b Department of Applied Chemistry, College of Environmental and Chemical Engineering, Nanchang Hangkong University, Nanchang 330063, China;
c Department of Chemistry, Texas A & M University, College Station, Texas 77843, United States;
d School of Resources, Environmental and Chemical Engineering, Nanchang University, Nanchang 330031, China
With the rapid development of human society, the increasing discharge of pollutants into the environment poses a serious hazard to all living things. Organic pollutants and heavy metals are among the most concerning contemporary contaminants due to their high toxicity and persistence in the environment. Consequently, methods for the detection and removal of these pollutants are important. Colorimetric detection has attracted much attention because it is intuitive and requires relatively simple instrumentation. Adsorption is widely used to remove heavy metals, and its low-cost and practicality make it suitable for use in rural and remote areas [1, 2]. The photocatalytic removal of organic pollutants has seen rapid development in recent years due to its low cost, environmental-friendliness, and ease of operation [3-6].
Organic pollutants and heavy metals are two important classes of contaminants [7-9], and remediation strategies that only target one type of them are usually ineffective in treating complex pollution systems. Consequently, different methods are typically coupled together as a broad treatment strategy. However, the multiple treatment steps and materials employed result in tedious operations and high costs [10-12]. In this context, the emergence of novel multifunctional materials that couple high performance adsorption, detection and photocatalysis are exciting prospects for the future of environmental remediation technology. Oxygen vacancy defects can confer materials with beneficial properties, such as plentiful adsorption active sites, enhanced conductivity and suppression of electron-hole pair (e−-h+) recombination. Accordingly, they have been widely engineered into adsorbents [13], detectors [14] and photocatalysts [15]. With this potential in mind, we envisaged that the introduction of oxygen vacancies could endow a material with the multifunctionality of adsorption, detection and photocatalysis.
With the aim to effectively treat organic and heavy metal contaminants, an oxygen-vacancy-rich nanocomposite of phenanthroline (Phen) modified TiO2 (Phen/TiO2) was synthesized by a facile single-step hydrothermal method. Methyl orange (MO) and Cr3+ were selected as the organic and heavy metal model contaminants due to their toxicity and ubiquity.
Scanning electron microscopy and transmission electron microscopy were used to analyze the nanoparticle morphology of Phen/TiO2 (Figs. S2a and b in Supporting information). The elemental mapping analysis (Figs. S2c-g in Supporting information) demonstrated that C, N, O and Ti were evenly distributed on the Phen/TiO2 surface, suggesting the successful integration of Phen and TiO2. The high-resolution X-ray photoelectron spectroscopy (XPS) spectra of the Ti 2p and O 1s regions of TiO2 and Phen/TiO2 exhibited a significant binding energy decrease in both Ti 2p and Ti−O of Phen/TiO2 (Figs. 1a and b). This phenomenon indicated the existence of oxygen vacancies in Phen/TiO2 [16]. The additional e− remaining after the O atoms were removed from the surface of TiO2 increases the e− cloud density around the Ti and O atoms close to the oxygen vacancies, thus decreasing the binding energies of Ti and O [16]. The N 1s high-resolution XPS spectrum of Phen/TiO2 could be resolved into three peaks at 397.49, 399.27 and 400.20 eV, corresponding to Ti–N, C=N and N–O, respectively (Fig. 1c) [17-18]. The N–O and C=N originated from adventitious organic compounds and phenanthroline, respectively. The N-Ti bond energies of Phen/TiO2 indicate that the N atoms in phenanthroline were coordinated with the Ti atoms of TiO2. This interaction between N and Ti may reduce the strength of the O−Ti bond in TiO2, allowing the O atoms to leave, thereby promoting the formation of oxygen vacancies. To further confirm the existence of oxygen vacancies in Phen/TiO2, an electron paramagnetic resonance (EPR) spectroscopy investigation was carried out. Fig. 1d shows the EPR spectra of Phen/TiO2 and TiO2. The EPR spectrum of Phen/TiO2 exhibited a strong signal at g = 2.003, which was absent for TiO2. This signal could be attributed to the e− trapped in the oxygen vacancies and is strong evidence of their existence [19-20].

