Chiral compounds, especially the enantiomers of chiral drugs, may have great differences in their metabolism, pharmacodynamics and pharmacology, and therefore, the resolution of chiral compounds is of great significance [1]. However, it is difficult to separate enantiomers because of their similar physical and chemical properties [2]. Chromatographic techniques based on a chiral stationary phase (CSP) are some of the most attractive methods used to separate and obtain enantiomerically pure compounds [3, 4]. At present, chiral immobilization using cyclodextrins, polysaccharides, crown ethers, glycopeptides and other classical chiral material has been widely used [5]. However, with the development of materials science, especially the rapid development of chiral porous materials, many new CSPs, such as metal-organic frameworks (MOFs) [6, 7], porous organic cages (POCs) [8, 9], metal-organic cages (MOCs) [10, 11] and microporous organic networks (MONs) [12] have also received a great deal of attention.
Covalent organic frameworks (COFs) are a new class of crystalline organic polymer materials with highly ordered porous structures. Recently, COFs have become a research hotspot because of their large specific surface area, excellent thermal and chemical stability and predesignable structures [13]. The applications of COFs include gas storage and adsorption [14, 15], catalysis [16], biosensing [17], energy storage [18], separation [19], and drug delivery [20], among others [21, 22]. Recently, COFs have been explored as potential CSPs for chiral chromatographic separation [23]. For example, Han et al. [24] demonstrated the synthesis of 3D chiral COFs using imine condensation used as CSPs in HPLC for the separation of racemic alcohols with excellent repeatability and reproducibility. Qian et al. [25] reported a bottom-up strategy to synthesize chiral COFs used in gas chromatography (GC). These chiral COF-bound capillary columns were synthesized via an in situ growth approach, which achieved the baseline separation of (±)-methyl lactate, (±)-1-phenyl ethanol, (±)-limonene and (±)-1-phenyl-1-propanol within 5 min. Ji's group [26] prepared a novel COF incorporated chiral polymer monolithic column for CEC analysis, which exhibited excellent enantioseparation performance for eight pairs of different classes of chiral drugs including β-blockers, antihistamines and anticoagulants. However, the development of CSP using chiral COFs is still in its infancy due to the difficulties observed in the synthesis of chiral COFs [27].
Herein, we report a β-CD-COF-coated capillary column used for the GC separation of compounds. Although this material has been used in selective molecular adsorption and as a CSP in capillary electrochromatography, its application in GC has been ignored [28, 29]. β-CD-COF was synthesized using heptakis(6-amino-6-deoxy)-β-CD (Am7CD) and terephthalaldehyde (TPA) in an acetic acid solution [30]. Benefitting from the integration of β-CD chiral units, the CSP not only had permanent chiral space, but also exhibited high thermal stability and a large surface area. Ultimately, a variety of compounds, including linear alkanes, linear alcohols, fatty acid methyl esters, the Grob mixture, positional isomers and racemates were successfully separated on a β-CD-COF-coated open tubular column. This work will provide a new reference for the application of chiral COFs in gas chromatography.
The β-CD-COF was synthesized according to the method in the literature with some modifications (Supporting information). β-CD-COF was characterized using fourier transformed infrared (FTIR), powder X-ray powder diffraction (PXRD), thermo gravimetric analysis (TGA) and scanning electron microscopy (SEM). Fig. 1a shows the FT-IR spectra of β-CD-COF and Am7CD exhibit characteristic bands corresponding to C‒H at 1047 and 1153 cm–1, respectively. The intense peaks observed at 1565, 1644 and 1697 cm–1 were attributed to the stretching vibrations of the C=N bond, confirming that the covalent bond was successfully formed. The PXRD data (Fig. 1b) obtained for β-CD-COF exhibited prominent diffraction peaks at 6.72°, 11.54°, 13.16°, 17.56°, 20.04° and 24.08°, which was in good agreement with the simulated spectra data reported in the literature, and indicated the successful synthesis of β-CD COF [30]. The TGA curve showed that the β-CD-COF crystals were stable up to 230 ℃ (Fig. 1c), indicating its suitability as a GC stationary phase. The morphology of the β-CD-COF crystals and COF-coated column were characterized using SEM. Fig. 1d shows that β-CD-COF exhibits an aggregated short rod-like morphology, which was likely due to the inhibition of crystal growth upon stirring. The SEM images of the column cross-section (Figs. 1e and f) show that the COF-coated capillary column was successfully prepared and β-CD-COF was dispersed on the inner wall of the capillary column.

