaci.2013.04.048 |
| [49] |
A. Jeyaram, T.N. Lamichhane, S. Wang, et al., Mol. Ther. 28 (2020) 975-985. DOI:10.1016/j.ymthe.2019.12.007 |
| [50] |
O.M. Elsharkasy, J.Z. Nordin, D.W. Hagey, et al., Adv. Drug Deliv. Rev. 159 (2020) 332-343. DOI:10.1016/j.addr.2020.04.004 |
| [51] |
Z. Li, J. Huang, J. Wu, Biomater Sci. 9 (2021) 574-589. DOI:10.1039/d0bm01729a |
| [52] |
Q. Xu, L. Wang, T. Tong, et al., Appl. Mater. Today 21 (2020) 100849. DOI:10.1016/j.apmt.2020.100849 |
| [53] |
K. Ou, Y. Kang, L. Chen, et al., Biomater. Sci. 7 (2019) 2491-2498. DOI:10.1039/c9bm00344d |
| [54] |
N. Perets, O. Betzer, R. Shapira, et al., Nano Lett. 19 (2019) 3422-3431. DOI:10.1021/acs.nanolett.8b04148 |
| [55] |
R. He, Y. Jiang, Y. Shi, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 117 (2020) 111314. DOI:10.1016/j.msec.2020.111314 |
| [56] |
T.J. Antes, R.C. Middleton, K.M. Luther, et al., J. Nanobiotechnology 16 (2018) 61. DOI:10.1186/s12951-018-0388-4 |
| [57] |
F. Yan, Z. Zhong, Y. Wang, et al., J. Nanobiotechnology 18 (2020) 115. DOI:10.1186/s12951-020-00675-6 |
| [58] |
F. Royo, U. Cossio, A. Ruiz de Angulo, et al., Nanoscale 11 (2019) 1531-1537. DOI:10.1039/c8nr03900c |
| [59] |
M. Luo, X. Zhang, J. Wu, et al., Carbohydr. Polym. 266 (2021) 118097. DOI:10.1016/j.carbpol.2021.118097 |
| [60] |
R. Tamura, S. Uemoto, Y. Tabata, Acta. Biomater. 57 (2017) 274-284. DOI:10.1016/j.actbio.2017.05.013 |
| [61] |
J. Huang, Y. Li, A. Orza, et al., Adv. Funct. Mater. 26 (2016) 3818-3836. DOI:10.1002/adfm.201504185 |
| [62] |
X. Li, Y. Wang, L. Shi, et al., J. Nanobiotechnology 18 (2020) 113. DOI:10.1186/s12951-020-00670-x |
| [63] |
S. Liu, X. Chen, L. Bao, et al., Nat. Biomed. Eng. 4 (2020) 1063-1075. DOI:10.1038/s41551-020-00637-1 |
| [64] |
J. Wang, W. Li, L. Zhang, et al., ACS Appl. Mater. Interfaces 9 (2017) 27441-27452. DOI:10.1021/acsami.7b06464 |
| [65] |
X. Wang, Y. Chen, Z. Zhao, et al., J. Am. Heart Assoc. 7 (2018) e008737. DOI:10.1161/JAHA.118.008737 |
| [66] |
L. Alvarez-Erviti, Y. Seow, H. Yin, et al., Nat. Biotechnol. 29 (2011) 341-345. DOI:10.1038/nbt.1807 |
| [67] |
S. Ohno, M. Takanashi, K. Sudo, et al., Mol. Ther. 21 (2013) 185-191. DOI:10.1038/mt.2012.180 |
| [68] |
K.B. Johnsen, J.M. Gudbergsson, M.N. Skov, et al., Biochim. Biophys. Acta 1846 (2014) 75-87. |
| [69] |
M. Grapp, A. Wrede, M. Schweizer, et al., Nat. Commun. 4 (2013) 2123. DOI:10.1038/ncomms3123 |
| [70] |
T. Yong, D. Wang, X. Li, et al., J. Control. Release 322 (2020) 555-565. DOI:10.1016/j.jconrel.2020.03.039 |
| [71] |
A. Mullard, Nat. Biotechnol. 38 (2020) 1221-1223. DOI:10.1038/s41587-020-0724-8 |
