b Center of Chemistry for Frontier Technologies, Zhejiang University, Hangzhou 310027, China;
c Department of Chemistry, Zhejiang University, Hangzhou 310027, China;
d Department of Polymer Science and Engineering, College of Chemical Engineering, Dalian University of Technology, Dalian 116024, China
The alternating copolymerization of carbonyl sulfide (COS) with epoxides to synthesize functional poly(thiocarbonate)s, as an efficient method of introducing sulfur to backbone, has received considerable attention in the past several years [1-4]. The presence of thiocarbonate linkages endow it with some favorable properties [5-9], and thus poly(thiocarbonate)s can be utilized as heavy-metal scavengers, photoconductive fibers, solid polymer electrolytes, etc. [5, 6, 10, 11]. Recently, semicrystalline poly(trimethylene monothiocarbonate) (PTMMTC) with a melting temperature (Tm) above 120 ℃, derived from the copolymerization of COS with oxetane (OX), was reported [7, 12]. By contrast, its corresponding polycarbonate analogue, (poly(trimethylene carbonate) (PTMC) is crystalline only upon tensile loading [13]. We call this phenomenon "sulfur-substitution-enhanced crystallization", and it is common in polyether-polythioether and polyester-polythioester systems. For example, poly(ethylene sulfide) [14] and poly(ε-thiocaprolactone) [15] have higher Tms compared with their oxygen analogues i.e. poly(ethylene oxide) and polycaprolactone, respectively. In addition, Marchessault [16, 17] and Matsumura [18, 19] found that both the Tm and crystallization temperature (Tc) of various linear aliphatic polythioesters were always higher when compared to those of the corresponding polyoxyesters. Sasanuma and co-workers investigated the conformational characteristics of aliphatic polythioethers and aromatic polythioesters by a rotational isomeric state analysis of ab initio molecular orbital calculations and compared with those of their corresponding polyethers [20-24]. They found that the more trans conformers and a larger characteristic ratio were observed for sulfur-substituted polyethers due to the strong S···S repulsion along the polymer chains. It is worth noting that the steric S···S repulsion may only exist when adjacent S atoms are closely spaced. For example, the S···S repulsion is negligible in poly(trimethylene sulfide) with three bonds between adjacent sulfur atoms [21]. Therefore, unlike the poly(ethylene sulfide), the C-C bond adjacent to the C-S bond in poly(trimethylene sulfide) exhibits its inherent gauche conformation without the S···S repulsion [21]. On the other hand, Bhaumik and Mark [25] pointed out that the difference of intermolecular interaction energy between poly(ethylene sulfide) and poly(ethylene oxide) was primarily due to van der Waals interactions rather than dipolar effect. However, it is known that the S atom has a larger atomic radius than O atom and the bond length of the C-S bond is larger than that of C-O bond, which should result in a weaker intermolecular interaction. It seems that the mechanism for sulfur-substituted-enhanced crystallization of polymers is still controversial. Herein, we compared the crystallization behaviors of PTMMTC and PTMC using differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD). The crystal structure of PTMMTC was preliminarily determined as well.
As shown in Fig. 1, the synthesis of PTMMTC and PTMC were performed following the alternating copolymerization method described in our earlier work [26]. The details for synthesis were shown in the Supporting Information. The molecular weight and polydispersity index (PDI) of the samples were listed in Table S1 (Supporting information). Fig. 1 shows the typical DSC thermograms of PTMMTC and PTMC powders. It is observed that PTMMTC displays a strong exothermic peak at 88℃ when cooled from the melt at a rate of 10℃/min (Fig. 1a). The melting peak of PTMMTC is located at 133℃ with a melting enthalpy (ΔHm) of 74.4 J/g. In addition, PTMMTC can still crystallize even if it is quenched at a cooling rate (Rc) of 50℃/min (Fig. S3 in Supporting information). All of these results show that PTMMTC can crystallize quickly. On the contrary, only glass transition can be detected by DSC for PTMC upon both cooling and heating (glass transition temperature Tg = -17.7℃), indicating that PTMC cannot crystallize under the experimental conditions (Fig. 1b). It was reported that PTMC could crystallize only upon tensile loading and was amorphous in the relaxed state [13]. That is to say, PTMMTC possesses stronger crystallizability than corresponding PTMC. In the present work, the only difference of PTMMTC and PTMC chains lies in sulfur-substitution. Therefore, we call this phenomenon as "sulfur-substitution-enhanced crystallization". The stronger crystallizability of PTMMTC further leads to its better mechanical properties, as compared with PTMC (Figs. S4 and S5 in Supporting information).

