b Instrumental Analysis Center of Nanchang Hangkong University, Nanchang 330063, China;
c School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China;
d Research Institute of Aero-Engine, Beihang University, Beijing 100191, China;
e School of Ecology and Environment, Zhengzhou University, Zhengzhou 450001, China
With more and more electronic products into life, the microwave radiation generated by electronic devices has caused harm to human health and the environment. In order to reduce/avoid the harm of microwave radiation, intensive efforts have been made to develop advanced microwave absorbing materials [1-5]. At present, the widely used microwave absorbing materials are mainly divided into ferrites (Fe3O4, α-Fe2O3 and BaFe12O19, etc.) [6-8], magnetic metals (Ni, Co, α-Fe) [9-11], non-iron-based metal oxides (TiO2, NiO, CaBiNb2O4, ZnO and MoS2, etc.) [12-16], carbon-based materials (graphene, carbon nanotube and porous carbon, etc.) [17-20], polymers (polyaniline, polypyrrole, and polythiophene, etc.) [21-23], and other composites [24-31]. Among them, ferrites have attracted extensive interest owing to their abundant resources, low cost, and good absorption performance [32, 33].
Usually, ferrites have three structures, i.e., Fe3O4, γ-Fe2O3 and α-Fe2O3. Among them, Fe3O4 and γ-Fe2O3 belong to ferromagnetic materials, which have large saturation magnetization and high relative complex permeability suiting for microwave absorption. However, these two ferrites are easy to oxidization and agglomerate, leading to poor microwave absorption performance [2, 34]. By contrast, α-Fe2O3 provides more options for the microwave absorber design due to its stable chemical properties, environment-friendly feature, and controllable morphology.
Various morphologies of α-Fe2O3 have been widely reported, such as microspheres, microcubes, nanoparticles, and nanorods [35-42]. Coating an insulating layer on α-Fe2O3 particle surface is an effective strategy to adjust the particle-particle distance, enhance the anti-oxidation capability, reduce the density of particles, and improve the impedance matching between particles and free space. As a surface modification material, SiO2 is an ideal candidate due to its excellent chemical stability, nontoxicity, and easy conjugation with various functional groups. Various structures with α-Fe2O3 as the core and SiO2 as the shell have been fabricated for microwave absorption, such as core-shell structure, and yolk-shell structure [43-46]. However, the "structure-performance" relationship between shell thickness and microwave absorption performance is rarely reported.
Herein, we have synthesized the core-shell structural α-Fe2O3@SiO2 nanoparticles with a constant core size and changeable shell thickness to explore the "structure-performance" relationship between shell thickness and microwave absorption performance. The dependence of shell thickness on microwave absorption performance and the corresponding mechanisms were revealed and explained, respectively. The results indicated that α-Fe2O3@SiO2 of 35 nm shell-thickness presented a significant enhancement of absorption performance compared with that of pure α-Fe2O3. Although the absolute value of the reflection loss was relatively low (–4.3 dB), this study has shed an important reference on the design of next-generation advanced iron oxide-based materials for excellent microwave absorption by tuning the shell-thickness. Meanwhile, based on the "structure-performance" relationship between shell-thickness and microwave absorption performance, the Fe@SiO2, γ-Fe2O3@SiO2, Fe3O4@SiO2, and FeNx@SiO2 with excellent absorption performance will also be fabricated/developed from the core-shell structural α-Fe2O3@SiO2 nanoparticles by phase conversion of α-Fe2O3 through heat treatment.
