b Key Laboratory of Pesticides & Chemical Biology Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China
Anthrones are privileged scaffolds, and their derivatives possess multiple biological activities including anticancer and anti-inflammatory properties (Scheme 1a) [1–6]. For example, Dithranol has been used in the treatment of psoriasis, and Emodin plays an important role in antitumor immunity [4–6]. In recent years, considerable progress has been achieved for the enantioselective synthesis of anthrone derivatives especially by organocatalysts [7–14]. The established protocols mainly limited to 1,4-additions and Diels-Alder reaction of anthrones. For instance, in 2006, Tan and coworkers developed a highly enantioselective Diels-Alder reaction of anthrones with phenylmaleimides by chiral guanidine catalyst [14]. In contract, little attention has been paid on transition-metal catalyzed asymmetric reactions of anthrones. Until 2015, Lautens and coworkers realized the first rhodium(I)-catalyzed benzylic functionalization of anthrones via ring opening of oxabicycles (Scheme 1b) [15]. Thereafter, You and Wu et al. disclosed a highly enantioselective iridium-catalyzed allylation of anthrones Scheme 1b [16]. Despite these indisputable advances, earth-abundant-metal-catalyzed asymmetric functionalization of anthrones continue to be scarce [17]. Therefore, the use of earth-abundant metals to realize asymmetric functionalization of anthrones remains a challenging but highly desirable goal.
|
Download:
|
| Scheme 1. (a) Representative biological anthrone derivatives; (b) Previous studies; (c) This work. | |
In the meantime, enantioselective copper catalyzed transformations have ermerged as powerful protocols in organic synthesis [18–29]. For examples, copper-catalyzed asymmetric propargylic substitution [30–35] is a powerful approach for the construction of C–C [36–47] or C–X [48–53] (X = N, O) bonds. A diverse range of propargylic compounds have been synthesized through the addition of nucleophiles to Cu-allenylidene intermediates [54–61]. However, to our knowledge, the use of anthrones as C-nucleophile for propargylic alkylation has not been realized, which may be attributed to the more challenging site- and enantio-selectivitive control. Within the program on asymmetric propargylic substitutions [62–64], we now report the asymmetric copper-catalyzed propargylic substitution of anthrones and propargylic esters utilizing a chiral N, N, P-ligand (Scheme 1c).
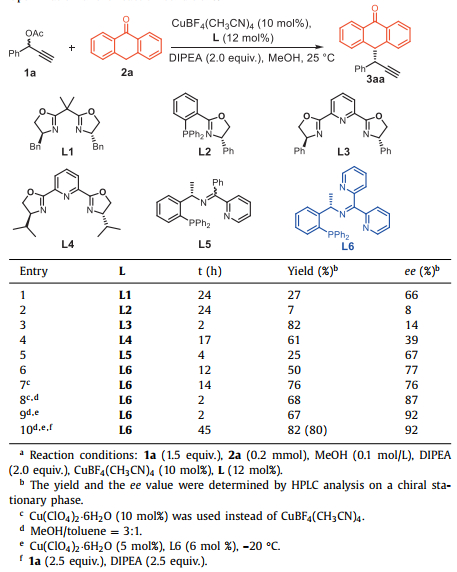
We initiated our investigation using phenyl-2-propynyl acetate (1a) and anthrone (2a) as model substrates to explore the use of various chiral ligands in the presence of diisopropylethylamine (DIPEA) as a base (Table 1 and Tables S1 in Supporting information). The desired product 3aa was obtained in 27% yield with 66% ee using Bn-BOX (L1) as ligand at room temperature (entry 1). Other bidentate ligand such as PHOX (L2) led to almost completely racemic product (entry 2). Afterwards we turned our attention to use tridentate ligand for turning chirality environment of the copper catalyst. A range of PyBOX analogues were screened, and Ph-PyBOX (L3) and iPr-PyBOX (L4) catalyzed the reaction smoothly (entries 3 and 4), delivering 3aa in high yield with lower ee. We further probed tridentate ligand L5 developed by Hu group [61], affording the desired product in moderate enantioselectivity (entry 5, 67% ee). Notably, the enantioselectivity of the target molecule was increased to 77% and the yield was improved considerably using dipyridyl N, N, P-ligand L6, which was developed by our group (entry 6). For the copper catalyst, Cu(ClO4)2·6H2O gave the best results (entry 7) (For details, see Supporting information). The ee of the product was increased to 87% with mixture solvents (MeOH/toluene = 3:1) (entry 8).
|
|
Table 1 Optimization of the reaction conditions.a |
Interestingly, the asymmetric propargylic substitution occurred efficiently with catalyst loadings as low as 5 mol% (entry 9). Finally, the desired product 3aa could be isolated in 80% yield with 92% ee (entry 10).
