b School of Pharmacy and State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macao, China
Hydrogels are three-dimensional cross-linked polymeric networks with a large amount of water or aqueous fluids. Owing to the high hydration level and excellent biocompatibility, hydrogels demonstrate broad application prospects in various biomedical and industrial fields [1–9]. In particular, hydrogels may serve as an ideal candidate for artificial bio-tissues [10,11]. While the high level of similarities between synthetic hydrogels and bio-tissues are often highlighted in the literature, substantial differences occur in terms of micro- to macro-structures. Conventional hydrogels tend to manifest isotropic structures with randomly oriented three-dimensional polymer networks. Nonetheless, biological tissues, such as tendons, skeletal muscles, and cartilage, adopt anisotropic structures with hierarchically integrated building units. Such oriented structure typically plays essential roles for various critical biological functions, such as anisotropic mechanical performance, cell culture, and asymmetric mass transport [12].
Considering this aspect, the fabrication and application of anisotropic hydrogels have undoubtedly attracted considerable attention during the past two decades. Scientists have conceived numerous approaches to construct anisotropic hydrogels, including with the aid of shear forces [13,14], magnetic or electronic field [15,16], directional pre-orientation [17–19], selective mass diffusion [20,21], directional crystallization [22,23], and 3D print [24]. Among all these methods, directional pre-orientation, especially the prestretching treatment serves as a simple yet effective way and has been widely applied in the preparation of anisotropic hydrogels. Via this approach, hydrogels are first deformed to give oriented structures, followed by post-crosslinking to immobilize the polymer alignments. For instance, Wang et al. [25] proposed a prestretching treatment of chitosan hydrogel with a post ionic crosslinking strategy to fabricate hierarchically anisotropic hybrid hydrogels with excellent mechanical performance. Similarly, Kim's group [26] developed an anisotropic double network hydrogel by prestretching and subsequent ionic crosslinking procedures of alginate hydrogels. In another work, Fujita's group [27] conceived a directional tension method together with a freeze-thaw process to physically immobilized semi-crystalline gels. Nevertheless, most anisotropic hydrogels with these approaches often require complicated preparation operations, as the post-crosslinking processes are typically time-consuming and labor-demanding [28]. This disadvantage severely restricts industrial production and the widespread application of such anisotropic hydrogels.
Clearly, the complex post-crosslinking method in the directional deformation strategy becomes a rate-determining step in the fabrication process of anisotropic hydrogels. The dry-induced crystallization phenomenon is a common feature for certain crystalline polymers, which can physically crosslink polymer networks and serve as a facile and effective approach to prepare hydrogels [29,30]. Inspired by this feature, herein we report a simple yet effective preparation approach of anisotropic hydrogels with a dry-induced crystallization process. Semi-crystalline hydrophilic polymer poly(vinyl alcohol) (PVA) is chosen as a model system to illustrate this concept. In brief, the preparation of PVA anisotropic hydrogels is realized with a facile prestretching-drying-swelling process. The prestretching process leads to the oriented polymer structure, the heating process immobilizes the alignment with crystalline domains and the swelling process endows the materials with considerable water content. Due to its distinct well-oriented micro-structure similar to bio-tissues, the hydrogels demonstrate excellent mechanical properties. Moreover, the mechanical performance manifests a noticeable anisotropic performance with the chain alignment. It is further verified that the fabrication parameters play a vital role in mechanical properties and anisotropicity: the large the pre-orientation strain and the longer the heating time give rise to better mechanical performance and more remarkable anisotropicity. Benefited from the anisotropically oriented feature, the hydrogels exhibited remarkably differential mass transportation, bringing in a 6.6-fold difference in ionic conductivity.