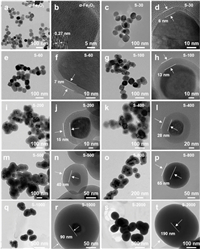
The core-shell structural α-Fe2O3@SiO2 nanoparticles were synthesized through the Stöber method (Fig. S1 in Supporting information) [47, 48]. By controlling the mass of tetraethyl orthosilicate (TEOS) during this reaction, the shell-thickness of SiO2 was finely regulated in the range of 0-190 nm (Fig. 1). Pure α-Fe2O3 cores present a uniformly dispersed size with 50 ± 10 nm (Figs. 1a and b, Fig. S2 in Supporting information). The lattice space of 0.27 nm corresponds to the (104) lattice plane of α-Fe2O3 (Fig. 1b). With increasing the mass of TEOS from 30 to 2000 mg, the shell-thickness of SiO2 increases from 6 ± 2 nm to more than 190 nm (Figs. 1a-t). Specifically, when the TEOS mass is less than 200 mg, the α-Fe2O3@SiO2 nanoparticles are granular (Figs. 1c-j). Then with increasing TEOS from 200 to 500 mg, the morphology of α-Fe2O3@SiO2 tends to be a sphere with a single core (Figs. 1k-n). And further increasing TEOS mass to 2000 mg, the agglomeration of α-Fe2O3 cores would be induced in α-Fe2O3@SiO2 particles (Figs. 1o-t, Fig. S3 in Supporting information). Based on the relationship between shell-thickness and TEOS mass, we can infer the shell growth rate is 0.091 nm/mg(TEOS), i.e., each 1 mg TEOS can increase the shell-thickness by 0.091 nm (Fig. S4 in Supporting information). This SiO2 shell growth rate maybe provides an important data reference for the fine synthesis of other core-shell structural M@SiO2 (M = metal oxides, metal particles, carbon materials, etc.) nanomaterials.
|
Download:
|
| Fig. 1. Regulation of shell-thickness in core-shell structural α-Fe2O3@SiO2 nanoparticles: (a, b) α-Fe2O3, (c, d) S-30, (e, f) S-60, (g, h) S-100, (i, j) S-200, (k, l) S-400, (m, n) S-500, (o, p) S-800, (q, r) S-1000, (s, t) S-2000. | |
Typically, the structure and components of α-Fe2O3, S-30, S-500, and S-1000 were characterized in detail (Fig. 2). Pristine α-Fe2O3 nanoparticle presents a series of X-ray diffraction (XRD) peaks at 24.1°, 33.1°, 35.6°, 40.9°, 49.5°, 54.09°, 57.59°, 62.45°, 63.99°, 71.94°, and 75.43°, corresponding to the (012), (104), (110), (113), (024), (116), (018), (214), (300), and (1010) lattice planes (JCPDS No. 33-0664) (Fig. 2a). These sharp and strong diffraction peaks indicate that the pristine α-Fe2O3 nanoparticles have high crystallinity and purity. When the added mass of TEOS was less than 500 mg, the as-prepared samples, i.e., S-30 and S-500, exhibit similar diffraction peaks with pristine α-Fe2O3, and no obvious SiO2 characteristic peak appeared due to the amorphous form of SiO2 shell. When the TEOS mass was 1000 mg, the amorphous SiO2 characteristic peak around 20°~30° began to appear in the corresponding sample, i.e., S-1000. The existence of SiO2 in S-500 and S-30 samples could be confirmed by Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and elemental mappings (Figs. 2b-j, Fig. S5 in Supporting information). For the S-500 sample, FT-IR absorption peaks at 1097, 949, and 789 cm–1 were caused by Si-O-Si, Si-OH, and Si-O vibrations from the SiO2 shell (Fig. S5 in Supporting information). After coating of the SiO2 shell, Fe 2p and Fe 3p peaks in XPS full spectra almost disappeared, but Si 2p and Si 2s appeared (Figs. 2b and c). Compared with pristine α-Fe2O3, the Fe 2p3/2, and Fe 2p1/3 peaks of S-500 all shifted to higher binding energy by ca. 0.4 eV in XPS fine spectra, confirming the interaction between α-Fe2O3 core and SiO2 shell (Fig. 2d). These results indicate the presence of SiO2-shell layers in S-500 [49, 50]. In order to further visually observe the core-shell structure, the S-30 sample with thin SiO2 layers was selected for elemental mapping characterization (Figs. 2e-j). Element mappings demonstrated that the outer shell layers were dominated by Si and O, and the inter cores by Fe and O (Figs. 2f-j).

