b State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
The rapid consumption of fossil resources and carbon emission derived environmental issues motivate the application of renewable resources. Biomass is a kind of abundant and renewable carbon containing material. The utilization of biomass is an alternative for the production of transport fuels and fine chemicals [1, 2]. Furfural (FF) production from biomass by acid-catalyzed dehydration of xylose is a major commercial process for the biomass valorization. As summarized in Fig. 1a, FF is used for the manufacture of furfuryl alcohol (FA), 2-methylfuran (MF), tetrahydrofurfuryl alcohol (THFA), 2-methyltetrahydrofuran (MTHF) and so on [3, 4]. FA is widely used as a raw material for the production of foundry resins, plastics, synthetic fibers and other fine chemicals [5]. Due to the extensive applications, FA production accounts for more than 65% of FF produced [6]. Thus, hydrogenation of FF to FA is one of the most valuable and practical routes.
|
Download:
|
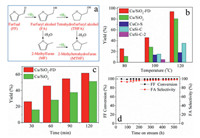
| Fig. 1. (a) FF hydrogenation chart. (b) Catalytic performance of Cu/SiO2-FD catalyst and reference catalysts at different reaction temperature (0.05 g catalyst, 2 g FF and 30 mL 1, 4-dioxane, 4 MPa H2, 600 rpm, 1 h). (c) Catalytic performance of Cu/SiO2-FD and Cu/SiO2 at different reaction time (0.05 g catalyst, 2 g FF and 30 mL 1, 4 dioxane, 100 ℃, 4 MPa H2, 600 rpm). (d) Stability test of Cu/SiO2-FD using trickle bed reactor (120 ℃, 4 MPa H2, 2 g FF-1, 4-dioxane/g-catalyst/h, H2/FF = 40). | |
Copper chromite is a commonly used commercial catalyst for the catalytic conversion of FF to FA [7]. The toxic chromium species is harmful to humans and the environment. Thus, the development of chromium-free catalyst attracts vast attention both from academia and industry. Several non-chromium catalysts have been developed for both gas phase and liquid phase hydrogenation processes, such as Ru, Pd, Pt, Cu, Ni [8, 9]. The copper based catalyst has been considered as the most promising for industrial application [10]. Recently, we reported that FF was hydrogenated to FA in the gas phase with higher stability by using ammonia evaporation derived silica supported copper catalyst and impregnation derived ethanolamine modified silica supported copper catalyst [10, 11]. Besides, ammonia evaporation derived silica supported copper catalysts have been reported to have good performances for ester hydrogenation, CO2 hydrogenation and FF hydrogenolysis to MF [12-14]. However, the gas phase hydrogenation technology for FF conversion needs more fixed investment in industrial scale production, which limits its application at present. In contrast, the liquid phase hydrogenation is extensively used in plants thanks to its simple process and low fixed input for same scale of capacity.
Thus, an efficient silica supported copper catalyst for FF hydrogenation in liquid phase is developed in the present study. The catalyst (Cu/SiO2-FD) is synthesized by ammonia evaporation using freeze drying (FD) technology during the preparation process. The performance of Cu/SiO2-FD is superior to that of conventional ammonia evaporation using oven drying derived catalyst (Cu/SiO2), commercial copper chromite catalyst purchased from Strem Chemicals (CuCr-S) and a commercial copper catalyst supplied by FA production plant (CuSi-C). The promotion effect of freeze drying on the catalytic performance is also explained by various characterization results.
The detailed information of materials, catalyst preparation, catalyst characterization, catalyst evaluation, product analyses and some characterization results are described in Supporting information.
Fig. 1b shows the FF hydrogenation performances over the Cu/SiO2, Cu/SiO2-FD and reference commercial catalysts. The selectivity to FA is nearly 100% in all cases. The Cu/SiO2-FD exhibits higher FA yield at 80 ℃, 100 ℃ and 120 ℃ compared to Cu/SiO2. And the activity of Cu/SiO2-FD is much higher than that of commercial CuCr-S catalyst and CuSi-C catalyst. For the commercial CuSi-C catalyst on the equal copper amount of Cu/SiO2-FD, its performance as that marked as CuSi-C-2 is considerably far inferior to that of the Cu/SiO2-FD. Take the Cu/SiO2 and Cu/SiO2-FD for comparison (Fig. 1c), FA yield increases with the increasing of reaction time, the Cu/SiO2-FD performs better than Cu/SiO2 in all cases. The performance of Cu/SiO2-FD catalyst is also superior to majority of the copper based catalysts that were reported previously (Table S1 in Supporting information). And the composition of the Cu/SiO2-FD is much simpler compared to the reference catalysts summarized in Table S1. The stability of the Cu/SiO2-FD was further assessed using a trickle bed reactor in continuous mode, and the result is depicted in Fig. 1d. There is no obvious decrease of FF conversion and FA selectivity during the whole reaction (~550 h). The above results demonstrate that the silica supported copper catalyst prepared by ammonia evaporation using freeze drying as drying technology (Cu/SiO2-FD) is more active than that of the silica supported copper catalyst synthesized by ammonia evaporation using conventional oven drying (Cu/SiO2), commercial CuCr-S catalyst and commercial CuSi-C catalyst supplied by a FA plant. And the Cu/SiO2-FD catalyst shows excellent stability. Therefore, the freeze drying promotes the performance of silica supported copper catalyst for FF hydrogenation in liquid phase.