| [72] |
X. Xu, C. Liu, Y. Wang, et al., Adv. Drug Deliv. Rev. (2021) 113891. |
| [73] |
M.A. Islam, J. Rice, E. Reesor, et al., Biomaterials 266 (2021) 120431. DOI:10.1016/j.biomaterials.2020.120431 |
| [74] |
G.H. Nam, Y. Choi, G.B. Kim, et al., Adv. Mater. 32 (2020) e2002440. DOI:10.1002/adma.202002440 |
| [75] |
T. Huang, C. Song, L. Zheng, et al., Mol. Cancer 18 (2019) 62. |
| [76] |
J. Xie, Y. Lu, B. Yu, et al., Chin. Chem. Lett. 31 (2020) 1173-1177. DOI:10.1016/j.cclet.2019.10.030 |
| [77] |
H. Xie, K. Di, R. Huang, et al., Chin. Chem. Lett. 31 (2020) 1737-1745. DOI:10.1016/j.cclet.2020.02.049 |
| [78] |
J. Zhang, M. Hou, G. Chen, et al., Chin. Chem. Lett. 32 (2021) 3474-3478. DOI:10.1016/j.cclet.2021.04.056 |
| [79] |
A. Bronisz, Y. Wang, M.O. Nowicki, et al., Cancer Res. 74 (2014) 738-750. DOI:10.1158/0008-5472.CAN-13-2650 |
| [80] |
X. Zhang, X. Zhang, S. Hu, et al., Nucleic Acids Res. 45 (2017) 5930-5944. DOI:10.1093/nar/gkx317 |
| [81] |
M. Fareh, F. Almairac, L. Turchi, et al., Cell Death Dis. 8 (2017) e2713. DOI:10.1038/cddis.2017.117 |
| [82] |
F.M. Lang, A. Hossain, J. Gumin, et al., Neuro. Oncol. 20 (2018) 380-390. DOI:10.1093/neuonc/nox152 |
| [83] |
S.G. Lei Yu, Yawei Liu, Xiaoyu Qiu, et al., Aging-US 11 (2019) 5300-5318. DOI:10.18632/aging.102092 |
| [84] |
H. Sung, J. Ferlay, R.L. Siegel, et al., CA Cancer J. Clin. 71 (2021) 209. DOI:10.3322/caac.21660 |
| [85] |
S. Mohammadi-Yeganeh, V. Hosseini, M. Paryan, J. Cell Physiol. 234 (2019) 18317-18328. DOI:10.1002/jcp.28465 |
| [86] |
Y. Yu, W. Luo, Z.J. Yang, et al., Mol. Cancer 17 (2018) 70. DOI:10.1109/imcec.2018.8469488 |
| [87] |
Y. Gao, X. Li, C. Zeng, et al., Adv. Sci. (Weinh) 7 (2020) 2002518. DOI:10.1002/advs.202002518 |
| [88] |
M. Han, J. Hu, P. Lu, et al., Cell Death Dis. 11 (2020) 43. DOI:10.1007/s43236-019-00013-6 |
| [89] |
S. Shojaei, S.M. Hashemi, H. Ghanbarian, et al., Stem Cell Rev. Rep. 17 (2021) 1027-1038. DOI:10.1007/s12015-020-10089-4 |
| [90] |
E.J. Park, H.J. Jung, H.J. Choi, et al., FASEB J. 34 (2020) 3379-3398. DOI:10.1096/fj.201902434r |
| [91] |
M. Moradi-Chaleshtori, M. Bandehpour, S. Soudi, et al., Cancer Immunol. Immunother. 70 (2020) 1341. |
| [92] |
H. Wu, X. Mu, L. Liu, et al., Cell Death Dis. 11 (2020) 801. DOI:10.1049/iet-cta.2019.1172 |
| [93] |
H. Zhang, J. Wang, T. Ren, et al., Cancer Lett. 490 (2020) 54-65. DOI:10.1016/j.canlet.2020.07.008 |
| [94] |
Y. Jia, X. Ding, L. Zhou, et al., Oncogene 40 (2020) 246. |
| [95] |
Z. He, W. Li, T. Zheng, et al., J. Exp. Clin. Cancer Res. 39 (2020) 140. DOI:10.1186/s13046-020-01631-w |
| [96] |