|
Download:
|
| Fig. 2. Structure and components characterization. (a) XRD patterns. (b) XPS full spectra. (c, d) XPS fine spectra of Si 2p and Fe 2p. (e) High-angle annular dark-field (HAADF) image. (f-j) Elemental mappings of Fe, O, Si, Fe + O, and Fe + Si. | |
The static magnetic properties of as-prepared α-Fe2O3@SiO2 nanoparticles were measured using a vibrating sample magnetometer (VSM). For pure α-Fe2O3, it does not reach saturation magnetization even under a very high applied magnetic field of –80~80 kOe, suggesting its weak ferromagnetic behavior [35, 37, 51] (Fig. S6 in Supporting information). Therefore, the α-Fe2O3@SiO2 samples also do not reach saturation magnetization, and only possess narrow hysteresis features (Fig. S7 in Supporting information). Among them, the S-500 sample has a maximum area indicating higher hysteresis loss of energy. The detailed remanent magnetization (Mr) and coercivity (Hc) values of these samples were summarized in Table S1 (Supporting information). With increasing SiO2-shell thickness, Mr and Hc first increase and then decrease. The Mr value increases from 0.030 emu/g (for pure α-Fe2O3) to 0.045 emu/g (for S-500), then decreases to 0.022 emu/g (for S-1000). Similarly, the Hc value increases from 71 Oe to 1100 Oe, then decreases to 458 Oe (Table S1). The first increment resulted from the internal stress on the core generated by the shell, which hindered the rotation of the magnetic domain during the magnetization process [7, 52]. The subsequent decline was attributed to the fact that quite large amount of non-magnetic SiO2 shell layer reduced the content of α-Fe2O3 core. Besides, whether the agglomeration of α-Fe2O3 cores in single particles leads to the deterioration of Hc still needs further study (Fig. S7 in Supporting information).
For microwave absorbing materials, the electromagnetic parameters, i.e., relative complex permittivity (ɛr = ɛr′ + ɛr″) and relative complex permeability (μr = μr′ + μr″), directly influence their microwave absorbing performances. The real parts (ɛr′ and μr′) represent the storage capability of electric and magnetic energy, and the imaginary parts (ɛr″ and μr″) represent the loss ability of electric and magnetic energy [21, 53]. Specifically, with increasing thickness of the SiO2 shell, the ɛr′ values of these four samples gradually decreased from ca. 5.7 to ca. 3.5, in the order of α-Fe2O3 > S-30 > S-500 > S-1000 (Fig. 3a). Finally, the ɛr′ value of S-1000 was close to that of bulk SiO2 (ca. 4.0), due to the ~90 nm thick insulating layer of SiO2 [54]. However, with the increase of SiO2-shell thickness, the ɛr″ values first increased and then decreased (Fig. 3b). The corresponding dielectric loss tangent (tan δɛ = ɛr″/ɛr′) values also presented a similar evolution, from 0.02 (α-Fe2O3) to 0.08 (S-500), then to 0.06 (S-1000) (Fig. 3c). The evolutions of ɛr′ and tan δɛ may be due to the influence of SiO2-shell thicknesses, which was explained in the section for relationships of "structure-performance". For relative complex permeability, with increasing the SiO2-shell thickness, the μr′ values in these four samples ranged from 0.99 to 1.13 (Fig. 3d), and the μr″ values presented a gradually decreasing trend from 0.08 to 0.02 (Fig. 3e). The corresponding magnetic loss tangent (tan δμ = μr″/μr′) values gradually decreased from 0.08 (α-Fe2O3) to 0.02 (S-1000), indicating the decrease of magnetic loss capability (Fig. 3f). Compared with the electromagnetic parameters, i.e., relative complex permittivity and relative complex permeability, the values of ɛr′, ɛr″ and tan δɛ were all higher than those of μr′, μr″ and tan δμ. This result indicated that the contributions of dielectric loss were more than that of magnetic loss in the microwave absorption process.