|
Download:
|
| Fig. 1. DSC thermograms of (a) PTMMTC and (b) PTMC powders. | |
In order to clarify the mechanism of sulfur-substitution-enhanced crystallization, the mobility of chain segments and sub-segment units in PTMMTC and PTMC were investigated using dynamic mechanical analysis (DMA). The tanδ and loss modulus (E″) of PTMMTC and PTMC as a function of temperature are shown in Fig. 2. It is observed that, both tanδ~T and E″~T curves of PTMMTC and PTMC exhibit a strong peak at high temperature and a weak peak at low temperature, which are ascribed to α-transition (glass transition) and β-transition, respectively. In general, Tg reflects the mobility of chain segments in middle length scale, which involves several repeating units. The β-transition reflects the local mobility of PTMMTC and PTMC chains in short length scale, which usually involves 2-4 bonds, possibly originating from the vibration or rotation of thiocarbonate and carbonate groups. The values of Tg and the β-transition temperature (Tβ) determined by different methods are summarized in Table S2 (Supporting information). The Tg of the PTMMTC obtained by DMA is higher than that of PTMC, which is consistent with the result of DSC. This indicates that the chain segments of PTMC possess higher mobility than those of PTMMTC. As shown in Table S2, the Tβs of PTMC, determined from both tanδ ~T and E″~T curves, are lower than those of PTMMTC. This shows that the carbonate groups exhibit higher mobility than monothiocarbonate groups.

|
Download:
|
| Fig. 2. DSC thermograms of (a) PTMMTC and (b) PTMC powders. | |
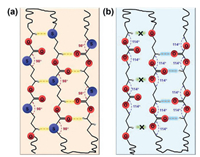
The configurations of two small molecule model compounds (Fig. S7 in Supporting information) comprising the same repeating unit of PTMMTC and PTMC were also simulated using Gaussian 09 software based on the density functional theory (DFT) [27, 28]. One can see that there is a striking difference in the bond angles of C-S-C and C-O-C, which are 98.08° and 114.28°, respectively. Similar phenomena were reported in the polyether-polythioether systems. The bond angle of C-S-C (99.45° ~ 99.5°) in polythioethers is also smaller than that of C-O-C (111.5° ~ 112.8°) in polyethers [20, 21]. The smaller bond angle of C-S-C may result in a larger antiparallel dipole-dipole interaction along the bisector of the C-S-C angle (Fig. S7). In addition, it is reported that C-S bond in the polythioether systems possess bigger dipole moment than C-O bond [20, 21]. That is to say, the total intermolecular dipole-dipole interaction energy in PTMMTC is larger than that in PTMC. On the other hand, the smaller bond angle of C-S-C and the larger atomic radius of S atom will lead to more protrusion of S atoms along the direction vertical to polymer main chain. As a result, besides the C=O and C-H intermolecular interaction, S atoms can interact with the H atoms in -CH2-O- of other polymer chains more easily due to a shorter distance in PTMMTC (Fig. 3a). By contrast, in PTMC, the intermolecular interaction between O atoms in C-O-C and the H atoms in -CH2-O- may be absent because of the long distance (Fig. 3b). Combining the above two aspects, the intermolecular interaction in PTMMTC with the planar zig-zag conformation (this will be confirmed from the crystal structure of PTMMTC discussed below) is stronger than that of PTMC.
|
Download:
|
| Fig. 3. Possible interactions for (a) PTMMTC and (b) PTMC with extended chain conformation. | |
After clarifying the chain flexibility and intermolecular interaction of PTMMTC and PTMC, their different crystallizability and the origin of sulfur-substitution-enhanced crystallization can be understood from the thermodynamic viewpoint. The equilibrium melting temperature (Tm0) of polymers can be written as:
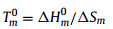
|
(1) |
where ΔHm0 is the melting enthalpy of crystals. The value of ΔHm0 is mainly determined by the intermolecular interaction of polymers. Above results indicate that PTMMTC possesses the stronger intermolecular interaction than PTMC, which will lead to a larger ΔHm of PTMMTC than the corresponding PTMC. In general, the entropy change upon melting (ΔSm) of polymer can be expressed as:

|
(2) |
where Sm and Sc are the entropies of polymer chains in the amorphous melt and in the crystal, respectively. Since polymer chains can hardly change their conformation in crystal, Sc is approximately zero. The DSC and DMA results show that more rigid chain conformation of PTMMTC. That is to say the Sm of PTMMTC is smaller, thus its ΔSm is also smaller accordingly. As a consequence, PTMMTC possesses higher Tm than that of corresponding PTMC.