|
Download:
|
| Fig. 1. Characterization of β-CD-COF and the β-CD-COF coated column. (a) FT-IR spectrum, (b) PXRD pattern and (c) TGA curve and (d) SEM image obtained for β-CD-COF. (e) and (f) SEM images of the cross-section of the β-CD-COF coated capillary column. | |
The column efficiency and McReynolds constants are basic parameters used to determine the overall performance of a chromatographic column [31, 32]. The column efficiency of the β-CD-COF-coated capillary column was obtained using n-dodecane as the test compound at 120 ℃ and the number of theoretical plates was determined to be 2700 plates/m at the optimal flow rate. The McReynolds constants of the β-CD-COF-coated capillary column were evaluated using benzene (X′), 1-butanol (Y′), 2-pentanone (Z′), 1-nitropropane (U′) and pyridine (S′) as probe molecules. Table 1 gives the McReynolds constants obtained for the five probe compounds, which show that the polarity of the β-CD-COF-coated capillary column was moderate. The elution sequence of the probes was benzene, 2-pentanone, 1-butanol, nitropropane and pyridine.
|
|
Table 1 McReynolds constants obtained for the β-CD-COF-coated column at 120 ℃. |
In this work, linear alkanes, linear alcohol, fatty acid methyl esters and the Grob mixture were chosen as test compounds for separation on the β-CD-COF-coated column [33]. Fig. 2 shows that all of these compounds were well separated on the β-CD-COF-coated capillary column. Figs. 2a–c show that baseline separation was achieved for linear alkanes (n-C12 to n-C17) and linear alcohols (n-C8-OH to n-C12-OH) on the stationary phase, as well as a mixture of fatty acid methyl esters (C6:0 to C10:0) with slight tailing; thus, indicating that this CSP exhibits good resolution performance for these mixtures. The separation performance of the β-CD-COF-coated capillary column for the Grob mixture was also tested. Fig. 2d shows the β-CD-COF-coated capillary column achieved fair resolution and peak shapes for most of the analytes except the obvious tailing and overlapping of 2, 6-xylenol and 2, 6-dimethylaniline. These results proved that β-CD-COF has the potential to be used as a stationary phase in GC.

|
Download:
|
| Fig. 2. GC chromatograms obtained using the β-CD-COF-coated capillary column for: (a) Linear alkanes and (b) linear alcohols (temperature program: 50 ℃ for 1 min, followed by heating to 200 ℃ at 30 ℃/min under a flow of 1.0 mL/min N2); (c) fatty acid methyl ester mixture (Peaks: (1) methyl caproate, (2) methyl heptanoate, (3) methyl octanoate, (4) methyl nonanoate and (5) methyl caprate; temperature program: 50 ℃ for 1 min, followed by heating to 150 ℃ at 20 ℃/min under a flow of 1.0 mL/min N2); (d) Grob mixture (Peaks: (1) decane, (2) undecane, (3) 1-octanol, (4) 2, 6-dimethylphenol, (5) 2, 6-dimethylaniline, (6) methyl decanoate, (7) methyl undecanoate and (8) methyl laurate; temperature program: 50 ℃ for 1 min, followed by heating to 200 ℃ at 30 ℃/min under a flow of 0.5 mL/min N2). | |
The β-CD-COF-coated capillary column provided good resolution and selectivity during the separation of alkylbenzenes and positional isomers (Fig. 3). Fig. 3a shows a mixture of alkylbenzenes containing four compounds (toluene, ethylbenzene, n-propylbenzene and n-butylbenzene) that were separated on the β-CD-COF-coated column and eluted in the order of their boiling points. Figs. 3b–f show the separation of the positional isomers of ionone, nitroaniline, trichlorobenzene, dichlorobenzene and dibromobenzene on the β-CD-COF-coated column. The elution sequence of the nitroaniline, trichlorobenzene and dibromobenzene isomers followed the order of their boiling points, for example 2-nitroaniline (284 ℃) < 3-nitroaniline (306 ℃) < 4-nitroaniline (332 ℃). The broader peaks observed for the positional isomers of dichlorobenzene and dibromobenzene may be attributed to the stronger dipole-dipole interactions formed between the isomers and β-CD-COF.