|
Download:
|
| Fig. 3. Electromagnetic parameters: (a) ɛr′, (b) ɛr″, (c) tan ɛɛ, (d) ɛr′, (e) μr″, (f) tan δμ. | |
In general, the dielectric loss of material stems from conduction loss and polarization loss (e.g. ionic polarization, electric polarization, dipolar polarization, and interfacial polarization) [55, 56]. In our case, the ionic polarization and electron polarization would be excluded, because they generally exist in a much higher frequency region (103~106 GHz) [54]. Wave-like shapes of the curves were a typical behavior of dielectric relaxation (Figs. 3a-c), which was responsible for intrinsic dipolar polarization of the core structure [55, 57]. Generally, the insulator SiO2 possesses lower conductivity than that of the semiconductor α-Fe2O3. With the increase of SiO2 content, the reduced conductivity of α-Fe2O3@SiO2 led to the degradation of ɛr′ (Fig. 3a). According to the equation (ɛr″ ≈ σ/(2πɛ0f), where σ is the conductivity, ε0 is the permittivity in the free space, and f is the frequency), the ɛr″ theoretically should also decrease with the reduced conductivity [3, 34]. However, the ɛr″ of core-shell structural α-Fe2O3@SiO2 nanoparticles (S-30 and S-500) was higher than that of pure α-Fe2O3 (Fig. 3b). The phenomena may be possibly attributed to the compensation effect from the interfacial polarization, which originates from the charge buildup around the core-shell interface [7, 53, 55]. Therefore, the variations feature of dielectric loss of α-Fe2O3@SiO2 samples came from the dipolar polarization of the core structure, decrease of overall conductivity, and introduction of interfacial polarization.
Microwave absorption performances of α-Fe2O3@SiO2 nanoparticles can be evaluated by the reflection loss (RL), which is derived from the following formulas [58, 59].
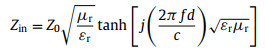
|
(1) |
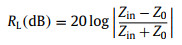
|
(2) |
where Z0 is the characteristic impedance of free space, Zin is the input impedance of the microwave absorber, d is the thickness of the absorber, c is the speed of light, and f is the frequency of the microwave. Using the measured electromagnetic parameters in Fig. 3, the RL of core-shell structural α-Fe2O3@SiO2 nanoparticles with different shell thicknesses can be calculated from formulas 1 and 2.
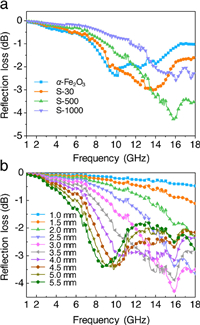
To more clearly reflect the influence of SiO2-shell thickness, the RL of α-Fe2O3@SiO2 nanoparticles with fixed d (sample thickness) of 3.0 mm were evaluated in Fig. 4a. With increasing the SiO2-shell thickness, the minimum value of RL first decreased and then increased (Fig. 4a). S-500 sample possessed the strongest microwave absorption with the lowest RL value of –4.3 dB. Thus, the microwave absorption performance of α-Fe2O3@SiO2 would be enhanced under proper SiO2-shell thickness. Fig. 4b shows the performances of S-500 under different d of 1.0~5.5 mm. As d was in the range of 1.0~2.0 mm, the RL decreased monotonously with the increase of frequency from 1~18 GHz. In the range of 2.5~5.5 mm, each curve presented a sharp or broad peak. As d was 3.0 mm, the RL achieved the minimum value of –4.3 dB at 16 GHz, which was superior to that of pure α-Fe2O3 (Fig. 4b and Fig. S8 in Supporting information). Additionally, it is interesting to note that most of the strong peaks were constrained in 6~11 and 13~17 GHz. This phenomenon may be generated from the wave character of dielectric loss (Figs. 3b, c and 4b).