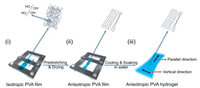
As depicted in Fig. 1, the solution is dehydrated at a low temperature to afford isotropic PVA films (ⅰ). Once cast, the films undergo uniaxial deformation at high temperature prior to cooling, which endows the polymer mobility and induces chain alignment along the stretching direction (ⅱ). Benefitted from its well-oriented hydroxy side groups in PVA structures, crystalline domains are formed and immobilize the oriented structure [29]. Subsequently, the material is immersed in water for swelling (ⅲ). During this process, the polymer crystalline domains remain relatively stable, which serves as physical crosslinking points to maintain the materials at solid-state; the polymer amorphous domains mix with solvent owing to their high hydrophilicity, which absorbs a large amount of liquid by osmotic pressure. Therefore, anisotropic PVA hydrogels are obtained with a well-oriented structure by this simple prestretching-drying-swelling process.
|
Download:
|
| Fig. 1. Schematic illustrations of the preparation process of anisotropic PVA crystalline hydrogels. (ⅰ) A piece of PVA dry film is fixed in a homemade stretcher at its two ends. Note: The purple arrow represents the direction of prestretching. (ⅱ) The film experiences tensile along the parallel direction, which affords an anisotropic PVA film with well-aligned polymer chains in the tensile direction. (ⅲ) Subsequently, the above prepared PVA film undergoes a swelling process in pure water to obtain an anisotropic PVA hydrogel. Note that parallel direction is defined as along the pretension direction, while the vertical direction is defined as along the orthogonal direction to pretension direction. | |
To confirm the anisotropic structure of PVA hydrogels, a polarizing optical microscopic (POM) was selected to observe the aligned microstructure of PVA chains under different prestretching strains (Fig. 2). Obviously, when hydrogel without experiencing pre-orientation strain process (0%, ⅰ), the parallel and vertical directions presented no visible color variation, implying an isotropic structure. When a prestretched strain is imposed, the hydrogels demonstrated evident distinction in color aspect (ⅱ-ⅳ), indicating the unidirectional orientation. Moreover, the color divergence became increasingly important with the growth of pretension strains (50%−150%), which again emphasized the importance of prestretching on the anisotropic performance and stayed in good agreement with previous studies with prestretching-induced anisotropicity [31]. The anisotropically aligned structures have been further confirmed with scanning electron microscope (SEM, Fig. S1 in Supporting information) and Atomic Force Microscope (AFM, Fig. S2 in Supporting information).

|
Download:
|
| Fig. 2. In-situ observation of anisotropic hydrogels with well-aligned structures. Polarizing optical microscopic (POM) images of anisotropic hydrogels with different prestretching strains: (ⅰ) 0, (ⅱ) 50, (ⅲ) 75, and (ⅳ) 150% (The scale bars represent a length of 50 µm). Note that the first line photos display the color variation of anisotropic hydrogel along the parallel direction, while the second line photos are along the vertical direction. Moreover, the olive arrows indicate prestretching direction (parallel direction), and the gold arrows show vertical direction. | |
According to the previous observation, the well-orientation of polymer chains was formed in the hydrogels network by prestretching. This polymer alignment may endow the hydrogels with excellent mechanical properties. Meanwhile, the presence of crystallization domains may also contribute to the materials' mechanical performance. Therefore, in the next step, PVA anisotropic hydrogels' mechanical properties along the parallel direction were investigated by uniaxial tensile tests. As shown in Fig. 3a, The mechanical performance of anisotropic hydrogels was significantly improved with the enhancing of prestretching strains. To clarify this phenomenon, we summarized Young's modulus and fracture stress of all samples, as is demonstrated in Fig. 3b. In general, both parameters demonstrated evident positive dependence on the prestretching ratio. In a typical example, an anisotropic hydrogel with 200% of pretension strain along the parallel direction manifested Young's modulus and fracture stress as high as 3.6 MPa and 6.2 MPa, respectively, which are more than 3 and 4 folds higher than that PVA hydrogel without any prestretching treatment. Consequently, anisotropic PVA hydrogels with considerable mechanical performance were well prepared by a simple yet effective method. In addition, this preparation method demonstrates good stability as it has almost no shape dependence (Fig. S3 in Supporting information).