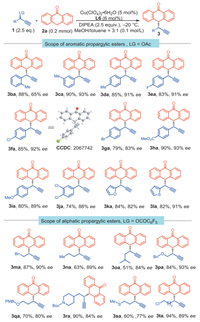
With the optimized conditions in hand, we then explored the versatility of the reaction with respect to the propargylic esters (Scheme 2). A variety of aryl propargylic esters were tolerated and participated in the reaction smoothly. For example, substituents on the meta- and ortho-positions proved to be compatible with this reaction, producing the corresponding products 3ba and 3ca with 65% and 93% ee, respectively. Similarly, the introduction of either electron-withdrawing or electron-donating groups at the para-position of the phenyl group delivered the products 3da – 3ja with 83%–93% ee. Notably, heteroaromatic esters were also efficiently converted with high selectivity control, providing the desired products 3ka and 3la in 84% and 82% yield with 82% and 91% ee, respectively. The absolute configuration of chiral 10-propargylanthrone 3fa was unambiguously confirmed by X-ray analysis, and the configurations of the other products were assigned by analogy (For details, see Supporting information.). Aliphatic-substituted propargylic substrates with perfluorobenzoyl as the leaving group also reacted smoothly with anthrone (2a) under our optimized reaction conditions. A variety of secondary propargylic esters reacted well, and excellent enantioselectivities of the products 3ma – 3pa was observed. The ee values of the compounds 3na and 3oa were assigned by Cu-catalyzed azide-alkyne cycloaddition. (For details, see Supporting information). The functional groups, such as ether, amine, thioether, and chloro, were fully tolerated, which is invaluable for further late-stage diversification.
|
Download:
|
| Scheme 2. Scope of the reaction with propargylic esters. | |
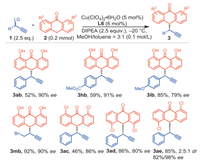
To our delight, anthrones bearing hydroxyl groups (anthralin) and chlorines proceeded smoothly under the standard reaction conditions (Scheme 3). The reactivity of anthralin also favoured carbon over oxygen attack, providing the benzylic functionalization product 3ab in 52% yields with 90% ee. Electron rich and electron deficient aryl propargylic esters proceeded smoothly under the standard reaction conditions, generating the desired products 3hb-3ib in good yields with good to excellent enantioselectivities. Again, aliphatic propargylic ester was the suitable substrate (3mb). Interestingly, the reaction with 1,8-dichloroanthrone and 4,5-dichloroanthrone gave the corresponding products 3ac and 3ad in 46% and 86% yield with 86% and 80% ee, respectively. Additionally, unsymmetrical anthrone 1,5-dichloroanthrone 2e was also performed to deliver 3ae with moderate diastereoselectivity of 2.5:1 d.r. in 85% yield with 82%/98% ee.
|
Download:
|
| Scheme 3. Scope of the reaction with anthrones. | |
To demonstrate the potential synthetic power of the present asymmetric copper-catalyzed propargylic substitution in practical organic synthesis, we performed various transformations with substituted anthrone 3aa (Scheme 4). Bromo-alkyne 4 was easily prepared via bromination of terminal alkyne with NBS and AgNO3. Cu-catalyzed azide-alkyne cycloaddition of 3aa with optically active 1-azido-1-deoxy-β-D-flucopyranoside tetraacetate generated cycloadduct 6 with greater than 20:1 diastereoselectivity ratio. Sonagoshira coupling between iodobenzene and 3aa delivered compound 7 in 60% yield. The terminal alkyne group could be hydrogenated with Pd/C under H2, delivering the target hydrogenation product 8 in 85% yield.