Z. Chen, Y. Xie, W. Chen, et al., Life Sci. 284 (2021) 119222. DOI:10.1016/j.lfs.2021.119222 |
| [97] |
M. Kobayashi, K. Sawada, M. Miyamoto, et al., Biochem. Biophys. Res. Commun. 527 (2020) 153-161. DOI:10.1016/j.bbrc.2020.04.076 |
| [98] |
X. Zhang, J. Bai, H. Yin, et al., Mol. Oncol. 14 (2020) 2589-2608. DOI:10.1002/1878-0261.12765 |
| [99] |
M.S. Phipps, C.A. Cronin, BMJ 368 (2020) l6983. DOI:10.1136/bmj.l6983 |
| [100] |
H. Xin, Y. Li, Z. Liu, et al., Stem Cells 31 (2013) 2737-2746. DOI:10.1002/stem.1409 |
| [101] |
G.J. Moon, J.H. Sung, D.H. Kim, et al., Transl. Stroke Res. 10 (2019) 509-521. DOI:10.1007/s12975-018-0668-1 |
| [102] |
S. Huang, X. Ge, J. Yu, et al., FASEB J. 32 (2018) 512-528. DOI:10.1096/fj.201700673r |
| [103] |
Y. Song, Z. Li, T. He, et al., Theranostics 9 (2019) 2910-2923. DOI:10.7150/thno.30879 |
| [104] |
J. Yang, L.L. Cao, X.P. Wang, et al., Cell Death Dis. 12 (2021) 23. DOI:10.1038/s41419-020-03310-2 |
| [105] |
H. Xin, M. Katakowski, F. Wang, et al., Stroke 48 (2017) 747-753. DOI:10.1161/STROKEAHA.116.015204 |
| [106] |
M. Haupt, X. Zheng, Y. Kuang, et al., Stem Cells Transl. Med. 10 (2021) 357-373. DOI:10.1002/sctm.20-0086 |
| [107] |
J. Yang, X. Zhang, X. Chen, et al., Mol. Ther. Nucleic Acids 7 (2017) 278-287. DOI:10.1016/j.omtn.2017.04.010 |
| [108] |
F. Ferrantelli, C. Chiozzini, P. Leone, et al., Pharmaceutics 12 (2020) 529. DOI:10.3390/pharmaceutics12060529 |
| [109] |
P. Scheltens, B. De Strooper, M. Kivipelto, et al., The Lancet 397 (2021) 1577. DOI:10.1016/S0140-6736(20)32205-4 |
| [110] |
D. Liu, D. Fu, L. Zhang, et al., Chin. Chem. Lett. 32 (2021) 1066-1070. DOI:10.1016/j.cclet.2020.09.009 |
| [111] |
J. Cao, M. Huang, L. Guo, et al., Mol. Psychiatry 26 (2021) 4687-4701. DOI:10.1038/s41380-020-0824-3 |
| [112] |
N. Chopra, R. Wang, B. Maloney, et al., Mol. Psychiatry 26 (2021) 5636-5657. DOI:10.1038/s41380-019-0610-2 |
| [113] |
A.T. Barros-Viegas, V. Carmona, E. Ferreiro, et al., Mol. Ther. Nucleic Acids 19 (2020) 1219-1236. DOI:10.1016/j.omtn.2020.01.010 |
| [114] |
Y. Jahangard, H. Monfared, A. Moradi, et al., Front. Neurosci. 14 (2020) 564. DOI:10.3389/fnins.2020.00564 |
| [115] |
H. Wei, Y. Xu, Q. Chen, et al., Cell Death Dis. 11 (2020) 290. DOI:10.1038/s41419-020-2490-4 |
| [116] |
A.S. Aghabozorgi, N. Ahangari, T.E. Eftekhaari, et al., J. Cell Physiol. 234 (2019) 21796-21809. DOI:10.1002/jcp.28942 |
| [117] |
W. Wang, N. Zhu, T. Yan, et al., Cell Commun. Signal. 18 (2020) 119. DOI:10.1186/s12964-020-00581-2 |
| [118] |
L. Wang, Q. Jia, C. Xinnong, et al., J. Cell Mol. Med. 23 (2019) 7124-7131. DOI:10.1111/jcmm.14562 |