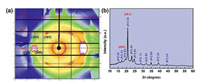
The crystal structure of PTMMTC was analyzed using the 2 dimensional wide-angle X-ray diffraction (2D-WAXD) pattern as shown in Fig. 4a. Combined with the fiber pattern and 1D-WAXD curve (Fig. 4b), the unit cell of the PTMMTC crystal structure model are estimated by the constrained least-squares method. PTMMTC can be indexed by an orthorhombic unit cell with parameters a = 10.74 Å, b = 4.79 Å, and c (fiber axis) =7.74 Å (Fig. S8 in Supporting information). The unit cell of PTMMTC includes two molecular chains with one repeating unit in each chain. In the ideal extended chain of PTMMTC, the length of one repeating units is 7.62 Å, which is very close to the value of c axis, further indicating the planar zig-zag conformation of PTMMTC (Fig. 3). Furthermore, the planar zig-zag conformation in polymers can easily lead to the odd-even effect, which means that the thermal properties of polymers fluctuate with the number of methylene unit in the chain and is widely observed for polyamides [29]. This agrees well with our previous result that PTMMTC with 3 methylene units in each repeating unit has a higher Tm than the poly(ethylene monothiocarbonate) with 2 methylene units in each repeating unit [26]. By contrast, the stretched PTMC forms an orthorhombic unit cell of a = 7.02 Å, b = 5.81 Å and c (fiber axis) = 12.30 Å, in which there are also two PTMC chain but the PTMC chains adopt a helical conformation [13]. The cell parameters of PTMMTC also calculated based on the powder X-ray diffraction data (Fig. 4b) using the Jade software, and the obtained unit cell parameters are given in Table S3 (Supporting information). It is found that the cell parameters obtained by two methods are similar. This also indicates that there is no obvious change in the crystal structure of PTMMTC after stretching.
|
Download:
|
| Fig. 4. (a) 2D WAXD pattern and (b) 1D-WAXD curve of PTMMTC. | |
In this study, the crystallization properties of PTMMTC and PTMC were contrastively investigated. It is found that PTMMTC possesses stronger crystallizability than corresponding PTMC. DSC and DMA results reveal that the PTMMTC chains are more rigid than PTMC. Computer simulation shows that the bond angle of C-S-C bond is evidently smaller than that of C-O-C, leading to stronger intermolecular dipole-dipole and -S-/H2C-O interactions in PTMMTC. The sulfur-substitution-enhanced crystallization phenomenon in PTMMTC can be interpreted from above two aspects. The crystal structure of PTMMTC was also preliminarily determined. The unit cell of PTMMTC crystals is orthorhombic and contains two PTMMTC chains with one repeating unit in the c-axis. The PTMMTC chains in the crystals adopt an extended conformation.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 21774108 and 21574116), the Distinguished Young Investigator Fund of Zhejiang Province (No. LR16B040001), the China Postdoctoral Science Foundation (No. 2020M681818) and the Center of Chemistry for Frontier Technologies of Zhejiang University. We also thank beamline 16B1 at Shanghai Synchrotron Radiation Facility (SSRF) for providing beamtime and kind help for WAXD experiments.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.014.
| [1] |
M. Luo, X.H. Zhang, B.Y. Du, Q. Wang, Z.Q. Fan, Macromolecules 46 (2013) 5899-5904. DOI:10.1021/ma401114m |
| [2] |
M. Luo, Y. Li, Y.Y. Zhang, X.H. Zhang, Polymer 82 (2016) 406-431. DOI:10.1016/j.polymer.2015.11.011 |
| [3] |
J.L. Yang, H.L. Wu, Y. Li, X.H. Zhang, D.J. Darensbourg, Angew. Chem. Int. Ed. 56 (2017) 5774-5779. DOI:10.1002/anie.201701780 |
| [4] |
C.J. Zhang, H.L. Wu, Y. Li, J.L. Yang, X.H. Zhang, Nature Commun 9 (2018) 2137. DOI:10.1038/s41467-018-04554-5 |
| [5] |
A. Kultys, Sulfur-Containing Polymers in Encyclopedia of Polymer Science and Technology, 4th ed., John Wiley & Sons, Inc., Hoboken, 2010.