|
Download:
|
| Fig. 3. (a) Uniaxial tensile stress-strain curves of anisotropic hydrogels with different prestretching strains along the parallel direction. (b) Young's modulus and fracture stress of PVA hydrogels with prestretching strains range from 0 to 200% in the parallel direction. (c) The polymer fraction, Young's modulus, and normalized modulus (E/φ) of anisotropic hydrogels with different prestretching strains. (d) XRD spectra of anisotropic hydrogels with different prestretching strains. Note that the PVA films were prestretched to given strain at 120 ℃, and no waiting time is placed for all the films prior to reswell in water at room temperature. | |
From the above results, it is obvious that the mechanical properties of anisotropic hydrogels manifested large-scale tunability just by imposing different pretension strains. However, the reinforcement mechanism of mechanical strength demands further investigation. When the samples are treated with different prestretching strains, certainly that this process can affect the hydration capacity of hydrogels, resulting in the difference of polymer fraction. Based on classical rubber elasticity theory [32], hydrogels' polymer volume fraction directly affects its Young's modulus permanent entanglement, that is:

|
(1) |
where φ is defined as the volume fraction of polymer, νe is defined as at the solvent-free state effectively elastic polymer chain density, R is gas constant and T is the absolute temperature. Undoubtedly, Young's modulus of all the hydrogels presented a positive correlation with polymer fraction (Fig. 3c). On the whole, the higher polymer fraction of samples can endow better mechanical strength. To exclude the role played by polymer fraction variation, we further normalized Young's modulus (E/φ) as a function of pretension strains. According to Eq. 1, it is clear that this value should stay constant for all the hydrogels if no other factor governs. However, this value experimentally exhibited nonlinearly increased tendency with the enhancement of prestretching strains, from 2.9 MPa to 8.4 MPa, indicating polymer fraction only partly factor for hydrogels' toughening. Indeed, amorphous and crystalline regions coexist for such as PVA semi-crystalline hydrogels. Therefore, we speculate that the crystalline regions may also play a prominent role in toughening of hydrogels. As seen in Fig. 3d, X-ray diffraction (XRD) measurement markedly signified that the pretension strains could conspicuously affect hydrogels' crystalline levels. While the sample stretched to 200% showed a sharp crystalline peak at 18°–21°, which is a characteristic peak from PVA crystallization, the sample without prestretching treatment displayed a rather slight signal peak at the same range. All these results indicate that both amorphous and crystalline domains are strikingly affected by imposing different pretension strains, therefore the hydrogels are tunably strengthened to different levels.
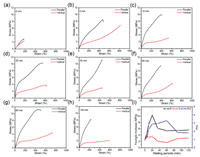
From the previous report, extending the heating time is able to enhance the crystalline ratio of PVA-type polymer [33]. Therefore, we systematically investigated the heating time in the following studies. A series of PVA hydrogels have been prepared with a fixed prestretching strain of 100% but varied waiting periods from 0 to 60 min at high temperature. As shown in Fig. 4a, uniaxial tensile stress-strain curves of hydrogel without waiting time exhibited only a slight variation in mechanical performance between the parallel and vertical directions. Moreover, the samples demonstrated a classical entropy-induced elasticity with a fairly limited elongation ratio. By placing certain stages of waiting periods, the gels manifested evident differences in the parallel and vertical directions. As seen in Figs. 4b–h, the rigidity of the parallel direction surpasses that of the vertical direction. Additionally, the materials in both directions manifested much better extensibility and toughness. This distinction gets increasingly prominent by raising the waiting time. In addition, the gels with waiting periods manifested an obvious strain-softening and strain-hardening performance, which resembles the results with physical interactions reinforced hydrogels [34,35]. To better evaluate the waiting time effect, we further extract Young's modulus of two directions for all samples as a function of waiting periods (Fig. 4i). With heating time rising from 0 to 20 min, Young's modulus of hydrogels along the parallel direction (P) augmented by a large margin from 2.3 MPa to 10.4 MPa, while the same parameter in the vertical direction (V) slighted increased from 2.1 MPa to 3.8 MPa. At the same time, the polymer volume fraction experienced a steady increase with extending the heating time (Fig. S4 in Supporting information). Interestingly, the rigidity at both directions experienced noticeable decreases with a long-term period in the oven (20–120 min), which may due to the possible degradation of polymer in such a harsh environment. On the basis of the rigidity at two directions, anisotropicity ratio, which corresponding to Young's modulus ratio in parallel and vertical direction (P/V), is defined to better characterize the unidirectional performance. From Fig. 4i, this value keeps monotonous growth from 1.1 to 3.3 with the improvement of waiting time from 0 to 60 min. This increase is especially remarkable at short periods from 0 to 20 min, while the more extended period only brings in a slight increase in the anisotropicity ratio. From this point, the hydrogels can embrace a more evident anisotropic feature owing to the longer waiting time of samples at high temperature. When continuously enhancing the heating time, the anisotropicity ratio becomes less prominent together with the dropping of rigidity in both directions. In contrast to the huge impact of the heating condition, the cooling temperature displayed a relatively limited influence on the mechanical performance. Almost no difference in terms of rigidity and extensibility appears between three samples that were directly quenched to different cooling temperatures (Fig. S5 in Supporting information). Although the programmable cooling process can lead to differences in mechanical performance (Fig. S6 in Supporting information), this variation may mainly come from the different heating periods, which is in good agreement with the previous discussion.
