b College of Chemistry, and Green Catalysis Center, Zhengzhou University, Zhengzhou 450001, China
Inspired by information technology, molecular logic gates are important molecular devices to generate outputs with one or multiple chemical or physical inputs [1,2]. Molecular logic gates could be constructed by covalent conjugation or supramolecular complexation [3–6]. Molecular logic gates have been used for information processing, drug delivery, and chemical or biological sensing [7–10].
Supramolecular complexation is a strategy to construct complex systems with simple building blocks [11–20]. To this date, it is challenging to develop supramolecular logic gates, which are operational in the complex biological environment. Researchers are interested in special physiological and pathological conditions, which are crucial for diagnostic and therapeutic uses. Mildly acidic condition and reactive oxygen species (ROSs) are two important biomarker signals. ROS is existing in tumor and inflammatory tissues [21,22]. Mildly acidic condition is often found in tumor extracellular environment or endosomal compartments [23]. Molecular or nanoscale structures responsive to acidic pH, ROS or other biomarker signals are valuable for disease diagnostics or the targeted delivery of pharmaceutical drugs [24,25]. Nevertheless, there are only limited examples of biological system-operational supramolecular logic gates [26].
To construct sophisticated supramolecular logic gates, the major challenge is to introduce two or multiple types of stimuli-responsiveness. We recently develop a type of stimuli-reactive host molecules, which could be used to construct stimuli-responsive supramolecular assemblies [27–30]. By introducing one type of stimuli-responsiveness to the host molecule and the other type of stimuli-responsiveness to the guest molecule, the resulting host-guest complexation-based molecular logic gate may be responsive to two input types. This supramolecular molecular logic gate will be composed of simple-to-synthesize building blocks. Host-guest interaction has been proved to be a useful method to build supramolecular assembly for biological applications [31,32]. Therefore, this new strategy combines simplicity in structure and complexity in function.
Here, we report a new strategy to construct host-guest complexation-based AND molecular logic gates. As a proof-of-concept system, this AND molecular logic gate is composed of an acid-labile acyclic cucurbit[n]uril (CB[n]) host molecule and a ROS-responsive guest dye. By introducing pH-responsiveness to the host molecule and ROS-responsiveness to the guest dye, supramolecular AND logic gate is conveniently assembled by modular design. In the presence of both chemical inputs, fluorescence output is turned on. Mechanistic study is carried out for AND logic gate. This supramolecular complex demonstrates operational AND logic gate in live human cancer cells, which may be used for sophisticated biological sensing.
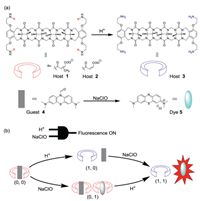
The design of supramolecular logic gate involves host-guest complexation between hosts 1, 2 and guest 4. As shown in Fig. 1a, hosts 1 and 2 are acid-labile acyclic CB[n] molecular containers, previously developed by us [28]. These host molecules undergo acid-induced conversion from anionic hosts 1 and 2 to cationic host 3. The degradation rate is tunable depending on different molecular structures: citraconic amide-based host 1 degrades faster than maleic amide-based host 2 under the same condition. For both host molecules, a higher degradation rate is achieved at a lower pH. Guest 4 is developed by the Yi group, which possesses stimuli-responsiveness towards ROS (NaClO): the presence of NaClO is capable of converting guest 4 to fluorescent dye 5 [33].
|
Download:
|
| Fig. 1. (a) Acid-induced conversion from anionic hosts 1 and 2 to cationic host 3, and NaClO-induced conversion from guest 4 to dye 5. (b) Schematic representation for AND logic gate, and the fluorescence "switch on" in the presence of both acidic condition and NaClO (1, 1). | |
We expect supramolecular complexes based on hosts 1-2 and guest 4 demonstrate AND logic gate. As shown in Fig. 1b, there are four types of input patterns: (1) in the absence of both low pH and NaClO (0, 0); (2) in the presence of NaClO (0, 1); (3) in the presence of low pH (1, 0); (4) in the presence of both low pH and NaClO (1, 1). When NaClO is absent (0, 0), guest 4 is non-fluorescent under our experimental condition (λex 620 nm, λem 690 nm), and no output is observed. Similarly, when only low pH is present (1, 0), guest 4 is unable to convert to be fluorescent dye 5, and the system is non-fluorescent. When only NaClO is present (0, 1), guest 4 is encapsulated by the host molecule, which inhibits NaClO-induced degradation and results in a slowed formation of fluorescent dye 5. Even if dye 5 is formed under this condition, this fluorescent dye is encapsulated by anionic host, and the fluorescence of the dye is switched off. Therefore, the sole logic operation to generate fluorescence output is (1, 1): acid-induced degradation of hosts 1 and 2 leads to release of guest 4, which allows it to convert to be fluorescent dye 5 in the non-complexed form.