|
Download:
|
| Fig. 3. GC chromatograms obtained using the β-CD-COF-coated capillary column for the separation of alkylbenzenes and positional isomers: (a) alkylbenzenes (1-toluene; 2-ethylbenzene; 3-propylbenzene; 4-butylbenzene; temperature program: 50 ℃ for 1.0 min, followed by heating to 200 ℃ at 30 ℃/min under a flow of 0.5 mL/min N2), (b) α-/β-ionone (N2 flow rate of 1.0 mL/min at 150 ℃); (c) o-, m- and p-nitroaniline (N2 flow rate of 1.0 mL/min at 200 ℃); (d) 1, 2, 3-, 1, 2, 4- and 1, 3, 5-trichlorobenzene (N2 flow rate of 1.0 mL/min at 130 ℃); (e) o-, m- and p-dichlorobenzene (N2 flow rate of 0.5 mL/min at 50 ℃); (f) o-, m- and p-dibromobenzene (N2 flow rate of 1.0 mL/min at 75 ℃). | |
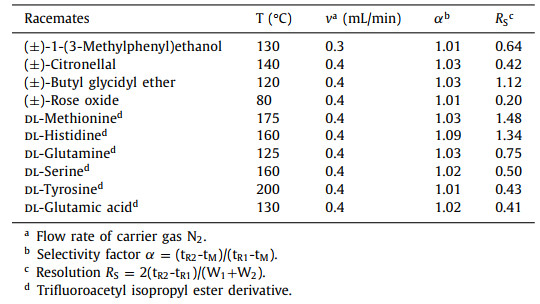
To evaluate the chiral recognition capability of the β-CD-COF-coated capillary column, a variety of racemates were used as probe compounds. The following ten racemates, including chiral alcohols, aldehydes, ethers, and amino acid derivatives, were separated on the column: 1-(3-methylphenyl) ethanol, citronellal, butyl glycidyl ether, (±)-rose oxide, dl-methionine derivative, dl-histidine derivative, dl-glutamine derivative, dl-serine derivative and dl-tyrosine derivative (molecular structures see Fig. S1 in Supporting information). The chromatograms are shown in Fig. 4 and the details are provided in Table 2. These results demonstrate that the β-CD-COF stationary phase displays good chiral recognition ability in GC.

|
Download:
|
| Fig. 4. GC chromatograms obtained using the β-CD-COF-coated capillary column for the separation of racemates: (a) (±)-1-(3-methylphenyl)ethanol, (b) (±)-citronellal, (c) (±)-butyl glycidyl ether, (d) (±)-rose oxide, (e) dl-methionine, (f) dl-histidine, (g) dl-glutamine, (h) dl-serine; (i) dl-tyrosine and (j) dl-glutamic acid. The separation conditions are reported in Table 2. | |
|
|
Table 2 Separation of racemates on the β-CD-COF-coated column. |
The chiral recognition mechanism on a CSP is usually complicated because the molecular recognition process is often the result of a variety of interactions [25]. Such as hydrogen bonds, dipole-dipole, van der Waals, π-π interactions as well as a steric effect [34, 35]. Besides, another outstanding feature of the cyclodextrin stationary phase was it can form inclusion complexes with a wide variety of guest compounds [36].
In order to demonstrate that the steric effect of β-CD-COF will affect the separation of enantiomers, Am7CD was used as a stationary phase for the control group to separate the racemates mentioned above. However, all 10 racemates could not be separated on this column (Fig. S2 in Supporting information). This difference of separation performance would be due to the cyclodextrin units of β-CD-COF crystal materials were arranged more ordered (Fig. S3 in Supporting information), similar as to the ordered cyclodextrin liquid crystal has better recognition ability [37]. In addition, the asymmetry of cyclodextrin ligands was enhanced by the formation of imine bond (-N=H-) may be another reason for the better resolution ability of β-CD-COF [38]. These results indicate that the chiral microenvironment of the β-CD-COF crystals is important for the chiral separation of racemates.