|
Download:
|
| Fig. 4. Uniaxial tensile stress-strain curves of anisotropic PVA hydrogels along the parallel direction and vertical direction with pretension strain of 100% upon PVA films were placed oven at 120 ℃ for different waiting periods of (a) 0, (b) 5, (c) 10, (d) 20, (e) 35, (f) 60, (g) 90 and (h) 120 min. (ⅰ) Young's moduli in the parallel direction (P), the vertical direction (V), and the anisotropicity ratio (denoted as the P/V) of hydrogels with different waiting periods. | |
Inspired by the above results, we further investigated the mechanical properties of anisotropic hydrogels with different prestretching strains upon samples with an optimal waiting time of 60 min. As depicted in Fig. 5a, the typical uniaxial stress-strain curves of hydrogel without prestretching strain along parallel and vertical directions showed high similarity. At the same time, the hydrogel with a pretension strain of 25% presented evident variation in the stress-strain curves along with two directions (Fig. 5b). The difference became increasingly remarkable with a longer prestretching ability (Figs. 5c–e). The gel with 100% prestretching strain revealed excellent mechanical performance, with maximum fracture stress as high as 9.6 MPa. By using the similar analytical method in Fig. 4f, we also calculated the elastic modulus of hydrogels with different prestretching strains ranged from 0 to 100%. Evidently, As seen in Fig. 5f, Young's modulus along the parallel direction and vertical direction of hydrogel without any treatment presented a pretty similar value. With the growth of prestretching strains, Young's modulus of all anisotropic hydrogels along parallel direction show a sharp and monodirectional increase, while the ones in vertical direction exhibited a one-way decreased tendency. As the result, the anisotropicity ratio (P/V) demonstrated a directional ascent ranged from 1.0 to 3.3 with prestretching strain enhancement.

|
Download:
|
| Fig. 5. Stress-strain curves of anisotropic PVA hydrogels along the parallel direction and vertical direction upon films were heated for 60 min with different prestretching strains of (a) 0%, (b) 25%, (c) 50%, (d) 75% and (e) 100%, respectively. (f) Young's moduli in the parallel direction (P), the vertical direction (V), and the anisotropicity ratio (denoted as the P/V) of samples along two directions with prestretching strains range from 0 to 100%. | |
Benefitted from the well-oriented structure, the hydrogels embraced distinct differential ionic conductivity perior to treated with 1 mol/L CaCl2 solution. As shown in Fig. 6 and Fig. S7 (Supporting information), the electrical resistance of anisotropic hydrogels along the parallel direction displayed relatively low values (80–170 kΩ, Fig. 6a and Figs. S7a and c). This value kept decreasing with the increase of waiting time. In contrast, the values in the vertical direction are much higher and more stable, ranging of ca. 300–500 kΩ (Fig. 6b, Figs. S7b and d). Therefore, the resistance gap between the two directions became increasingly remarkable by enhancing the waiting periods from 5 min to 60 min. The comparability is more prominent by comparing the ionic conductivity (Fig. 6c). While the conductivity in the parallel direction experienced a gradual and sharp increase from 0.08 S/m to 0.32 S/m, the values demonstrated a smooth and steadily decrease trend in the vertical direction. For better comparison, we divide the conductivity value from the two directions to characterize the anisotropicity. Similar to the value from mechanical performance, the ionic conductivity anisotropicity experienced a 3-fold sharp increase with the extending of the heating period at 120 ℃ from 5 min to 60 min. The anisotropicity value reached a maximum value of 6.6 with 100% prestretching ratio and 60 min waiting period. As the prominent difference of resistance of anisotropic hydrogels along two directions plays a vital role in the field of electrochemistry, the hydrogels are expected to manifest interesting application prospects in the fields of gel polymer electrolyte and supercapacitor.