First, we investigated host-guest interaction between hosts 1-3 and guest/dye 4 and 5. While guest 4 is poorly soluble in water, dye 5 has decent solubility in water for 1H NMR spectroscopy. As shown in Figs. S1 and S2 (Supporting information), upfield shifted proton resonances indicated supramolecular encapsulation of dye 5 by the cavity of hosts 1 and 2. In contrast, when mixed with host 3, proton resonances of dye 5 shifted downfield, indicating the supramolecular complexation was out of the cavity (Figs. S3 and S4 in Supporting information). The poor aqueous solubility of guest 4 renders it impossible for 1H NMR study. As shown in Figs. S7 and S8 (Supporting information), the addition of hosts 1 and 2 could significantly enhance the weak fluorescence of guest 4, which indicated supramolecular complexation. Direct UV-vis or fluorescence titration and indicator displacement assay were used to determine the value of binding constant Ka (Figs. S7-S16 in Supporting information). As summarized in Table 1, anionic hosts 1 and 2 could encapsulate guest 4 and dye 5 with modest affinity. The value of Ka for the complex of anionic host 3 and guest 4/dye 5 was smaller compared to that for hosts 1 and 2. Mildly acidic condition of pH 6.0 was also used to determine the binding affinity for hosts 1-3. Anionic hosts 1 and 2 could bind guest 4 and dye 5 at pH 6.0 with similar binding affinity as that at pH 7.4. The binding affinity between cationic host 3 and guest 4/dye 5 was also significantly weaker compared to that for hosts 1 and 2 at pH 6.0. Therefore, anionic hosts 1 and 2 could encapsulate guest 4 and dye 5 under neutral or acidic condition. After the acid-induced conversion from hosts 1 and 2 to cationic host 3, binding affinity was significantly reduced to release encapsulated guest or dye.
|
|
Table 1 Binding constant value (Ka, L/mol) determined for supramolecular complexation between hosts 1-3 and guest/dye 4-5. |
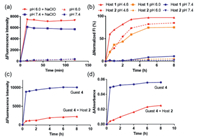
Next, AND molecular logic gate behavior was studied by fluorescence spectroscopy. Excess quantity of host molecule was used to ensure a high ratio of host-guest complexation. Guest 4 was incubated with host 1 (20 equiv.) at 37 ℃ under four different conditions: (1) phosphate buffered saline (PBS, pH 7.4) (0, 0); (2) NaClO (20 µmol/L) at pH 7.4 (0, 1); (3) mildly acidic buffer (pH 6.0) (1, 0); (4) NaClO (20 µmol/L) at pH 6.0 (1, 1). All four solutions were colorless in the beginning. After 30 min, the solution in the presence of both chemical inputs (1, 1) turned blue (Fig. 2a). UV-vis absorbance was consistent with naked-eye observations, which showed the significantly enhanced absorbance at 670 nm in the presence of both chemical inputs (1, 1) (Fig. 2b). Fluorescence spectroscopy showed that the solution in the presence of both chemical inputs (1, 1) had the significantly higher fluorescence intensity compared to the other three input patterns with an 8.2-fold difference. As summarized in Figs. 2c and d, the complex of host 1 and guest 4 demonstrates AND logic gate with high fluorescence output in the presence of both chemical inputs (1, 1).