| [119] |
S. Sahoo, D.W. Losordo, Circ. Res. 114 (2014) 333-344. DOI:10.1161/CIRCRESAHA.114.300639 |
| [120] |
M. Cheng, J. Yang, X. Zhao, et al., Nat. Commun. 10 (2019) 959. DOI:10.1038/s41467-019-08895-7 |
| [121] |
F. Xu, J.Y. Zhong, X. Lin, et al., J. Pineal. Res. 68 (2020) e12631. |
| [122] |
N. Zhang, J. Zhu, Q. Ma, et al., Stem Cell Res. Ther. 11 (2020) 273. DOI:10.1186/s13287-020-01782-9 |
| [123] |
Y. Song, C. Zhang, J. Zhang, et al., Theranostics 9 (2019) 2346-2360. DOI:10.7150/thno.29945 |
| [124] |
Z. Wen, Z. Mai, X. Zhu, et al., Stem Cell Res. Ther. 11 (2020) 36. DOI:10.1186/s13287-020-1563-8 |
| [125] |
T.M. Ribeiro-Rodrigues, T.L. Laundos, R. Pereira-Carvalho, et al., Cardiovasc. Res. 113 (2017) 1338-1350. DOI:10.1093/cvr/cvx118 |
| [126] |
C. Gollmann-Tepekoylu, L. Polzl, M. Graber, et al., Cardiovasc. Res. 116 (2020) 1226-1236. DOI:10.1093/cvr/cvz209 |
| [127] |
S.W. Youn, Y. Li, Y.M. Kim, et al., Antioxidants (Basel) 8 (2019) 18. DOI:10.3390/antiox8010018 |
| [128] |
N. Abbas, F. Perbellini, T. Thum, Basic. Res. Cardiol. 115 (2020) 52. DOI:10.1007/s00395-020-0816-0 |
| [129] |
L.L. Wang, Y. Liu, J.J. Chung, et al., Nat. Biomed. Eng. 1 (2017) 983-992. DOI:10.1038/s41551-017-0157-y |
| [130] |
M. Khan, E. Nickoloff, T. Abramova, et al., Circ. Res. 117 (2015) 52-64. DOI:10.1161/CIRCRESAHA.117.305990 |
| [131] |
X. Zhang, Y. Li, Y.E. Chen, et al., Nat. Commun. 7 (2016) 10376. DOI:10.1038/ncomms10376 |
| [132] |
Y. Xiong, L. Chen, C. Yan, et al., J. Nanobiotechnology 18 (2020) 66. DOI:10.1186/s12951-020-00622-5 |
| [133] |
S. Chen, Y. Tang, Y. Liu, et al., Cell Prolif. 52 (2019) e12669. |
| [134] |
H. Hu, L. Dong, Z. Bu, et al., J. Extracell. Vesicles 9 (2020) 1778883. DOI:10.1080/20013078.2020.1778883 |
| [135] |
M. Zhai, Y. Zhu, M. Yang, et al., Adv. Sci. (Weinh) 7 (2020) 2001334. DOI:10.1002/advs.202001334 |
| [136] |
L. Huang, J. Zhang, X. Liu, et al., Chin. Chem. Lett. 32 (2021) 234-238. DOI:10.1016/j.cclet.2020.11.046 |
| [137] |
Y. Zhao, L. Meng, K. Zhang, et al., Chin. Chem. Lett. 32 (2021) 266-270. DOI:10.1016/j.cclet.2020.10.031 |
| [138] |
X. Gao, Z. Xu, G. Liu, et al., Acta. Biomater. 119 (2021) 57-74. DOI:10.13173/centasiaj.64.1-2.0057 |
| [139] |
K. Huang, G. Liu, Z. Gu, et al., Chin. Chem. Lett. 31 (2020) 3190-3194. DOI:10.1016/j.cclet.2020.07.002 |
| [140] |
P.H.L. Tran, D. Xiang, T.T.D. Tran, et al., Adv. Mater. 32 (2020) e1904040. DOI:10.1002/adma.201904040 |
| [141] |
Y. Zhang, J. Bi, J. Huang, et al., Int. J. Nanomedicine 15 (2020) 6917-6934. DOI:10.2147/ijn.s264498 |
 2022, Vol. 33
2022, Vol. 33 

