b Institute of Zhejiang University - Quzhou, Quzhou 324000, China
With the development of modern society, the application of critical metals in high-tech materials is increasing year by year, making a great gap between the demand and metal mineral reserves [1, 2]. One approach is to recover critical metals from the secondary resources [3-5]. Taking rare earth elements (REEs), one of typical critical metals, as an example, the most important rare earth material in recent years is NdFeB permanent magnet, which contains about 10%–15% of REEs including Nd, Pr, Dy, and Tb [6]. However, these REEs occupy less than 30% of REEs in the minerals such as monazite, bastnasite, and ion adsorption deposit [7-10]. In this case, when extracting Nd from the minerals, the large amount of less valuable REEs like La and Ce will also be co-extracted. As a result, the operation and stock cost will be raised significantly. In addition, large amounts of waste gas, wastewater, and radioactive waste will generate during the refinery of minerals, which has become a serious threat to the environment [11]. Recovering REEs from waste NdFeB can partly solve the resource and environmental problems, but the volatile organic solvent and inorganic acids adopted in the state of art technique will also produce secondary environmental pollution [12, 13]. An environmentally friendly recovery process based on green solvents is essential for the sustainable utilization of critical metals.
Various novel separation methods, including ionic liquids (ILs), deep eutectic solvents, supercritical carbon dioxide, and mesoporous material adsorption have been introduced in the critical metal recovery area [14-17]. Among them, the application of trihexyl(tetradecyl)phosphonium chloride (P66614Cl, a phosphonium salt IL) as extractant in the recovery of NdFeB has attracted much attention for its advantages of low vapor pressure, good thermal stability, high ion conductivity, and specifical selectivity towards Fe in the solvent extraction [18-22]. However, the viscosity of P66614Cl would increase to a very high value when loading with large concentrations of metals, which made it unfavorable for the industrial operation [22, 23]. Recently, an alternative extraction system based on the tributyl(tetradecyl)phosphonium chloride (P44414Cl), has been reported [24]. The P44414Cl molecule is a water-soluble IL, which could not be applied in the traditional liquid-liquid extraction process. However, it is found that this IL could form aqueous biphasic system (ABS) with NaCl solution or hydrochloric acid when the temperature exceeds the upper critical solution temperature [24, 25]. This ABS is aqueous solution that has a much lower viscosity compared with P66614Cl. Furthermore, the unique phase transfer mechanism makes it possible to be applied in the one-pot leaching-extraction recovery process, which can greatly simplify the traditional hydrometallurgical process [26, 27]. In previous researches about the REEs separation, the ABSs based on phosphonium nitrate, imidazolium IL or polymers were just introduced to replace traditional organic solvent [28-32]. To the best of our knowledge, the one-pot leaching-extraction process and the extraction and phase transfer mechanism of the P44414Cl-based ABS towards critical metals especially REEs are still lack of exploration.
In this work, the NdFeB was adopted as an example for the study of P44414Cl-HCl ABS in the REEs recovery process. The extraction of individual Nd and Fe in respective chloride solutions by P44414Cl-HCl ABS was carried out first. Then the extraction mechanism was discussed with the Raman and UV–vis spectra. With the P44414Cl-HCl system, a one-pot leaching-extraction process was established to recover and separate Fe and REEs from the roasted NaFeB powder. The influences of acidity, IL concentration, contact time, temperature and solid/liquid ratio were systematically investigated to obtain the optimistic condition.
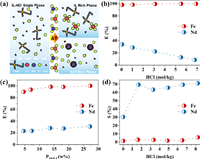
An IL ABS is composed of two immiscible aqueous phases, the salt-rich phase and the IL-rich phase. As illustrated in Fig. 1a, the original single phase P44414Cl-HCl solution can divide into two immiscible phases by rising temperature. During this process, the Nd (a represent of REEs) and Fe would be distributed in different phases. The influence of acidity and P44414Cl concentration on the extraction of Nd and Fe was very important. As shown in Fig. 1b, the extraction efficiency of Fe kept over 99.9% in the experimental acidity range, but the extraction of Nd decreased as the acidity increased. And from Fig. 1c, when the concentration of IL was 5%, the extraction efficiency of Fe was lower than 90%, and then increased to 99%, while the extraction efficiency of Nd increased very slightly with the increasing of IL concentration. As the P44414Cl showed strong extraction ability towards Fe while only a few Nd was extracted, high acidity and moderate IL concentration were favorable for the separation between Nd and Fe. Furthermore, the extracted Nd in the IL could be stripped by using a high concentration of HCl to increase the total recovery of Nd. The stripping efficiency of Nd could reach over 70% when the acidity exceeded 1.5 mol/kg (Fig. 1d).