|
Download:
|
| Fig. 4. Microwave absorption performances. (a) RL curves of α-Fe2O3, S-30, S-500, and S-1000 under d of 3.0 mm. (b) RL curves of S-500 with different d in the range of 1.0~5.5 mm. | |
Fig. 5 illustrates the analysis of "structure-performance" relationships in the core-shell structural α-Fe2O3@SiO2 nanoparticles. Based above results, the microwave absorption performances for the α-Fe2O3@SiO2 nanoparticles mainly come from the dielectric loss rather than the magnetic loss. The evolution of dielectric loss is mainly influenced by three factors: dipolar polarization of the α-Fe2O3 core, interfacial polarization between α-Fe2O3 core and SiO2 shell, and conductivity loss from the α-Fe2O3 core (Fig. 5a). The former factor may lead to the wave-like shapes of curves ɛ′, ɛr″ and tan δɛ, and the others will be conducive for the microwave absorption enhancement. Therefore, the microwave absorption performance of α-Fe2O3@SiO2 can be adjusted by tuning the SiO2-shell thickness, and achieve the best under a proper SiO2-shell thickness (Fig. 5b).
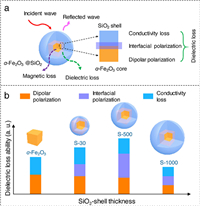
|
Download:
|
| Fig. 5. The "structure-performance" relationships. (a) Schematic of the possible microwave absorbing mechanism. (b) The evolution of the dielectric loss ability with the increase of SiO2-shell thickness. | |
Specifically, for the pure α-Fe2O3 nanoparticle, there are dipolar polarization and conductivity loss contributing to the dielectric loss. Although the dipolar polarization interaction and conductivity loss slightly decrease with the slight increase of the SiO2-shell thickness, the appearances of interfacial polarization lead to an enhancement of the overall dielectric loss ability, e.g., the case of S-30 (Fig. 5b). With the continuous increase of the SiO2-shell thickness, e.g., the case from S-30 to S-500, the contributions of both dipolar polarization and conductivity loss all gradually decrease. But the interfacial polarization continuously increases so that the dielectric loss ability reaches the optimum level at a proper SiO2-shell thickness, i.e., in the case of S-500 (Fig. 5b). Further increasing the thickness of SiO2 shells, e.g., the case from S-500 to S-1000, the dipolar polarization, interfacial polarization, and conductivity loss all gradually decrease to the minimum, e.g., the case of S-1000. It is due to the mismatched thickness of SiO2 insulation layers resulting in that the overall dielectric loss ability presents a decreased trend and unlimitedly approaches to that of pure SiO2 nanoparticles (Fig. 5b) [54]. Therefore, at the proper SiO2-shell thickness of 35 nm, the S-500 sample achieved the strongest microwave absorbing ability.
In summary, we have synthesized the core-shell structural α-Fe2O3@SiO2 nanoparticles with a constant α-Fe2O3-core size and changeable SiO2-shell thickness to explore the "structure-performance" relationship between shell thickness and microwave absorption performance. The shell thickness increases nearly linearly as the TEOS addition increases, with a slope of about 0.091 nm/mg(TEOS). There is a strong "structure-performance" dependence between microwave absorption performance and SiO2-shell thickness. With increasing the SiO2-shell thickness, the microwave absorption ability first increase and then decrease. This indicates that the microwave absorption performance of α-Fe2O3@SiO2 can be enhanced under proper SiO2-shell thickness. With a proper SiO2-shell thickness of 35 nm, the S-500 sample achieved the strongest microwave absorbing ability with a minimum RL value of –4.3 dB under a sample thickness of 3 mm, higher than that of pure α-Fe2O3 (–3.8 dB with 2.5 mm). This enhanced microwave absorption performance is mainly derived from dielectric loss including conductivity loss, interfacial polarization, and dipolar polarization. Although the absolute value of the reflection loss was relatively low (–4.3 dB), this study shed an important reference on designing next-generation advanced iron oxide-based materials for microwave absorption by tuning the shell thickness. Meanwhile, based on the "structure-performance" relationship between SiO2-shell thickness and microwave absorption performance, the M@SiO2 (M = Fe, γ-Fe2O3, Fe3O4 and FeNx) with excellent absorption performance will also be fabricated/developed from the core-shell structural α-Fe2O3@SiO2 nanoparticles by phase conversion of α-Fe2O3 through heat treatment.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21667019, 22066017, 52001156, and 52000163), the Key Project of the Natural Science Foundation of Jiangxi Province (No. 20171ACB20016), the Jiangxi Province Major Academic and Technical Leaders Cultivating Object Program (No. 20172BCB22014), the Science and Technology Department of Jiangxi Province (Nos. 20181BCB18003, 20181BAB216012, 20181ACG70025, CK202002473, and 20192BAB216003), the Key Laboratory of Photochemical Conversion and Optoelectronic Materials, TIPC, CSA (No. PCOM201906), and the Key Project of Science and Technology Research of the Jiangxi Provincial Department of Education (Nos. DA201602063, GJJ191044), the Aviation Science Foundation of China (No. 2017ZF56020) and the Program B for Outstanding Ph.D. Candidate of Nanjing University (No. 202002B076), and the Natural Science Foundation of Henan Province (No. 202300410423).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.027.