|
Download:
|
| Fig. 6. Anisotropic differential conductivity of PVA hydrogels. Photos of the electrical resistance of anisotropic hydrogels with prestretching strain of 100% and waiting time 60 min along the parallel (a) and vertical (b) directions. Note that the value in (a) is in the kΩ unit while in (b) in the MΩ unit. Both measured samples are of similar length, width, and thickness. (c) Ionic conductivity of PVA hydrogels in the parallel, vertical directions and the ratio of two directions (P/V) with prestretching strain of 100% as a function of waiting periods. | |
In this work, the construction of anisotropic crystalline hydrogels has been successfully realized by a simple yet effective prestretching-drying-swelling process, which is attributed to the dry-induced crystallization phenomenon of PVA polymers. Due to their distinct well-oriented micro-structure, the hydrogels demonstrate excellent mechanical properties with noticeable directional distinction. Fabrication parameters, such as the pre-orientation strain and the heating time, play a critical role in tuning hydrogels' mechanical properties and anisotropicity. Owing to the anisotropically aligned polymer structure, the hydrogels exhibited remarkably differential mass transportation, which leads to a 6.6-fold difference in ionic conductivity. This method proves to be a facile yet effective approach in anisotropic hydrogels fabrication, which may become highly useful for guiding the oriented structure for other hydrogel systems. We anticipate that this method may endow the materials with more interesting application prospects as bio-inspired materials in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the National Key Research and Development Program of China (No. 2019YFE0111000), the National Natural Science Foundation of China (NSFC, Nos. 51903253, 51903257), Natural Science Foundation of Guangdong Province of China (Nos. 2019A1515011150, 2019A1515011258), Macau University of Science and Technology Foundation (No. FRG-19-003-SP), and the Science and Technology Development Fund of Macao (Nos. FDCT 0009/2019/A, 0083/2019/A2, 0007/2019/AKP).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.010.
| [1] |
G. Su, S. Yin, Y. Guo, et al., Mater. Horiz. 8 (2021) 1795-1804. DOI:10.1039/D1MH00085C |
| [2] |
X. Pei, H. Zhang, Y. Zhou, L. Zhou, J. Fu, Mater. Horiz. 7 (2020) 1872-1882. DOI:10.1039/D0MH00361A |
| [3] |
S. Xiao, M. Zhang, X. He, et al., ACS Appl. Mater. Interfaces 10 (2018) 21642-21653. DOI:10.1021/acsami.8b06169 |
| [4] |
X. Wang, O. Ronsin, B. Gravez, et al., Adv. Sci. 8 (2021) 2004213. DOI:10.1002/advs.202004213 |
| [5] |
H. Guo, N. Sanson, A. Marcellan, D. Hourdet, Macromolecules 49 (2016) 9568-9577. DOI:10.1021/acs.macromol.6b02188 |
| [6] |
H. Guo, C. Mussault, A. Marcellan, D. Hourdet, N. Sanson, Macromol. Rapid Commun. 38 (2017) 1700287. DOI:10.1002/marc.201700287 |
| [7] |
C. Xian, Q. Yuan, Z. Bao, G. Liu, J. Wu, Chin. Chem. Lett. 31 (2020) 19-27. DOI:10.1016/j.cclet.2019.03.052 |
| [8] |