|
Download:
|
| Fig. 2. (a) Image of an aqueous solution for the 1·4 complex under different conditions ([1] = 200 µmol/L, [4] = 10 µmol/L, [NaClO] = 20 µmol/L, pH 6.0 or 7.4, under 37 ℃, after 0.5 h). (b) UV-vis absorbance for the 1·4 complex under four different conditions. (c) Fluorescence intensity for the 1·4 complex under four different conditions (λex 620 nm, λem 695 nm). The fluorescence intensity of (1, 0) is 9.2 times that of (0, 1). (d) Logic gate matrix of four input combinations, and their corresponding fluorescence outputs. | |
Similarly, host 2 also demonstrated AND logic gate towards low pH and NaClO (Fig. S22 in Supporting information). Since host 2 was more stable under acidic condition compared to host 1, a lower pH of 4.6 was used. As a supramolecular system, host/guest ratio could be varied. As shown in Fig. S22, a lower host/guest ratio resulted in a less obvious AND logic gate effect due to the partial complexation between the host and the guest/dye. With a host/guest ratio of 5 or higher, a decent AND logic gate effect was observed. A similar ratio-dependent logic gate effect was observed for host 1 as well (Fig. S21 in Supporting information).
We are interested in the mechanism of these supramolecular logic gates. Stimuli-responsive degradation of host and guest was studied. As shown in Fig. 3a, NaClO triggered the degradation of guest 4, which led to an enhancement in fluorescence intensity. Acidic pH slightly accelerated degradation rate for guest 4. By contrast, when NaClO was absent, negligible fluorescence intensity enhancement was observed either at pH 7.4 or pH 6.0. The encapsulation of dye 5 by hosts 1 and 2 was confirmed to significantly reduce its fluorescence intensity (Fig. S5 in Supporting information). Therefore, we used dye 5 as an indicator for the host degradation and the release of the encapsulated dye. As shown in Fig. 3b, under neutral condition, minimal dye release was observed. By contrast, acidic condition significantly enhanced the dye release. As reported previously, host 1 was less stable at acidic pH compared to host 2. Consequently, supramolecular AND logic gates are tunable by choosing hosts with different acid-responsiveness.
|
Download:
|
| Fig. 3. (a) Degradation curve for guest 4 under four different conditions ([4] = 10 µmol/L, [NaClO] = 20 µmol/L). (b) Degradation curve for hosts 1-2 with dye 5 under different pH conditions ([1] = 200 µmol/L, [2] = 200 µmol/L, [5] = 10 µmol/L). (c) Fluorescence intensity change of guest 4 and NaClO with/without host 2 ([2] = 200 µmol/L, [4] = 10 µmol/L, [NaClO] = 20 µmol/L, λex = 620 nm, λem = 695 nm). (d) UV-vis absorbance change of guest 4 and NaClO with/without host 2 at 635 nm ([2] = 200 µmol/L, [4] = 10 µmol/L, [NaClO] = 20 µmol/L). | |
The encapsulation of guest 4 by host may inhibit its NaClO-induced degradation. A solution of guest 4 was incubated with the relatively stable host 2 in a neutral solution of NaClO (0, 1), and the fluorescence intensity was monitored. As shown in Fig. 3c, the fluorescence intensity grew at a much slower pace compared to that for guest 4 alone. Since supramolecular encapsulation resulted in fluorescence quenching, UV-vis absorbance was monitored. While guest 5 showed no absorbance at visible wavelength, dye 4 has strong visible absorbance. An isosbestic point was found at 635 nm based on UV-vis titration of host 2 and dye 5 (Fig. S6 in Supporting information). Therefore, we monitor UV-vis absorbance at 635 nm to avoid the impact of supramolecular encapsulation toward absorbance. As shown in Fig. 3d, degradation of guest 4 was inhibited in the presence of host 2. Based on the above mechanistic study, we confirmed that there are two reasons for the AND logic gate: (1) supramolecular encapsulation causes the inhibition of NaClO-induced guest degradation; (2) dye fluorescence is "switched off" by supramolecular complexation.