|
Download:
|
| Fig. 1. (a) Schematic of the separation of Nd and Fe by P44414-based ABS. (b) Effect of acidity on the extraction efficiency of Fe and Nd, (c) effect of P44414Cl concentration on the extraction efficiency of Fe and Nd, and (d) effect of acidity on the stripping ratio of Fe and Nd. | |
The huge difference between the extraction efficiency of Fe and Nd might be attributed to the different hydration potential of these two metals. The extraction mechanism of metal cations by phosphonium salt is considered as follows (Reaction 1):
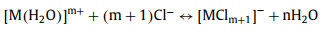
|
(1) |
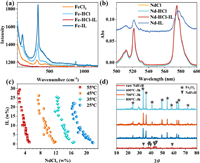
The critical process of this reaction is the exchange between water and Cl− on the coordination shell. The water-exchange enthalpy of Nd3+ and Fe3+ was 7.0 ± 2.0 and 41.4 kJ/mol, which indicated the Nd would keep in water complexes while the Fe might form Cl− coordination species in chloride solution [33, 34]. The Raman spectra of Fe (Fig. 2a) and UV–vis spectra of Nd (Fig. 2b) in the single chloride solution, hydrochloric acid, P44414Cl-HCl solution, and P44414Cl were analyzed to support this assumption. The Raman spectra of Fe in solution exhibited a clear Fe–Cl bond in all the four samples [6]. Comparing the spectra of Fe in HCl and individual chloride solution, a new band around 115 cm−1 appeared which could be attributed to the [FeCl4]− [35, 36]. This new band was also found in the IL, indicative of the generation of [FeCl4]−. The strong attraction of [FeCl4]− with P44414+ in HCl might be the reason for the high extractability of Fe [37]. The strong band around 334 cm−1 could be assigned to the FeCl3. It should be noticed that the bands from 850 to 1100 cm−1 in the spectra of FeCl3 and HCl solution vanished in the IL, which was an evidence of the disappearance of Fe–H2O coordination [38, 39]. In the UV-vis spectra, the absorption bands of Nd(Ⅲ) in all the three aqueous solutions kept at 575 nm while it shifted to 576 nm in the P44414Cl, which might be an indication of forming new Nd(Ⅲ) complexes [40]. However, the red-shift was so weak, indicating that some Nd(Ⅲ) species similar to the original coordination environment still existed. Thus, we think that this portion of Nd(Ⅲ) might be extracted as original complexes through a solvation effect by the water dissolved in IL-rich phase. This result can well explain that the extraction of Nd decreased as the acidity increased. The increasing acidity decreased the water dissolved in the IL-rich phase, thus decreased the amount of dissolved [Nd(H2O)n]3+.
|
Download:
|
| Fig. 2. (a) Raman spectra of Fe in FeCl3 solution, HCl, P44414Cl-HCl, and P44414Cl (band positions of each spectra were listed in Table S1 in Supporting information). (b) UV-vis spectra of Nd in NdCl3 solution, HCl, P44414Cl-HCl, and P44414Cl. (c) Phase diagram of P44414Cl-NdCl3 ABS, and (d) PXRD patterns of NdFeB roasted at different temperature (A full pattern of raw NdFeB was given in Fig. S1 in Supporting information.). | |
P44414Cl forms ABS with various amounts of chlorides and HCl because of good interfacial activity provided by the long alkyl chain [41-43]. Considering the low extraction of Nd by P44414Cl, the NdCl3 might act as a salting-out agent in a P44414Cl-NdCl3 system. The P44414Cl-NdCl3 phase diagram was titrated and shown in Fig. 2c. At each temperature, the critical concentration of NdCl3 varied in a small range, which was similar to other chloride salts like NaCl and MgCl2 rather than HCl [41]. The average critical concentration of NdCl3 at 25 ℃ was 0.74 mol/kg, which was even less than the strongest salting-out agent, CaCl2. This phenomenon could be interpreted by the high charge density and strong water coordination of Nd3+. The strong salting-out effect of Nd suggested that the original single phase solution could be transformed to biphasic system when the HCl was changed to NdCl3. The salting-out effect of FeCl3 was also tested. When high concentration FeCl3 solution was added into P44414Cl water solution, surprisingly, some water insoluble droplets would appear. It might be due to the formation of P44414[FeCl4], which had strong hydrophobicity and forms the hydrophobic phase instead of ABS.