|
Download:
|
| Fig. 1. High-resolution of XPS in the (a) Ti 2p, (b) O 1s regions of TiO2 and Phen/TiO2. (c) High-resolution of XPS in the N 1s region of Phen/TiO2. (d) EPR spectra of Phen/TiO2 and TiO2. | |
It was anticipated that Phen/TiO2 would adsorb Cr3+ via chelation with the Lewis base N centers of Phen along with the electrostatic attraction from hydroxyl groups on the surface of TiO2. The coordination event could be transduced into a colorimetric signal via metal-ligand charge transfer allowing the nanocomposite to operate as a chemosensor and an adsorbent. The isothermal and kinetic studies of Cr3+ adsorption by Phen/TiO2 are given in Figs. S3 and S4 (Supporting information), and Tables S1 and S2 (Supporting infromation); the experimental details are given in Supporting information. The maximum adsorption capacity of Cr3+ was calculated to be 12.76 mg/g based on the Langmuir isotherm model, which is comparable with those of reported benchmark adsorbents for Cr3+ (Table S3 in Supporting Infromation). The kinetic study revealed pseudo-first order kinetic behavior (rate constant, k = 1 × 10−2 g mg−1 min−1) depending on the concentrations of Cr3+ and Phen/TiO2. In mixture of co-existing ions, Phen/TiO2 exhibited high adsorption ability towards Cr3+, indicating its highly selective adsorption performance (Fig. S8 in Supporting infromation).
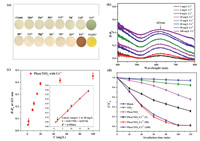
To demonstrate the qualitative detection capability of Phen/TiO2 towards Cr3+, the nanocomposite was used to adsorb different metal ions, and its color-change was observed visually. Fig. 2a shows that the color of the Phen/TiO2 nanocomposite was light yellow. When it captured Cr3+, its color rapidly changed to green. The color-change was highly specific to Cr3+. Other metal ions, such as Mn2+, Zn2+, Pb2+, Co2+, Ag+, Cd2+, Bi2+, Ce2+, Hg2+, In2+, Ni2+, and Zr2+ did not induce a color change, while Fe3+ and Cr2O72− induced color changes of red and bright yellow, respectively, even at concentrations five times than that of Cr3+. These results demonstrated that Cr3+ could be visually and qualitatively detected by Phen/TiO2. In order to quantitatively detect Cr3+, Phen/TiO2 was used to adsorb varying concentrations of Cr3+. The chroma of Cr3+ adsorbed on Phen/TiO2 was analyzed using visible-light difference diffuse reflectance spectroscopy (DRS). Fig. 2b shows the absorbance peak at λ = 621 nm from the diffuse reflectance spectra (DRS) of Cr3+ adsorbed Phen/TiO2 at pH 7 and a temperature of 30 ℃; the intensity of the peak at 621 nm increased with increasing concentration of Cr3+. The intensity of the peak at 621 nm in the difference DRS was plotted against the Cr3+ concentration (Fig. 2c) and a linear relationship (calibration curve) was obtained over the concentration range 1-20 mg/L (insert in Fig 2c). Using this method, the Cr3+ concentration could be quantitatively determined from the color change of Phen/TiO2 measured with DRS. The limit of detection (LD) for Cr3+ was estimated to be 0.42 mg/L from the blank response according to Eq. 1:
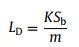
|
(1) |
|
Download:
|
| Fig. 2. (a) Photographic images of Phen/TiO2 powders after being treated with different metal ions. Mn2+, Zn2+, Pb2+, Co2+, Ag+, Bi2+, Ce2+, Hg2+, In2+, Ni2+, Zr2+, Fe3+, Cr2O72− and Cd2+: 100 mg/L; Cr3+: 20 mg/L; Adsorbent dosage: 1 g/L. (b) Concentration-dependent changes in visible difference DRS of Phen/TiO2 during the detection of Cr3+. (c) Concentration-dependent changes at λ = 621 nm (Adsorbent dosage was 1 g/L; The concentrations of Cr3+ were from 1 mg/L to 100 mg/L. Temperature was 30 ℃, pH was 7. The experiments were performed in triple and the related error bars are indicated). (d) Photocatalytic degradation of MO over the bare TiO2, Phen/TiO2 and Phen/TiO2-Cr3+ (The photocatalyst dosage was 1 g/L and the concentration of MO was 10 mg/L, room temperature.). | |
where K = 3, Sb was the standard deviation (SD) for the blank, and m was the slope of the linear calibration curve. The influence of pH, temperature and co-existing ions towards Phen/TiO2 detecting Cr3+ are shown in Figs. S14-S16 (Supporting infromation).