| [1] |
S. Li, Q. Li, J. Xu, et al., Adv. Funct. Mater. 26 (2016) 3738-3744. DOI:10.1002/adfm.201600122 |
| [2] |
M. Javid, Y. Zhou, D. Wang, et al., ACS Appl. Nano Mater. 1 (2018) 1309-1320. DOI:10.1021/acsanm.8b00055 |
| [3] |
J. Luo, K. Zhang, M. Cheng, M. Gu, X. Sun, Chem. Eng. J. 380 (2020) 122625. DOI:10.1016/j.cej.2019.122625 |
| [4] |
R. Peymanfar, A. Ahmadi, E. Selseleh-Zakerin, J. Alloys Compd. 848 (2020) 156453. DOI:10.1016/j.jallcom.2020.156453 |
| [5] |
Z. Jia, D. Lan, K. Lin, et al., J. Mater. Sci.: Mater. Electron. 29 (2018) 17122-17136. DOI:10.1007/s10854-018-9909-z |
| [6] |
B. Qu, C. Zhu, C. Li, X. Zhang, Y. Chen, ACS Appl. Mater. Interfaces 8 (2016) 3730-3735. DOI:10.1021/acsami.5b12789 |
| [7] |
G. Liu, L. Wang, Z. Yang, R. Wu, J. Alloys Compd. 718 (2017) 46-52. DOI:10.1016/j.jallcom.2017.05.100 |
| [8] |
J. Zhao, Y. Xie, D. Guan, et al., Sci. Rep. 5 (2015) 12544. DOI:10.1038/srep12544 |
| [9] |
L. Sha, P. Gao, T. Wu, Y. Chen, ACS Appl. Mater. Interfaces 9 (2017) 40412-40419. DOI:10.1021/acsami.7b07136 |
| [10] |
T. Liu, Y. Pang, M. Zhu, S. Kobayashi, Nanoscale 6 (2014) 2447-2454. DOI:10.1039/c3nr05238a |
| [11] |
Q. Liu, Z. Zi, D. Wu, Y. Sun, J. Dai, J. Mater. Sci. 47 (2011) 1033-1037. |
| [12] |
V.D. Phadtare, V.G. Parale, T. Kim, et al., J. Alloys Compd. 823 (2020) 153847. DOI:10.1016/j.jallcom.2020.153847 |
| [13] |
P. Liu, V.M.H. Ng, Z. Yao, et al., ACS Appl. Mater. Interfaces 9 (2017) 16404-16416. DOI:10.1021/acsami.7b02597 |
| [14] |
V.D. Phadtare, V.G. Parale, G.K. Kulkarni, H.H. Park, V.R. Puri, Ceram. Int. 44 (2018) 7515-7523. DOI:10.1016/j.ceramint.2018.01.150 |
| [15] |
Y. Song, F. Yin, C. Zhang, et al., Nano-Micro Lett. 13 (2021) 76. DOI:10.1007/s40820-021-00601-x |
| [16] |
X. Lin, J. Wang, Z. Chu, et al., Chin. Chem. Lett. 31 (2020) 1124-1128. DOI:10.1016/j.cclet.2019.07.003 |
| [17] |
Y. Zhang, Y. Huang, T. Zhang, et al., Adv. Mater. 27 (2015) 2049-2053. DOI:10.1002/adma.201405788 |
| [18] |
J. Zhao, Y. Xie, Z. Le, et al., Synthetic Met 181 (2013) 110-116. DOI:10.1016/j.synthmet.2013.08.015 |
| [19] |
R. Yang, X. Gui, L. Yao, et al., Nano-Micro Lett. 13 (2021) 66. DOI:10.1007/s40820-021-00597-4 |
| [20] |
Y. Jiang, X. Fu, Z. Zhang, et al., J Mater. Sci.: Mater. Electron. 30 (2019) 19173-19181. DOI:10.1007/s10854-019-02274-0 |