|
| [6] |
B. Ochiai, T. Endo, Prog. Polym. Sci. 30 (2005) 183-215. DOI:10.1016/j.progpolymsci.2005.01.005 |
| [7] |
M. Luo, X.H. Zhang, D.J. Darensbourg, Acc. Chem. Res. 49 (2016) 2209-2219. DOI:10.1021/acs.accounts.6b00345 |
| [8] |
G. Montaudo, C. Puglisi, C. Berti, E. Marianucci, F. Pilati, J. Polym. Sci. Part A: Polym. Chem. 27 (1989) 2277-2290. DOI:10.1002/pola.1989.080270712 |
| [9] |
E. Marianucci, C. Berti, F. Pilati, et al., Polymer 35 (1994) 1564-1566. DOI:10.1016/0032-3861(94)90361-1 |
| [10] |
X.H. Cao, J.L. Yang, R.Y. Wang, X.H. Zhang, J.T. Xu, Polymer 180 (2019) 121745. DOI:10.1016/j.polymer.2019.121745 |
| [11] |
X.H. Cao, J.H. Li, M.J. Yang, et al., Macromol. Rapid Comm. 12 (2020) 1900622. DOI:10.1002/marc.201900622 |
| [12] |
H.L. Wu, J.L. Yang, M. Luo, et al., Macromolecules 49 (2016) 8863-8868. DOI:10.1021/acs.macromol.6b02285 |
| [13] |
Y. Takahashi, R. Kojima, Macromolecules 36 (2003) 5139-5143. DOI:10.1021/ma030076q |
| [14] |
Y. Takahashi, H. Tadokoro, Y. Chatani, J. Macromol. Sci. B 2 (1968) 361-367. DOI:10.1080/00222346808212456 |
| [15] |
C.G. Overberger, J. Weise, J. Polym. Sci. Part B: Polym. Lett. 2 (1964) 329-331. DOI:10.1002/pol.1964.110020403 |
| [16] |
J. Kawada, T. Lutke-Eversloh, A. Steinbuchel, R.H. Marchessault, Biomacromolecules 4 (2003) 1698-1702. DOI:10.1021/bm0341327 |
| [17] |
T. Lutke-Eversloh, A. Fischer, U. Remminghorst, et al., Nat. Mater. 1 (2002) 236-240. DOI:10.1038/nmat773 |
| [18] |
M. Kato, K. Toshima, S. Matsumura, Macromol. Rapid Comm. 27 (2006) 605-610. DOI:10.1002/marc.200500836 |
| [19] |
M. Kato, K. Toshima, S. Matsumura, Biomacromolecules 8 (2007) 3590-3596. DOI:10.1021/bm700623s |
| [20] |
Y. Sasanuma, H. Ohta, I. Touma, et al., Macromolecules 35 (2002) 3748-3761. DOI:10.1021/ma012027o |
| [21] |
Y. Sasanuma, A. Watanabe, Macromolecules 39 (2006) 1646-1656. DOI:10.1021/ma0523623 |
| [22] |
Y. Sasanuma, Y. Hayashi, H. Matoba, et al., Macromolecules 35 (2002) 8216-8226. DOI:10.1021/ma020730m |
| [23] |
D. Abe, Y. Sasanuma, Polym. Chem. 3 (2012) 1576-1587. DOI:10.1039/c2py20118f |
| [24] |
Y. Sasanuma, A. Watanabe, K. Tamura, J. Phys. Chem. B 112 (2008) 9613-9624. DOI:10.1021/jp800597s |
| [25] |
D. Bhaumik, J.E. Mark, Macromolecules 14 (1981) 162-165. DOI:10.1021/ma50002a033 |
| [26] |
X.H. Cao, J.L. Yang, H.L. Wu, et al., Polymer 165 (2019) 112-123. DOI:10.1016/j.polymer.2019.01.030 |
| [27] |
J.P. Gu, F.F. Zhang, Z.M. Zheng, et al., Chin. Chem. Lett. 32 (2021) 87-91. DOI:10.1016/j.cclet.2020.09.021 |
| [28] |
W.J. Yang, Y.T. Zhu, J.J. Li, et al., Chin. Chem. Lett. 32 (2021) 286-290. DOI:10.1016/j.cclet.2020.10.040 |
| [29] |
B. Wunderlich, Macromolecular Physics, Volume 1: Crystal Structure, Morphology, Defects Academic Press, New York, 1973.
|
 2022, Vol. 33
2022, Vol. 33 