To better explain the remarkable effect of freezing drying on the performance, detailed physicochemical characterizations were conducted. As shown in Fig. S1a (Supporting information), the diffraction peaks at 31.0°, 35.6°, 57.1°, 63.3° and 72.0° are observed for both of the calcined Cu/SiO2-FD and Cu/SiO2 samples. These characteristic diffraction peaks correspond to the formation of copper phyllosilicate [11, 15]. The vibrations of 673 cm−1 and 1033 cm−1 in fourier transform infrared spectroscopy (FT-IR) spectra (Fig. S1b in Supporting information) of calcined sample verify the existence of copper phyllosilicate [15, 16]. The existence of copper phyllosilicate is also verified by the transmission electron microscope (TEM) images (Figs. S1c and d in Supporting information), in which the lamellar structure is observed. Based on the results of XRD, FT-IR and TEM, the copper phyllosilicate exists in both of the calcined Cu/SiO2 and Cu/SiO2-FD catalysts.
As listed in Table 1, the copper loading determined by ICP is 17.4 wt% and 17.3 wt% for Cu/SiO2 and Cu/SiO2-FD, respectively. The SBET, VP and DP of SiO2 support are 190 m2/g, 0.46 cm3/g and 9.4 nm. The SBET and VP of Cu/SiO2 increase to 416 m2/g and 0.82 cm3/g. A similar increase was reported for ammonia evaporation derived silica supported copper catalyst that contained copper phyllosilicate [17, 18]. Surprisingly, the VP and DP of calcined Cu/SiO2-FD are 1.87 cm3 and 14.3 nm, which are much higher than that of calcined Cu/SiO2. The SBET (449 m2/g) of Cu/SiO2-FD is a little higher than that of Cu/SiO2 as well. The observation means that freeze drying promotes the pore expansion during the preparation. The SBET and VP of fresh Cu/SiO2 and Cu/SiO2-FD samples decrease due to the decomposition of phyllosilicate during the reduction [19]. However, the VP and DP of fresh Cu/SiO2-FD are also higher than that of fresh Cu/SiO2. The higher VP and DP might be beneficial to the mass transfer, which leads to the higher activity. The similar loosely packed platelet structure and higher methane dry reforming performance of freeze dried Ni/MgAlOX catalyst compared to oven dried sample was reported previously [20].
|
|
Table 1 Textural properties of the copper catalysts. |
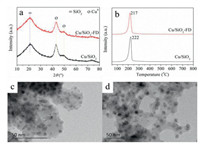
As shown in Fig. 2a, the diffraction peaks (2θ = 43.2° and 50.2°) ascribing to Cu0 are observed in the XRD patterns of freshly reduced catalysts [21]. The average particle size of Cu0 determined by Scherrer equation is 4.2 nm and 3.5 nm for Cu/SiO2 and Cu/SiO2-FD, respectively. The small average particle size of the copper in Cu/SiO2-FD compared to Cu/SiO2 is also verified by particle size distribution histograms (Fig. S2 in Supporting information). Moreover, the copper particles are distributed uniformly on the silica support after reduction (Figs. 2c and d). From the H2-temperature programmed reduction (H2-TPR) profile (Fig. 2b), it can be seen that the H2 consumption peak of Cu/SiO2-FD is slightly lower than that of Cu/SiO2. This reveals that the calcined Cu/SiO2-FD is easier to reduce than Cu/SiO2, which indicates that freeze drying decreased the size of copper particles [22]. Accordingly, copper particles with relatively small size were formed in the fresh Cu/SiO2-FD.