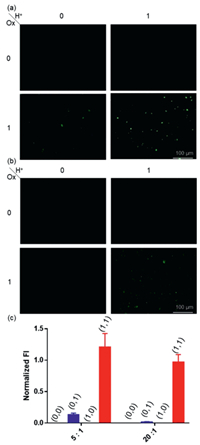
Lastly, human cancer MCF-7 and HeLa cells were used to validate the operation of AND molecular logic gate in vitro. HeLa cells were incubated with guest 4 and excess host 1 (5 equiv. or 20 equiv.) for 2 h at pH 7.4 or 6.5 to mimic neutral or acidic extracellular environment. The host/guest ratio was varied to investigate its impact towards logic gate effect. Subsequently, cells were washed, and further incubated for another 0.5 h in the presence or absence of NaClO to mimic pathological (ROS) or physiological condition. Cells were then thoroughly washed and imaged by fluorescence microscopy. As shown in Figs. 4 and 5, for both cell lines and both host/guest ratios, fluorescence was only turned on when both acidic pH (6.5) and NaClO were present. ImageJ was used to quantify fluorescence intensity, which showed fluorescence intensity in the presence of both biomarker signals (1, 1) was at least 38.1-fold higher compared to that for any other condition when the host/guest ratio is 20:1. For the host/guest ratio of 5:1, fluorescence intensity of (1, 1) was also at least 9.2-fold higher than the other groups. The results showed that logic gate effect was robust to certain extent of host/guest ratio. Therefore, we confirm that AND logic gates are operational in both types of cancer cells.
|
Download:
|
| Fig. 4. Fluorescence microscopic images of MCF-7 cells treated with host 1 and guest 4 under four different logic gate chemical input patterns. (0, 0): pH 7.4; (0, 1): NaClO at pH 7.4; (1, 0): pH 6.5; (1, 1): NaClO at pH 6.5 when host: guest molar ratio is 5:1 (a) or 20:1 (b). [4] = 10 µmol/L, [NaClO] = 50 µmol/L. (c) Fluorescence intensity statistics based on ImageJ. | |

|
Download:
|
| Fig. 5. Fluorescence microscopic images of HeLa cells treated with host 1 and guest 4 under four different logic gate chemical input patterns. (0, 0): pH 7.4; (0, 1): NaClO at pH 7.4; (1, 0): pH 6.5; (1, 1): NaClO at pH 6.5 when host: guest molar ratio is 5:1 (a) or 20:1 (b). [4] = 10 µmol/L, [NaClO] = 50 µmol/L. (c) Fluorescence intensity statistics based on ImageJ. | |
In summary, we develop supramolecular AND logic gates based on acid-labile host molecules and ROS-responsive guest, which is operational in live cells. Oxidation inhibition and encapsulation-induced fluorescence quenching were discovered to be the reasons for AND logic gate. Convenient-assembled and highly tunable supramolecular design render these AND logic gates to be further developed for diagnostic and therapeutic applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors are grateful to National Natural Science Foundation of China (Nos. 21921003 and 21672042) for financial support. Guest 4 was kindly provided by Prof. Tao Yi and Dr. Peng Wei.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.021.
| [1] |
U. Pischel, Angew. Chem. Int. Ed. 46 (2007) 4026-4040. DOI:10.1002/anie.200603990 |
| [2] |
A.P. De Silva, N.D. McClenaghan, Chem. Eur. J. 10 (2004) 574-586. DOI:10.1002/chem.200305054 |
| [3] |
S.J. Langford, T. Yann, J. Am. Chem. Soc. 125 (2003) 11198-11199. DOI:10.1021/ja036909+ |
| [4] |
A. Okamoto, K. Tanaka, I. Saito, J. Am. Chem. Soc. 126 (2004) 9458-9463. DOI:10.1021/ja047628k |
| [5] |