| [21] |
C. Tian, Y. Du, P. Xu, et al., ACS Appl. Mater. Interfaces 7 (2015) 20090-20099. DOI:10.1021/acsami.5b05259 |
| [22] |
N. Mokhtar, P.Y. Wong, G.B. Teh, S.W. Phang, Polym. Bull. 78 (2021) 6351-6365. DOI:10.1007/s00289-020-03432-9 |
| [23] |
J. Zhao, Y. Xie, Z. Le, et al., Polym. Compos. 34 (2013) 1801-1808. DOI:10.1002/pc.22584 |
| [24] |
J. Luo, L. Yue, H. Ji, K. Zhang, N. Yu, J. Mater. Sci. 54 (2019) 6332-6346. DOI:10.1007/s10853-018-03305-7 |
| [25] |
Y. Wang, X. Di, Z. Lu, X. Wu, J. Colloid Interface Sci. 589 (2021) 462-471. DOI:10.1016/j.jcis.2021.01.013 |
| [26] |
J. Sun, Y. Shen, X.S. Hu, Polym. Bull. 75 (2018) 653-667. DOI:10.1007/s00289-017-2060-9 |
| [27] |
J.M.A. Sulaiman, M.M. Ismail, S.N. Rafeeq, Appl. Phys. A 126 (2020) 236. DOI:10.1007/s00339-020-3413-z |
| [28] |
X. Zhao, Y. Huang, J. Yan, et al., J. Colloid Interface Sci. 595 (2021) 78-87. DOI:10.1016/j.jcis.2021.03.109 |
| [29] |
J. Zhao, Y. Xie, C. Yu, et al., Mater. Chem. Phys. 142 (2013) 395-402. DOI:10.1016/j.matchemphys.2013.07.035 |
| [30] |
X. Di, Y. Wang, Y. Fu, X. Wu, P. Wang, Carbon 173 (2021) 174-184. DOI:10.1016/j.carbon.2020.11.006 |
| [31] |
X. Zhao, Y. Huang, J. Yan, et al., Compos. Sci. Technol. 210 (2021) 108801. DOI:10.1016/j.compscitech.2021.108801 |
| [32] |
K. Jia, R. Zhao, J. Zhong, X. Liu, J. Magn. Magn. Mater. 322 (2010) 2167-2171. DOI:10.1016/j.jmmm.2010.02.003 |
| [33] |
B. Lu, X.L. Dong, H. Huang, et al., J. Magn. Magn. Mater. 320 (2008) 1106-1111. DOI:10.1016/j.jmmm.2007.10.030 |
| [34] |
P. Liu, S. Gao, Y. Wang, et al., ACS Appl. Mater. Interfaces 11 (2019) 25624-25635. DOI:10.1021/acsami.9b08525 |
| [35] |
J. Lian, X. Duan, J. Ma, et al., ACS Nano 3 (2009) 3749-3761. DOI:10.1021/nn900941e |
| [36] |
S. Cao, Y. Zhu, G. Cheng, Y. Huang, J. Phys. Chem. Solids 71 (2010) 1680-1683. DOI:10.1016/j.jpcs.2010.09.006 |
| [37] |
H. Jiao, G. Jiao, Mater. Lett. 63 (2009) 2725-2727. DOI:10.1016/j.matlet.2009.09.054 |
| [38] |
X. Zheng, J. Cai, W. Zhao, et al., Chin. Chem. Lett. 32 (2021) 2143-2150. DOI:10.1016/j.cclet.2020.11.017 |
| [39] |
B. Paul, S. Vadivel, S.S. Dhar, Chin. Chem. Lett. 27 (2016) 1725-1730. DOI:10.1016/j.cclet.2016.07.005 |
| [40] |
H. Nagabhushana, S.S. Saundalkar, L. Muralidhar, et al., Chin. Chem. Lett. 22 (2011) 143-146. DOI:10.1016/j.cclet.2010.09.020 |