To verify the accuracy of the colorimetric Cr3+ analysis obtained using the Phen/TiO2 nanomaterial, the results were compared with those obtained by atomic absorption spectrometry (AAS) (Table S4 in Supporting Infromation). The relative standard deviation (SD) of the difference between the absolute values obtained by each method were < 3%, confirming the accuracy of the Phen/TiO2-based method.
The bright color exhibited by the Cr3+ adsorbed Phen/TiO2 (Phen/TiO2-Cr3+) indicated that the nanomaterial may possess enhanced light absorption capacity. It was envisioned that this feature could be exploited to enhance the photocatalytic activity, which could be applied to the degradation of organic pollutants. In this way, the adsorbed Cr3+ could act as a synergistic photocatalyst for the remediation of organic pollutants. To test this hypothesis, Phen/TiO2 was pre-adsorbed with 1, 20, 50 and 100 mg/L of Cr3+ and used to photocatalytically degrade MO under visible light irradiation (Fig. 2d). Phen/TiO2 exhibited higher photocatalytic capacity than TiO2, indicating that the nanomaterial had enhanced visible light activity. Adsorption of Cr3+ onto Phen/TiO2 further improved the photocatalytic activity. The photocatalytic degradation of MO increased as the Cr3+ concentration increased from 1 mg/L to 50 mg/L, reaching a plateau beyond 50 mg/L. Presumably the hyperchromic effect resulting from Cr3+ adsorption was almost saturated at a Cr3+ concentration of 50 mg/L, so further adsorption of Cr3+ had no additional influence. At an optimum Cr3+ adsorption of 50 mg/L, Phen/TiO2-Cr3+ could degrade ~98% of 10 mg/L MO solution within 100 min.
The X-ray diffraction (XRD) patterns of the crystal phases of TiO2, Phen/TiO2, and Phen/TiO2-Cr3+ are shown in Fig. S5 (Supporting infromation). The results showed that the incorporation of Phen changed the crystal phase of TiO2 from pure anatase to mixed phases of anatase, rutile and brookite. Mixtures of different crystal phases of TiO2 were shown to enhance visible light absorption due to the increased probability of forming heterojunctions in the TiO2, thereby boosting the related photocatalytic performance [21]. To understand the mechanism of the increased photocatalytic activity demonstrated by the nanomaterial, the photo-electric properties of Phen/TiO2 and Phen/TiO2-Cr3+ were characterized. Fig. 3a shows that the dimensions of the light absorption edges of Phen/TiO2, Phen/TiO2-Cr3+ (1), Phen/TiO2-Cr3+ (20), Phen/TiO2-Cr3+ (50) and Phen/TiO2-Cr3+ (100) were 408, 417, 423, 431 and 428 nm, respectively; (1), (20), (50) and (100) refer to the concentrations (mg/L) of adsorbed Cr3+. The hyperchromicity resulting from the adsorption of Cr3+ produced a redshift of the absorption edge of Phen/TiO2. However, when the concentration of Cr3+ was > 50 mg/L the hyperchromicity was saturated and the redshift of the adsorption edge ceased. These observations were consistent with the results of the photocatalytic degradation experiments. The nanomaterials were additionally characterized by electrochemical impedance spectroscopy (EIS) (Fig. 3b). Phen/TiO2 exhibited the highest impedance. The impedance decreased with the addition of Cr3+ over the concentration range 1-50 mg/L but increased at concentrations > 50 mg/L Cr3+. In other words, Phen/TiO2 in the presence of 50 mg/mL Cr3+ exhibited the smallest impedance among the samples, suggesting that the adsorption of Cr3+ significantly decreased the impedance of Phen/TiO2 [22]. The photocurrent (PC) spectroscopy responses and photoluminescence (PL) spectra of the samples are shown in Figs. 3c and d. Compared with EIS, the PC responses and PL spectra exhibited similar trends following Cr3+ adsorption by Phen/TiO2. The corresponding photo-electronic properties of the samples increased with the increasing Cr3+ adsorption to reach maximum values at a Cr3+ concentration of 50 mg/L and decreased thereafter. The magnitude of the current density in PC spectra reflects the number of e− produced in the sample by light irradiation [23]. In PL spectra, a low fluorescence intensity is indicative of the slow recombination rate of photo-generated charge carriers [24]. Phen/TiO2-Cr3+(50) had the highest PC density and lowest PL intensity.