J. Yang, D. Dai, L. Ma, Y.W. Yang, Chin. Chem. Lett. 32 (2021) 729-734. DOI:10.1016/j.cclet.2020.08.035 |
| [6] |
Y. Gao, Y. Gao, Y. Ding, et al., Chin. Chem. Lett. 32 (2021) 949-953. DOI:10.1016/j.cclet.2020.08.010 |
| [7] |
B.A. Badeau, M.P. Comerford, C.K. Arakawa, J.A. Shadish, C.A. Deforest, Nat. Chem. 10 (2018) 251-258. DOI:10.1038/nchem.2917 |
| [8] |
D. Margulies, C.E. Felder, G. Melman, A. Shanzer, J. Am. Chem. Soc. 129 (2007) 347-354. DOI:10.1021/ja065317z |
| [9] |
P. Díez, A. Sánchez, M. Gamella, et al., J. Am. Chem. Soc. 136 (2014) 9116-9123. DOI:10.1021/ja503578b |
| [10] |
T. Soboleva, H.J. Esquer, A.D. Benninghoff, L.M. Berreau, J. Am. Chem. Soc. 139 (2017) 9435-9438. DOI:10.1021/jacs.7b04077 |
| [11] |
F. Tian, D. Jiao, F. Biedermann, O.A. Scherman, Nat. Commun. 2 (2012) 2198. |
| [12] |
J. Zhou, G. Yu, F. Huang, Chem. Soc. Rev. 46 (2017) 7021-7053. DOI:10.1039/C6CS00898D |
| [13] |
S. Angelos, Y.W. Yang, N.M. Khashab, J.F. Stoddart, J.I. Zink, J. Am. Chem. Soc. 131 (2009) 11344-11346. DOI:10.1021/ja9042752 |
| [14] |
T.W. Bell, N.M. Hext, Chem. Soc. Rev. 33 (2004) 589-598. |
| [15] |
K. Yang, Y. Pei, J. Wen, Z. Pei, Chem. Commun. 52 (2016) 9316-9326. DOI:10.1039/C6CC03641D |
| [16] |
Y.M. Zhang, N.Y. Zhang, K. Xiao, Q. Yu, Y. Liu, Angew. Chem. Int. Ed. 57 (2018) 8649-8653. DOI:10.1002/anie.201804620 |
| [17] |
C. Gao, Q. Huang, Q. Lan, et al., Nat. Commun. 9 (2018) 2967. DOI:10.1038/s41467-018-05437-5 |
| [18] |
C. Kim, S.S. Agasti, Z. Zhu, L. Isaacs, V.M. Rotello, Nat. Chem. 2 (2010) 962-966. DOI:10.1038/nchem.858 |
| [19] |
L. Cao, G. Hettiarachchi, V. Briken, L. Isaacs, Angew. Chem. Int. Ed. 52 (2013) 12033-12037. DOI:10.1002/anie.201305061 |
| [20] |
J. Tian, T.Y. Zhou, S.C. Zhang, et al., Nat. Commun. 5 (2014) 5574. DOI:10.1038/ncomms6574 |
| [21] |
A.R. Lippert, G.C. van De Bittner, C.J. Chang, Acc. Chem. Res. 44 (2011) 793-804. DOI:10.1021/ar200126t |
| [22] |
Q. Chen, C. Liang, X. Sun, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 5343-5348. DOI:10.1073/pnas.1701976114 |
| [23] |
J.L. Wike-Hooley, J. Haveman, H.S. Reinhold, Radiother. Oncol. 2 (1984) 343-366. DOI:10.1016/S0167-8140(84)80077-8 |
| [24] |
Y. Lu, A.A. Aimetti, R. Langer, Z. Gu, Nat. Rev. Mater. 2 (2016) 1-17. DOI:10.1038/natrevmats.2016.75 |
| [25] |
G.C. van De Bittner, C.R. Bertozzi, C.J. Chang, J. Am. Chem. Soc. 135 (2013) 1783-1795. DOI:10.1021/ja309078t |
| [26] |
M.A. Romero, R.J. Fernandes, A.J. Moro, N. Basílio, U. Pischel, Chem. Commun. 54 (2018) 13335-13338. DOI:10.1039/C8CC07404F |
| [27] |
D. Ma, G. Hettiarachchi, D. Nguyen, et al., Nat. Chem. 4 (2012) 503-510. DOI:10.1038/nchem.1326 |
| [28] |
D. Mao, Y. Liang, Y. Liu, et al., Angew. Chem. Int. Ed. 56 (2017) 12614-12618. DOI:10.1002/anie.201707164 |
| [29] |
S. Jiang, S. Lan, D. Mao, et al., Chem. Commun. 54 (2018) 9486-9489. DOI:10.1039/C8CC05552A |
| [30] |
W. Mao, D. Mao, F. Yang, D. Ma, Chem. Eur. J. 25 (2019) 2272-2280. DOI:10.1002/chem.201804835 |
| [31] |
A. Hennig, H. Bakirci, W.M. Nau, Nat. Methods 4 (2007) 629-632. DOI:10.1038/nmeth1064 |
| [32] |
A. Norouzy, Z. Azizi, W.M. Nau, Angew. Chem. Int. Ed. 54 (2015) 792-795. DOI:10.1002/anie.201407808 |
| [33] |
P. Wei, W. Yuan, F. Xue, et al., Chem. Sci. 9 (2018) 495-501. DOI:10.1039/C7SC03784H |
 2022, Vol. 33
2022, Vol. 33