The reproducibility and stability are also important indexes to evaluate a chromatographic column. Here, α-/β-ionone isomers and a DL-glutamine derivative were used as probe compounds to characterize the stability of the chromatographic column. Fig. S4 (Supporting information) shows that the retention times of the analytes exhibited no significant changes after multiple injections (> 200). The thermal stability was be tested at 250 ℃ and shown in Fig. S5 (Supporting information). The smooth baseline revealed the β-CD-COF-coated column was well stabilized. These results indicate the good reproducibility and stability of the β-CD-COF-coated column for GC separations. These characteristics are helpful to explore the practical applications of chiral COFs as new chiral stationary phases used in GC.
In conclusion, we have reported a chiral COF based on β-CD as a novel CSP for gas chromatography separation. The β-CD-COF-coated capillary column showed excellent selectivity, not only for the separation of linear alkanes, linear alcohols, fatty acid methyl ester mixtures, the Grob mixture and positional isomers, but also for the resolution of chiral compounds, including chiral alcohols, aldehydes, ethers, and amino acid derivatives. In addition, β-CD-COF exhibits stronger chiral recognition ability and stability than Am7CD. The results show that as a new type of chiral stationary phase, chiral COFs have broad application prospects for enantiomeric separation in gas chromatography.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Key R&D Program of China (No. 2019YFA0904104). We are grateful to the Analysis & Testing Center of Beijing Institute of Technology, the Experimental Center of Advanced Materials of Beijing Institute of Technology and the Biological & Medical Engineering Core Facilities of Beijing Institute of Technology for their support with the characterization of materials.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.06.089.
| [1] |
M.M.M. Pinto, C. Fernandes, M.E. Tiritan, Molecules 25 (2020) 1931. DOI:10.3390/molecules25081931 |
| [2] |
Q. Wu, H. Lv, L. Zhao, TrAC Trend. Anal. Chem. 129 (2020) 115941. DOI:10.1016/j.trac.2020.115941 |
| [3] |
M. Zhou, Y. Long, Y. Zhi, et al., Chin. Chem. Lett. 29 (2018) 1399-1403. DOI:10.1016/j.cclet.2017.10.039 |
| [4] |
X. Wang, H. Li, K. Quan, et al., Talanta 225 (2021) 121987. DOI:10.1016/j.talanta.2020.121987 |
| [5] |
S.M. Xie, L.M. Yuan, J. Sep. Sci. 42 (2019) 6-20. DOI:10.1002/jssc.201800656 |
| [6] |
Z.R. Tao, J.X. Wu, Y.J. Zhao, et al., Nat. Commun. 10 (2019) 1-8. DOI:10.1038/s41467-018-07882-8 |
| [7] |
M.N. Corella-Ochoa, J.B. Tapia, H.N. Rubin, et al., J. Am. Chem. Soc. 141 (2019) 14306-14316. DOI:10.1021/jacs.9b06500 |
| [8] |
J.H. Zhang, S.M. Xie, L. Chen, et al., Anal. Chem. 87 (2015) 7817-7824. DOI:10.1021/acs.analchem.5b01512 |
| [9] |
J.H. Zhang, S.M. Xie, B.J. Wang, et al., J. Sep. Sci. 41 (2018) 1385-1394. DOI:10.1002/jssc.201701095 |
| [10] |
S.M. Xie, N. Fu, L. Li, et al., Anal. Chem. 90 (2018) 9182-9188. DOI:10.1021/acs.analchem.8b01670 |
| [11] |