|
Download:
|
| Fig. 3. (a) DRS of Phen/TiO2 and Phen/TiO2-Cr3+. (b) Electrochemical impedance spectroscopy of Phen/TiO2 and Phen/TiO2-Cr3+. (c) Photocurrent responses of Phen/TiO2 and Phen/TiO2-Cr3+ under visible light. (d) Photoluminescence spectra of Phen/TiO2 and Phen/TiO2-Cr3+. The values in the brackets mean the feeding concentration (mg/L) of Cr3+ in the materials preparation. | |
To determine the active species in the degradation of MO, various scavengers were added to the photocatalysis system. The results given in Fig. S6 (Supporting infromation) indicated that h+ and •O2− are the important active species in the degradation of MO while e− and •OH play a minor role.
Based on these results, a mechanism for the photocatalytic degradation of MO by Phen/TiO2-Cr3+ under visible light was proposed: Although pure anatase TiO2 cannot be excited under visible light, the incorporation of Phen modified the crystal phase of TiO2 from pure anatase to mixed phases of anatase, rutile and brookite; visible light absorbed by the modified phase could now excite e− from the valence band (VB) to the conduction band (CB) leaving h+ (on the VB) [21, 25]. Furthermore, the hyperchromic effect resulting from the N-chelation of Cr3+ by Phen enhanced the absorption of visible light, while also decreasing the impedance, which facilitated the photo-generation of charge carriers and depressed the recombination of charge carriers, all boosting the photocatalytic performance [26]. The photo-generated e− in the CB could then react with O2 to produce •O2−, which oxidized and degraded MO. The photo-generated h+ on the VB could also oxidize and degrade the pollutant.
An oxygen-vacancy-rich nanocomposite of Phen/TiO2, integrating the functions of detection, adsorption, and photocatalytic degradation of pollutants, was successfully prepared by a facile one-step hydro-thermal method. Phen/TiO2 could simultaneously adsorb and detect Cr3+ while the adsorbed heavy metal could synergistically enhance the photocatalytic degradation of MO under visible light irradiation. The integrated multi-functionality of the Phen/TiO2 demonstrated that these nanomaterials may offer a credible and efficient alternative to traditional adsorbents used for the remediation of complex pollutants. The strategy developed here may also provide a new outlook for the design of functional materials for practical applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe project is supported by the National Natural Science Foundation of China (Nos. 51978323, 42077162), the Key Research and Development Project of Jiangxi Province (No. 20203BBGL73229), and the Natural Science Foundation of Jiangxi Province (No. 20192ACBL20042).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.002.