|
Download:
|
| Fig. 2. (a) XRD patterns of freshly reduced samples. (b) H2-TPR profiles of calcined samples. TEM images of fresh samples for Cu/SiO2 (c) and Cu/SiO2-FD (d). | |
The Cu 2p and Cu LMM X-ray auger electron spectroscopy (XAES) spectra (Figs. S3A and B in Supporting information) of freshly reduced samples were collected in-situ by near ambient pressure X-ray photoelectron spectroscopy (XPS) instrument to analyze the copper state. As listed in Fig. S3A, the peak of Cu 2p3/2 at about 932.3 eV and a peak of Cu 2p1/2 at around 952.1 eV are observed. These peaks are assigned to Cu0 or Cu+ species [23]. The existence of these peaks suggests that the Cu+ or Cu0 formed during the reduction of calcined samples. Since the Cu 2p binding energy (BE) of Cu+ and Cu0 are almost identical from XPS spectra, the modified Auger parameter (Table S2 in Supporting information) is used to distinguish the Cu+ and Cu0 [24]. The modified Auger parameter equal to the sum of the Cu 2p3/2 BE and the kinetic energy (KE) of Cu LMM Auger electron. As seen from Table S2, the Cu+ content in Cu/SiO2-FD is lower than Cu/SiO2.
N2O titration was applied for the measurement of the exposed Cu0 sites, the results are listed in Table 2. The surface area of metallic copper (SCu) is 31.9 m2/g and 48.8 m2/g for Cu/SiO2 and Cu/SiO2-FD, respectively. The Cu0 dispersion (DCu) of Cu/SiO2-FD is higher than Cu/SiO2. The result reveals that the freeze drying promoted the dispersing of metallic copper. The particle size of metallic copper was calculated based on the N2O titration and XRD patterns. Relative small metallic copper particles were obtained when the freeze drying was used. Besides, the exposed Cu+ surface area (SCu) is also calculated based on the Cu+ content and SCu. Higher amount of exposed Cu+ sites are also obtained in the Cu/SiO2-FD sample. Then, the higher amount of Cu0 sites and higher amount of Cu+ sites gave rise to the higher activity of Cu/SiO2-FD.
|
|
Table 2 Physicochemical properties of the copper catalysts. |
The characterization results of calcined samples (Fig. S1 in Supporting information and Table 1) illustrate that the copper phyllosilicate existed in both of Cu/SiO2 and Cu/SiO2-FD samples, and the freeze drying promotes the pore expansion. The particles are uniformly distributed in the reduced samples (Fig. 2). The XRD, TEM, H2-TPR, in-situ XPS and N2O titration results (Fig. 2, Fig. S2, Table 2 and Table S2) suggest that the application of freeze drying during the preparation process promoted the dispersion of copper. Consequently, a relatively higher metallic copper surface area and Cu+ surface area were obtained. The Cu0 and Cu+ species were resulted from the reduction of highly dispersed CuO and copper phyllosilicate under the moderate conditions, respectively. As stated by the previous studies, the synergistic effect of Cu0 and Cu+ leads to the conversion of FF to FA [25-27]. In detail, H2 is adsorbed and activated at the Cu0 sites to form active H, the C=O bond in FF molecule is adsorbed at the Cu+ site and polarized through the oxygen electron lone pair. The active H attack the adjacent polarized C=O species. Then, the FF is hydrogenated to FA. Thus, the Cu/SiO2-FD exhibits the better performance due to its higher amount of Cu0 and Cu+ active sites. And the large VP and DP also facilitate the reaction by promoting the mass transfer.
In summary, the silica supported copper catalyst prepared by ammonia evaporation using freeze drying technology exhibits the better performance for FF hydrogenation in liquid phase than that of conventional ammonia evaporation using oven drying derived catalyst and representative commercial catalysts. The freeze drying promotes the copper dispersion, which leads to a higher amount of Cu0 and Cu+ active sites. In addition, the freeze drying also promotes the pore expansion, which boosts the mass transfer. Thus, the Cu/SiO2-FD exhibits the superior performance thanks to its higher number of active sites and developed pore structure.
Declaration of competing interestThere are no conflicts of interest to declare.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21721004, 21808217, 21932005), Natural Science Foundation of Liaoning Province (No. 2020-MS-018), Dalian Young Star of Science and Technology Project (No. 2020RQ023) and Dalian Institute of Chemical Physics (Nos. DICP ZZBS201812, DICPI201936).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.082.