In the practical recovery process, the NdFeB powder was roasted to transform the alloy into the corresponding metal oxides at first. The Fe and Nd might be oxidized to different phases as the temperature varied [44]. In this work, the NdFeB was roasted from 600 ℃ to 800 ℃. As the PXRD patterns illustrated (Fig. 2d), the Fe was all oxidized to α-Fe2O3 without any other phases, which was in accord with previous report [45]. To avoid the possible indissoluble NdFeO3 at higher temperature, the NdFeB roasted at 600 ℃ was adopted in the following recovery process. The metal composition of roasted NdFeB was given in Table S3 (Supporting information). Usually, the temperature change is essential in the one-pot recovery process of Co and Ni by P44414Cl-HCl ABS [26]. In our case, after dissolution of roasted NdFeB, the single phase P44414Cl-HCl solution was surprisingly divided into two immiscible aqueous phases at room temperature. It might be due to a combination of the strong salting-out effect of NdCl3 and hydrophobicity of P44414[FeCl4].
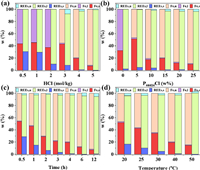
In ABS-based one-pot separation process, each element might distribute in three phases: The solid phase, the salt-rich phase (in this work, the lower phase), and the IL-rich phase (the upper phase). Various factors would influence the leaching and extraction, thus varied the distribution of different elements. As shown in Figs. 3a-d, all temperature, contact time, acidity and IL concentration had positive effect on the leaching of roasted NdFeB. The left Fe and REEs in the solid phase decreased obviously as all the factors increased. But the solid/liquid ratio had opposite effect. As illustrated in Fig. S2 (Supporting information), higher solid/liquid ratio would consume more H+ and reduce the balanced acidity, so the total leaching efficiency would decrease. It should be noted that when the acidity was lower than 3 mol/kg, the solution remained a single phase at room temperature. The extraction which determined the element distribution between upper and lower liquid phase was similar to the individual element extraction process. The extraction efficiency of Fe was very high (> 98%) under all conditions while the extraction of REEs was lower than 10%. The acidity had a negative effect on the extraction of REEs, while the increasing of P44414Cl concentration and contact time would slightly improve the extraction of REEs. With a comprehensive consideration of leaching and extraction, lower solid/liquid ratio, moderate contact time and IL concentration, higher temperature and acidity were beneficial for the recovery and separation of REEs and Fe.
|
Download:
|
| Fig. 3. Effect of acidity (a), P44414Cl concentration (b), contact time (c), and temperature (d) on the distribution of Fe and Nd in the upper phase, lower phase, and solid phase after the leaching-extraction of roasted NdFeB, where the w represents the mass fraction. | |
Based on the one-pot separation, a facile recovery process was illustrated in Scheme 1. The NdFeB was roasted firstly and reacted with P44414Cl-HCl aqueous solution to form an ABS. After reaction, most REEs were dissolved in the lower salt-rich phase and Fe was extracted into the upper IL-rich phase. The REEs loaded in the IL phase could be stripped by concentrated HCl with a stripping efficiency of 76%. These REEs containing HCl solution could be reused in the next one-pot separation cycle. The left Fe in IL phase could be precipitated out by using NaOH-NaCl solution. The P44414Cl would form ABS with NaCl solution and be recovered. The total Fe and REEs left in the IL was less than 4%, which promised the recycling of IL. In the total process, the recovery rate of REEs could reach 96% and the IL could be recycled.

|
Download:
|
| Scheme 1. Illustration of one-pot leaching-extraction recovery process for roasted NdFeB using an ABS based on P44414Cl. | |
In summary, we proposed a facile one-pot process based on ABS for recovering REEs from NdFeB. Due to the hydrophobic property of P44414[FeCl4] and salting-out effect of NdCl3, the leaching solution could form ABS at room temperature. The P44414Cl showed obviously different extraction abilities between Fe and REEs because of the coordination differences of Fe3+ and Nd3+ in chloride solution. REEs and Fe could be mutually separated in different phases during the one-pot process. The recovery of practical roasted NdFeB indicated the P44414Cl-based ABS had potential to be applied in the separation and recovery of REEs from other metals.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (No. U2067213), Natural Science Foundation of Zhejiang Province (No. LR21B060001), and the Fundamental Research Funds for the Central Universities (No. 2021QNA4029).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.026.