B. Tang, C.Y. Sun, W. Wang, et al., Chirality 32 (2020) 1178-1185. DOI:10.1002/chir.23263 |
| [12] |
Y.Y. Cui, C.X. Yang, X.P. Yan, ACS Appl. Mater. Interfaces 12 (2020) 4954-4961. DOI:10.1021/acsami.9b22023 |
| [13] |
K. Geng, T. He, R. Liu, et al., Chem. Rev. 120 (2020) 8814-8933. DOI:10.1021/acs.chemrev.9b00550 |
| [14] |
L. Zhu, Y.B. Zhang, Molecules 22 (2017) 1149. DOI:10.3390/molecules22071149 |
| [15] |
Y. Ding, Y. Wang, Y. Su, et al., Chin. Chem. Lett. 31 (2020) 193-196. DOI:10.1016/j.cclet.2019.05.012 |
| [16] |
Y. Yusran, H. Li, X. Guan, et al., Energy Chem. 2 (2020) 100035. DOI:10.1016/j.enchem.2020.100035 |
| [17] |
S. Bhunia, K.A. Deo, A.K. Gaharwar, Adv. Funct. Mater. 30 (2020) 2002046. DOI:10.1002/adfm.202002046 |
| [18] |
M.X. Wu, Y.W. Yang, Chin. Chem. Lett. 28 (2017) 1135-1143. DOI:10.1016/j.cclet.2017.03.026 |
| [19] |
N. Liu, L. Shi, X. Han, et al., Chin. Chem. Lett. 31 (2020) 386-390. DOI:10.1016/j.cclet.2019.06.050 |
| [20] |
G. Zhang, X. Li, Q. Liao, et al., Nat. Commun. 9 (2018) 2785. DOI:10.1038/s41467-018-04910-5 |
| [21] |
D. Rodríguez-San-Miguel, C. Montoro, F. Zamora, Chem. Soc. Rev. 49 (2020) 2291-2302. DOI:10.1039/c9cs00890j |
| [22] |
Y. Yan, X. Li, G. Chen, et al., Chin. Chem. Lett. 32 (2021) 107-112. DOI:10.1016/j.cclet.2020.11.063 |
| [23] |
S. Zhuo, X. Zhang, H. Luo, et al., Macromol. Rapid Commun. 41 (2020) 2000404. DOI:10.1002/marc.202000404 |
| [24] |
X. Han, J. Huang, C. Yuan, et al., J. Am. Chem. Soc. 140 (2018) 892-895. DOI:10.1021/jacs.7b12110 |
| [25] |
H.L. Qian, C.X. Yang, X.P. Yan, Nat. Commun. 7 (2016) 12104. DOI:10.1038/ncomms12104 |
| [26] |
S. Xu, Y. Wang, W. Li, et al., J. Chromatogr. A 1602 (2019) 481-488. DOI:10.1016/j.chroma.2019.06.018 |
| [27] |
X. Han, C. Yuan, B. Hou, et al., Chem. Soc. Rev. 49 (2020) 6248-6272. DOI:10.1039/d0cs00009d |
| [28] |
M. Ma, Y. Du, L. Zhang, et al., Microchim. Acta 187 (2020) 385. DOI:10.1007/s00604-020-04360-1 |
| [29] |
Y. Wang, S. Zhuo, J. Hou, et al., ACS Appl. Mater. Interfaces 11 (2019) 48363-48369. DOI:10.1021/acsami.9b16720 |
| [30] |
R.Q. Wang, X.B. Wei, Y.Q. Feng, Chem. Eur. J. 24 (2018) 10979-10983. DOI:10.1002/chem.201802564 |
| [31] |
Y. He, X. Yang, M. Qi, et al., Chin. Chem. Lett. 32 (2021) 2043-2046. DOI:10.1016/j.cclet.2020.11.009 |
| [32] |
J. He, M. Qi, Chinese Chem. Lett. 30 (2019) 1415-1418. DOI:10.1016/j.cclet.2019.03.008 |
| [33] |
Y. He, X. Yang, M. Qi, et al., Chin. Chem. Lett. 32 (2021) 2043-2046. DOI:10.1016/j.cclet.2020.11.009 |
| [34] |
Z.M. Wang, C.X. Yang, X.P. Yan, J. Chromatogr. A 1608 (2019) 460420. DOI:10.1016/j.chroma.2019.460420 |
| [35] |
G.K.E. Scriba, J. Chromatogr. A 1467 (2016) 56-78. DOI:10.1016/j.chroma.2016.05.061 |
| [36] |
N. Issaraseriruk, A. Shitangkoon, T. Aree, J. Mol. Graph. Model. 28 (2010) 506-512. DOI:10.1016/j.jmgm.2009.11.005 |
| [37] |
Y. Wang, Y. Sun, H. Bian, et al., ACS Appl. Mater. Interfaces 12 (2020) 45916-45928. DOI:10.1021/acsami.0c15836 |
| [38] |
T. Aree, J. Incl. Phenom. Macrocycl. Chem. 77 (2013) 439-445. DOI:10.1007/s10847-012-0264-z |
 2022, Vol. 33
2022, Vol. 33