G. Liu, Z. Bao, J. Wu, Chin. Chem. Lett. 31 (2020) 1817-1821. DOI:10.1016/j.cclet.2020.03.005 |
| [9] |
X. Lin, X. Wang, L. Zeng, et al., Chem. Mater. 33 (2021) 7633-7656. DOI:10.1021/acs.chemmater.1c01019 |
| [10] |
J.L. Drury, D.J. Mooney, Biomaterials 24 (2003) 4337-4351. DOI:10.1016/S0142-9612(03)00340-5 |
| [11] |
S. Sharma, R. Kumar, P. Kumari, et al., Int. J. Biol. Macromol. 125 (2019) 109-115. DOI:10.1016/j.ijbiomac.2018.12.018 |
| [12] |
K. Sano, Y. Ishida, T. Aida, Angew. Chem. Int. Ed. 57 (2018) 2532-2543. DOI:10.1002/anie.201708196 |
| [13] |
X.Y. Lin, Z.J. Wang, P. Pan, Z.L. Wu, Q. Zheng, RSC Adv. 6 (2016) 95239-95245. DOI:10.1039/C6RA17103F |
| [14] |
M.A. Haque, G. Kamita, T. Kurokawa, K. Tsujii, J.P. Gong, Adv. Mater. 22 (2010) 5110-5114. DOI:10.1002/adma.201002509 |
| [15] |
L. Wu, M. Ohtani, M. Takata, et al., ACS Nano 8 (2014) 4640-4649. DOI:10.1021/nn5003908 |
| [16] |
M. Liu, Y. Ishida, Y. Ebina, et al., Nature 517 (2015) 68-72. DOI:10.1038/nature14060 |
| [17] |
M.T.I. Mredha, Y.Z. Guo, T. Nonoyama, et al., Adv. Mater. 30 (2018) 1704937. DOI:10.1002/adma.201704937 |
| [18] |
P. Lin, T. Zhang, X. Wang, B. Yu, F. Zhou, Small 12 (2016) 4386-4392. DOI:10.1002/smll.201601893 |
| [19] |
L.E. Millon, H. Mohammadi, W.K. Wan, . Biomed. Mater. Res. B: Appl. Biomater. 79B (2006) 305-311. DOI:10.1002/jbm.b.30543 |
| [20] |
H. Bai, A. Polini, B. Delattre, A.P. Tomsia, Chem. Mater. 25 (2013) 4551-4556. DOI:10.1021/cm4025827 |
| [21] |
H. Guo, T. Nakajima, D. Hourdet, et al., Adv. Mater. 31 (2019) 1900702. DOI:10.1002/adma.201900702 |
| [22] |
J. Zhu, J. Wang, Q. Liu, et al., J. Mater. Chem. B 1 (2013) 978-986. DOI:10.1039/C2TB00288D |
| [23] |
X. Peng, C. He, J. Liu, H. Wang, J. Mater. Sci. 51 (2016) 5901-5911. DOI:10.1007/s10853-016-9891-x |
| [24] |
H. Arslan, A. Nojoomi, J. Jeon, K. Yum, Adv. Sci. 6 (2019) 1800703. DOI:10.1002/advs.201800703 |
| [25] |
Y. Wang, S. Liu, W. Yu, ACS Appl. Bio Mater. 3 (2020) 6959-6966. DOI:10.1021/acsabm.0c00828 |
| [26] |
S. Choi, Y. Choi, J. Kim, Adv. Funct. Mater. 29 (2019) 1904342. DOI:10.1002/adfm.201904342 |
| [27] |
Y. Nagakawa, M. Kato, S. Suye, S. Fujita, RSC Adv. 10 (2020) 38045-38054. DOI:10.1039/D0RA07322A |
| [28] |
Q. Sun, S. Ma, P. Lin, et al., ACS Appl. Polym. Mater. 2 (2020) 2142-2150. DOI:10.1021/acsapm.0c00096 |
| [29] |
E. Otsuka, A. Suzuki, J. Appl. Polym. Sci. 114 (2009) 10-16. DOI:10.1002/app.30546 |
| [30] |
P. Li, Z. Wang, X. Lin, X. Wang, H. Guo, Sci. China Mater. (2021). DOI:10.1007/s40843-021-1722-0 |
| [31] |
L. Zhang, J. Zhao, J. Zhu, C. He, H. Wang, Soft Matter 8 (2012) 10439-10447. DOI:10.1039/c2sm26102b |
| [32] |
S.P. Obukhov, M. Rubinstein, R.H. Colby, Macromolecules 27 (1994) 3191-3198. DOI:10.1021/ma00090a012 |
| [33] |
E. Otsuka, S. Komiya, S. Sasaki, et al., Soft Matter 8 (2012) 8129-8136. DOI:10.1039/c2sm25513h |
| [34] |
H. Guo, N. Sanson, D. Hourdet, A. Marcellan, Adv. Mater. 28 (2016) 5857-5864. DOI:10.1002/adma.201600514 |
| [35] |
H. Guo, C. Mussault, A. Brûlet, et al., Macromolecules 49 (2016) 4295-4306. DOI:10.1021/acs.macromol.6b00798 |
 2022, Vol. 33
2022, Vol. 33 