| [1] |
Z. Sun, H. Cao, Y. Xiao, et al., ACS Sustainable Chem. Eng. 5 (2016) 21-40. |
| [2] |
K. Binnemans, P.T. Jones, B. Blanpain, et al., J. Clean. Prod. 51 (2013) 1-22. DOI:10.1016/j.jclepro.2012.12.037 |
| [3] |
Z. Sun, Y.P. Xiao, H. Agterhuis, J. Sietsma, Y.X. Yang, J. Clean. Prod. 112 (2016) 2977-2987. DOI:10.1016/j.jclepro.2015.10.116 |
| [4] |
C.Y. Liu, Y.F. Deng, J. Chen, D. Zou, W.R. Su, Ind. Eng. Chem. Res. 56 (2017) 7551-7558. DOI:10.1021/acs.iecr.7b01427 |
| [5] |
T.C. Liu, J. Chen, H.L. Li, K. Li, Sep. Purif. Technol. 245 (2020) 116869. DOI:10.1016/j.seppur.2020.116869 |
| [6] |
M.A.R. Önal, S. Dewilde, M. Degri, et al., Green Chem. 22 (2020) 2821-2830. DOI:10.1039/d0gc00647e |
| [7] |
D.Q. Li, J. Rare Earths 37 (2019) 468-486. DOI:10.1016/j.jre.2018.07.016 |
| [8] |
L. Chen, Y.L. Wu, H.J. Dong, et al., Sep. Purif. Technol. 197 (2018) 70-85. DOI:10.1016/j.seppur.2017.12.053 |
| [9] |
D. Zou, J. Chen, K. Li, D.Q. Li, ACS Omega 3 (2018) 17036-17041. DOI:10.1021/acsomega.8b01140 |
| [10] |
J. Liu, L.Q. Zeng, S. Liao, et al., Chin. Chem. Lett. 31 (2020) 2849-2853. DOI:10.1016/j.cclet.2020.08.017 |
| [11] |
G.G. Zaimes, B.J. Hubler, S. Wang, V. Khanna, ACS Sustainable Chem. Eng. 3 (2015) 237-244. DOI:10.1021/sc500573b |
| [12] |
E.M. Iannicelli-Zubiani, M.I. Giani, F. Recanati, et al., J. Clean. Prod. 140 (2017) 1204-1216. DOI:10.1016/j.jclepro.2016.10.040 |
| [13] |
C.D. Deng, Y.J. Qiao, Q.D. Chen, X.H. Shen, Chin. Chem. Lett. 28 (2017) 19-23. DOI:10.1016/j.cclet.2016.06.020 |
| [14] |
C.Y. Liu, Q.B. Yan, X.W. Zhang, L.C. Lei, C.L. Xiao, Environ. Sci. Technol. 54 (2020) 10370-10379. DOI:10.1021/acs.est.0c03278 |
| [15] |
S. Riano, K. Binnemans, Green Chem. 17 (2015) 2931-2942. DOI:10.1039/C5GC00230C |
| [16] |
T. Vander Hoogerstraete, B. Blanpain, T. Van Gerven, K. Binnemans, RSC Adv. 4 (2014) 64099-64111. DOI:10.1039/C4RA13787F |
| [17] |
X.C. Li, T.R. Lu, Y. Wang, Y.F. Yang, Chin. Chem. Lett. 30 (2019) 2318-2322. DOI:10.1016/j.cclet.2019.05.056 |
| [18] |
R. Mohebat, A. Yazdani-Elah-Abadi, Chin. Chem. Lett. 28 (2017) 1340-1344. DOI:10.1016/j.cclet.2017.01.024 |
| [19] |
S.B. Yu, H.J. Zang, X.L. Yang, et al., Chin. Chem. Lett. 28 (2017) 1479-1484. DOI:10.1016/j.cclet.2017.02.016 |
| [20] |
Z.H. Weng, Y. Qi, L.S. Zong, et al., Chin. Chem. Lett. 28 (2017) 1069-1073. DOI:10.1016/j.cclet.2016.12.019 |
| [21] |
R. Hajian, Z. Alghour, Chin. Chem. Lett. 28 (2017) 971-975. DOI:10.1016/j.cclet.2016.12.003 |
| [22] |
T. Vander Hoogerstraete, K. Binnemans, Green Chem. 16 (2014) 1594-1606. DOI:10.1039/C3GC41577E |
| [23] |