| [1] |
Q. Wang, P. Chen, X. Zeng, J. Hazard. Mater. 381 (2020) 120954. DOI:10.1016/j.jhazmat.2019.120954 |
| [2] |
D. Kang, X. Yu, M. Ge, Chem. Eng. J. 330 (2017) 36-43. DOI:10.1016/j.cej.2017.07.140 |
| [3] |
X.F. Wu, Y. Sun, H. Li, J. Alloys Compd. 740 (2018) 1197-1203. DOI:10.1016/j.jallcom.2018.01.100 |
| [4] |
H. Jiang, X. Li, M. Li, Chem. Eng. J. 358 (2019) 58-66. DOI:10.1016/j.cej.2018.09.199 |
| [5] |
S. Zhang, J. Yi, J. Chen, Chem. Eng. J. 380 (2020) 122583. DOI:10.1016/j.cej.2019.122583 |
| [6] |
C. Cheng, S. Zong, J. Shi, Appl. Catal. B: Environ. 265 (2020) 118620. DOI:10.1016/j.apcatb.2020.118620 |
| [7] |
C. Liu, Y. Ding, W. Wu, Y. Teng, Chem. Eng. J. 306 (2016) 22-30. DOI:10.1016/j.cej.2016.07.043 |
| [8] |
F. Li, J. Huang, Q. Xia, Sep. Purif. Technol. 195 (2018) 83-91. DOI:10.1016/j.seppur.2017.11.058 |
| [9] |
W. Ding, J. Shi, W. Wei, C. Cao, H. Jiu, Int. J. Hydrog. Energy 46 (2021) 2899-2904. DOI:10.1016/j.ijhydene.2020.05.084 |
| [10] |
P.A. Neale, C. Feliers, L. Glauch, Environ. Sci. Wat. Res. 9 (2020) 2444-2453. DOI:10.1039/c9ew00987f |
| [11] |
J.J. Alvear-Daza, J. Sanabria, H.M. Gutiérrez-Zapata, J.A. Rengifo-Herrera, Sol. Energy 176 (2018) 581-588. DOI:10.1016/j.solener.2018.10.070 |
| [12] |
M. Li, S. Zhou, Y. Xu, Chem. Eng. J. 334 (2018) 1621-1629. DOI:10.1016/j.cej.2017.11.144 |
| [13] |
13.F. Ma, J. Yao, Y. Zhang, Y Wei, Chin. Chem. Lett. 29 (2018) 1689-1691. DOI:10.1016/j.cclet.2017.12.016 |
| [14] |
Z. Ni, S. Bao, X. Gong, Chin. Chem. Lett. 31 (2020) 1674-1679. DOI:10.1016/j.cclet.2019.10.027 |
| [15] |
X. Xie, Q.U. Hassan, H. Lu, Chin. Chem. Lett. 32 (2021) 2038-2042. DOI:10.1016/j.cclet.2020.10.002 |
| [16] |
Z. Hu, K. Lia, X. Wu, Appl. Catal. B: Environ. 256 (2019) 117860. DOI:10.1016/j.apcatb.2019.117860 |
| [17] |
K. Batalovic´, N. Bundaleski, J. Radakovic´, Phys. Chem. Chem. Phys. 19 (2017) 7062. DOI:10.1039/C7CP00188F |
| [18] |
Y. Wang, C. Feng, M. Zhang, J. Yang, Z. Zhang, Appl. Catal. B: Environ. 100 (2010) 84-90. DOI:10.1016/j.apcatb.2010.07.015 |
| [19] |
Z. Sun, R. Huo, C. Choi, Nano Energy 62 (2019) 869-875. DOI:10.1016/j.nanoen.2019.06.019 |
| [20] |
B. Wang, M. Zhang, X. Cui, Angew. Chem. Int. Ed. 132 (2020) 1628-1635. DOI:10.1002/ange.201910471 |
| [21] |
Y. Chu, L. Gu, H. Du, Int. J. Hydrog. Energ. 43 (2018) 21810-21823. DOI:10.1016/j.ijhydene.2018.09.147 |
| [22] |
J. Chen, J. Zhan, Y. Zhang, Y. Tang, Chin. Chem. Lett. 30 (2019) 735-738. DOI:10.3390/met9070735 |
| [23] |
H. Jiang, J. Liu, M. Li, Chinese J. Catal. 39 (2018) 747-759. DOI:10.1016/S1872-2067(18)63038-4 |
| [24] |
H. Jiang, M. Li, J. Liu, Ceram. Int. 44 (2018) 2709-2717. DOI:10.1016/j.ceramint.2017.10.225 |
| [25] |
K. Chalastara, F. Guo, S. Elouatik, Catalysts 10 (2020) 407. DOI:10.3390/catal10040407 |
| [26] |
Y. Shen, C. Zhu, B. Chen, Environ. Sci. Nano 7 (2020) 1525-1538. DOI:10.1039/d0en00120a |
 2022, Vol. 33
2022, Vol. 33 