| [41] |
M. Wang, M. Wang, Y. Fu, S. Shen, Chin. Chem. Lett. 28 (2017) 2207-2211. DOI:10.1016/j.cclet.2017.11.037 |
| [42] |
Z. Zhu, L. Zhong, Y. Wang, G. Zeng, W. Wang, Chin. Chem. Lett. 31 (2020) 2619-2622. DOI:10.1016/j.cclet.2020.01.038 |
| [43] |
M. Feyen, C. Weidenthaler, R. Guttel, et al., Chem. Eur. J. 17 (2011) 598-605. DOI:10.1002/chem.201001827 |
| [44] |
Z.M. Cui, Z. Chen, C.Y. Cao, L. Jiang, W.G. Song, Chem. Commun. 49 (2013) 2332-2334. DOI:10.1039/c3cc38649j |
| [45] |
X. Guo, Y. Deng, D. Gu, R. Che, D. Zhao, J. Mater. Chem. 19 (2009) 6706-6712. DOI:10.1039/b910606e |
| [46] |
J. Xu, J. Liu, R. Che, et al., Nanoscale 6 (2014) 5782-5790. DOI:10.1039/C4NR00158C |
| [47] |
W. Stober, A. Fink, E. Bohn, J. Colloid Interface Sci. 26 (1968) 62-69. DOI:10.1016/0021-9797(68)90272-5 |
| [48] |
J. Ge, L. Liu, Y. Cui, et al., Ceram. Int. 46 (2020) 15325-15332. DOI:10.1016/j.ceramint.2020.03.074 |
| [49] |
H. Lv, X. Liang, Y. Cheng, et al., ACS Appl. Mater. Interfaces 7 (2015) 4744-4750. DOI:10.1021/am508438s |
| [50] |
A.L. Morel, S.I. Nikitenko, K. Gionnet, et al., ACS Nano 2 (2008) 847-856. DOI:10.1021/nn800091q |
| [51] |
M. Tadic, N. Citakovic, M. Panjan, et al., J. Alloys Compd. 543 (2012) 118-124. DOI:10.1016/j.jallcom.2012.07.047 |
| [52] |
L. Yan, J. Wang, X. Han, et al., Nanotechnology 21 (2010) 095708. DOI:10.1088/0957-4484/21/9/095708 |
| [53] |
Y. Du, W. Liu, R. Qiang, et al., ACS Appl. Mater. Interfaces 6 (2014) 12997-13006. DOI:10.1021/am502910d |
| [54] |
R.R. Mishra, A.K. Sharma, Compos. Part A 81 (2016) 78-97. DOI:10.1016/j.compositesa.2015.10.035 |
| [55] |
B. Quan, X. Liang, G. Ji, et al., J. Alloys Compd. 728 (2017) 1065-1075. DOI:10.1016/j.jallcom.2017.09.082 |
| [56] |
N. Wu, C. Liu, D. Xu, et al., ACS Sustainable Chem. Eng. 6 (2018) 12471-12480. DOI:10.1021/acssuschemeng.8b03097 |
| [57] |
H.J. Yang, W.Q. Cao, D.Q. Zhang, et al., ACS Appl. Mater. Interfaces 7 (2015) 7073-7077. DOI:10.1021/acsami.5b01122 |
| [58] |
H. Sun, R. Che, X. You, et al., Adv. Mater. 26 (2014) 8120-8125. DOI:10.1002/adma.201403735 |
| [59] |
B. Zhang, Y. Feng, J. Xiong, Y. Yang, H. Lu, IEEE Trans. Magn. 42 (2006) 1778-1781. DOI:10.1109/TMAG.2006.874188 |
 2022, Vol. 33
2022, Vol. 33 