R. Banda, F. Forte, B. Onghena, K. Binnemans, RSC Adv. 9 (2019) 4876-4883. DOI:10.1039/c8ra09797f |
| [24] |
B. Onghena, T. Opsomer, K. Binnemans, Chem. Commun. 51 (2015) 15932-15935. DOI:10.1039/C5CC06595J |
| [25] |
M. Gras, N. Papaiconomou, N. Schaeffer, et al., Angew. Chem. Int. Ed. 57 (2018) 1563-1566. DOI:10.1002/anie.201711068 |
| [26] |
N. Schaeffer, M. Gras, H. Passos, et al., ACS Sustainable Chem. Eng. 7 (2019) 1769-1777. DOI:10.1021/acssuschemeng.8b05754 |
| [27] |
N. Schaeffer, H. Passos, M. Gras, et al., ACS Sustainable Chem. Eng. 8 (2020) 12260-12269. DOI:10.1021/acssuschemeng.0c04043 |
| [28] |
B. Dewulf, N.K. Batchu, K. Binnemans, ACS Sustain, ACS Sustainable Chem. Eng. 8 (2020) 19032-19039. DOI:10.1021/acssuschemeng.0c07207 |
| [29] |
S.J.R. Vargas, N. Schaeffer, J.C. Souza, L.H.M. da Silva, M.C. Hespanhol, Waste Manag. 125 (2021) 154-162. DOI:10.1016/j.wasman.2021.02.038 |
| [30] |
Y.H. Chen, H.Y. Wang, Y.C. Pei, J.J. Wang, Talanta 182 (2018) 450-455. DOI:10.1016/j.talanta.2018.02.018 |
| [31] |
V.A. Cocalia, M.P. Jensen, J.D. Holbrey, et al., Dalton Trans. (2005) 1966-1971. DOI:10.1039/b502016f |
| [32] |
N. Sui, K. Huang, C. Zhang, et al., Ind. Eng. Chem. Res. 52 (2013) 5997-6008. DOI:10.1021/ie4002553 |
| [33] |
M.L. Steele, D.L. Wertz, Inorg. Chem. 16 (1977) 1225-1228. DOI:10.1021/ic50171a050 |
| [34] |
D.T. Richens, The Chemistry of Aqua Ions. New York: Wiley, 1997.
|
| [35] |
S.K. Sharma, J. Chem. Phys. 60 (1974) 1368-1375. DOI:10.1063/1.1681206 |
| [36] |
L. Cui, F.Q. Cheng, J.F. Zhou, Ind. Eng. Chem. Res. 54 (2015) 7534-7542. DOI:10.1021/acs.iecr.5b01546 |
| [37] |
F.L. Jiang, H. Peng, C. Li, H.Y. Fu, G.Z. Wu, Chin. Chem. Lett. 31 (2020) 801-804. DOI:10.1016/j.cclet.2019.05.028 |
| [38] |
H. Kanno, J. Hiraishi, J. Raman Spectrosc. 12 (1982) 224-227. DOI:10.1002/jrs.1250120305 |
| [39] |
G. Oczko, L. Macalik, J. Legendziewicz, J. Hanuza, J. Alloys Compd. 380 (2004) 327-336. DOI:10.1016/j.jallcom.2004.03.009 |
| [40] |
G.X. Tian, S.J. Teat, L.F. Rao, Inorg. Chem. 53 (2014) 9477-9485. DOI:10.1021/ic5004484 |
| [41] |
N. Schaeffer, H. Passos, M. Gras, et al., Phys. Chem. Chem. Phys. 20 (2018) 9838-9846. DOI:10.1039/C8CP00937F |
| [42] |
X. Gong, L. Li, Chin. Chem. Lett. 28 (2017) 2045-2052. DOI:10.1016/j.cclet.2017.09.051 |
| [43] |
P. Han, T.C. Liu, X.W. Ji, S.K. Tang, Chin. Chem. Lett. 29 (2018) 1305-1309. DOI:10.1016/j.cclet.2017.10.042 |
| [44] |
H.S. Yoon, C.J. Kim, K.W. Chung, et al., Metals 5 (2015) 1306-1314. DOI:10.3390/met5031306 |
| [45] |
J.C. Lee, W.B. Kim, J.K. Jeong, I.J. Yoon, Korean J. Met. Mater. 36 (1998) 967-972. |
 2022, Vol. 33
2022, Vol. 33 