| [1] |
H. Du, X. Ma, M. Jiang, P. Yan, Z.C. Zhang, Energy 221 (2021) 119837. DOI:10.1016/j.energy.2021.119837 |
| [2] |
Z. Zhang, X. Tong, H. Zhang, Y. Li, Green Chem. 20 (2018) 3092-3100. DOI:10.1039/C8GC00852C |
| [3] |
K. Yan, G. Wu, T. Lafleur, C. Jarvis, Renew. Sust. Energ. Rev. 38 (2014) 663-676. DOI:10.1016/j.rser.2014.07.003 |
| [4] |
W. Gong, C. Chen, H. Wang, et al., Chin. Chem. Lett. 29 (2018) 1617-1620. DOI:10.1016/j.cclet.2018.03.030 |
| [5] |
Y. Wang, D. Zhao, D. Rodríguez-Padrón, C. Len, Catalysts 9 (2019) 796. DOI:10.3390/catal9100796 |
| [6] |
R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba, M.López Granados, Energ. Environ. Sci. 9 (2016) 1144-1189. DOI:10.1039/C5EE02666K |
| [7] |
G. Singh, L. Singh, J. Gahtori, et al., Mol. Catal. 500 (2021) 111339. DOI:10.1016/j.mcat.2020.111339 |
| [8] |
Y. Nakagawa, M. Tamura, K. Tomishige, ACS Catal. 3 (2013) 2655-2668. DOI:10.1021/cs400616p |
| [9] |
S. Thongratkaew, C. Luadthong, S. Kiatphuengporn, et al., Catal. Today 367 (2021) 177-188. DOI:10.1016/j.cattod.2020.04.066 |
| [10] |
H. Du, X. Ma, M. Jiang, P. Yan, Z.C. Zhang, Appl. Catal. A: Gen. 598 (2020) 117598. DOI:10.1016/j.apcata.2020.117598 |
| [11] |
H. Du, X. Ma, P. Yan, et al., Fuel Process. Technol. 193 (2019) 221-231. DOI:10.1016/j.fuproc.2019.05.003 |
| [12] |
Y. Zhao, Y. Zhang, Y. Wang, et al., Appl. Catal. A: Gen. 539 (2017) 59-69. DOI:10.1016/j.apcata.2017.04.001 |
| [13] |
Z. Wang, Z. Xu, S. Peng, et al., ACS Catal. 5 (2015) 4255-4259. DOI:10.1021/acscatal.5b00682 |
| [14] |
F. Dong, G. Ding, H. Zheng, et al., Catal. Sci. Technol. 6 (2016) 767-779. DOI:10.1039/C5CY00857C |
| [15] |
B. Li, L. Li, H. Sun, C. Zhao, ACS Sustain, Chem. Eng. 6 (2018) 12096-12103. DOI:10.1021/acssuschemeng.8b02425 |
| [16] |
Y. Zhao, S. Li, Y. Wang, et al., Chem. Eng. J. 313 (2017) 759-768. DOI:10.1016/j.cej.2016.12.027 |
| [17] |
X. Wang, M. Chen, X. Chen, et al., J. Catal. 383 (2020) 254-263. DOI:10.1016/j.jcat.2020.01.018 |
| [18] |
X. Zheng, H. Lin, J. Zheng, X. Duan, Y. Yuan, ACS Catal. 3 (2013) 2738-2749. DOI:10.1021/cs400574v |
| [19] |
H. Du, X. Ma, M. Jiang, et al., Catal. Today 365 (2021) 265-273. DOI:10.1016/j.cattod.2020.04.009 |
| [20] |
J. Huang, Y. Yan, S. Saqline, W. Liu, B. Liu, Appl. Catal. B: Environ. 275 (2020) 119109. DOI:10.1016/j.apcatb.2020.119109 |
| [21] |
Z. Wang, Z. Xu, M. Zhang, et al., RSC Adv. 6 (2016) 25185-25190. DOI:10.1039/C6RA02929A |
| [22] |
C. Chen, L. Lin, R. Ye, et al., Fuel 290 (2021) 120083. DOI:10.1016/j.fuel.2020.120083 |
| [23] |
J. Zheng, J. Zhou, H. Lin, et al., J. Phys. Chem. C 119 (2015) 13758-13766. DOI:10.1021/acs.jpcc.5b03569 |
| [24] |
X. Han, Q. Zhang, F. Feng, et al., Chin. Chem. Lett. 26 (2015) 1150-1154. DOI:10.1016/j.cclet.2015.04.031 |
| [25] |
S. Chen, R. Wojcieszak, F. Dumeignil, E. Marceau, S. Royer, Chem. Rev. 118 (2018) 11023-11117. DOI:10.1021/acs.chemrev.8b00134 |
| [26] |
R. Ye, L. Lin, Q. Li, et al., Catal. Sci. Technol. 8 (2018) 3428-3449. DOI:10.1039/C8CY00608C |
| [27] |
F. Hao, J. Zheng, S. He, et al., Catal. Commun. 151 (2021) 106266. DOI:10.1016/j.catcom.2020.106266 |
 2022, Vol. 33
2022, Vol. 33