|
Download:
|
| Scheme 4. Derivatization of the enantiomerically enriched anthrones. | |
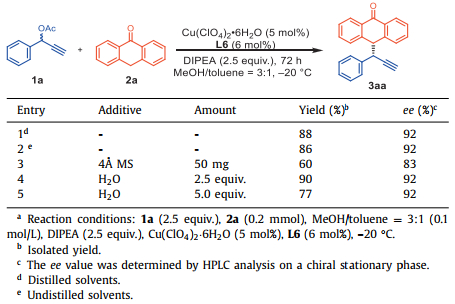
Several control experiments were conducted to test the effect of H2O on the reaction (Table 2). There was no obvious difference between the results obtained with freshly distilled and undistilled solvents, and the reaction proceeded smoothly to deliver the desired product in 86%–88% yields with 92% ee. However, the reactivity and the enantioselectivity of 3aa decreased when 4Å MS was added as an additive to remove trace amount of water in the system. Furthermore, addition of 2.5–5.0 equiv. of H2O to the model reaction led to the product 3aa in 77%–90% yield and 92% ee. The trance amount of H2O in the system is beneficial to both the yield and enantioselectivity of the reaction, which probably enhancing the proton shift in the catalytic cycles. Notably, these findings highlight the water-tolerant nature of the Cu-catalyzed alkylation, which, moreover, is a practical protocol in transition-metal asymmetric synthesis.
|
|
Table 2 The effect of water on the reaction.a |
Based on previously developed methods and past mechanistic studies [65,66], we anticipate that the reaction mechanism proceeds as depicted below (Scheme 5a). In the presence of base and the copper catalyst, the copper acetylide B was generated. Losing the acetate group formed Cu–allenylidene complex D which exists a resonance structure of E bearing a cationic γ-carbon. Subsequently, in the presence of DIPEA, anionic 2a′ was released from the anthrone 2a, which would then undergo a nucleophilic addition reaction with D, via a hydrogen atom shift, providing the Cu-π-alkyne complex G. After the ligand exchange, the desired product 3aa was formed, while regenerating the copper catalyst.

|
Download:
|
| Scheme 5. Proposed mechanism and model for enantioinduction. | |
Meanwhile, based on the observed absolute stereochemistry of the major enantiomer, we proposed a preliminary model for the enantioinduction (Scheme 5b). An edge-to-face aromatic interaction makes a phenyl group of the substrate close to a phenyl group of the ligand in the copper complex. Therefore, nucleophiles favorably attack γ-carbon atoms from Si surface to form (R)-products, while Re surface is hindered by steric hindrance of ligands.
In summary, the highly enantioselective propargylic substitution of propargylic esters with anthrones has been developed by employing a copper–N, N, P-ligand complex as catalyst. A series of anthrone derivatives bearing a terminal alkyne moiety were obtained in high yields with good to excellent enantioselectivities. The utility of this method has been demonstrated by derivatization on the terminal alkyne functionality of the substituted products.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsGenerous financial support from the National Natural Science Foundation of China (No. 21801087), Fundamental Research Funds for the Central Universities CCNU (No. CCNU19QN064), the Ministry of Science and Technology of China (No. 2016YFE0132600), Henan Center for Outstanding Overseas Scientists (No. GZS2020001) and the Key Scientific and Technological Project of Henan Province (No. 212102311068) is gratefully acknowledged.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.009.
| [1] |
H. Prinz, Y. Ishii, T. Hirano, et al., J. Med. Chem. 46 (2003) 3382-3394. DOI:10.1021/jm0307685 |
| [2] |
K. Müller, H. Prinz, J. Med. Chem. 40 (1997) 2780-2787. DOI:10.1021/jm9701785 |
| [3] |
D.W. Cameron, C.E. Skene, Aust. J. Chem. 49 (1996) 617-624. DOI:10.1071/CH9960617 |
| [4] |
D. Wahi, D. Soni, A. Grover, J. Cancer 12 (2021) 652-681. DOI:10.7150/jca.41160 |
| [5] |
Q. Li, J. Gao, X. Pang, A. Chen, Y. Wang, Front. Pharmacol. 11 (2020) 559607. DOI:10.3389/fphar.2020.559607 |
| [6] |
H. Myśliwiec, P. Myśliwiec, A. Baran, I. Flisiak, Adv. Med. Sci. 61 (2016) 207-211. DOI:10.1016/j.advms.2016.01.001 |
| [7] |
J. Kumagai, T. Otsuki, U.V.S. Reddy, et al., Tetrahedron Asymmetry 26 (2015) 1423-1429. DOI:10.1016/j.tetasy.2015.10.022 |
| [8] |
J.F. Bai, Y.L. Guo, L. Peng, et al., Tetrahedron 69 (2013) 1229-1233. DOI:10.1016/j.tet.2012.11.011 |
| [9] |
A. Zea, A.N.R. Alba, N. Bravo, et al., Tetrahedron 67 (2011) 2513-2529. DOI:10.1016/j.tet.2011.02.032 |
| [10] |
C. Wu, W. Li, J. Yang, X. Liang, J. Ye, Org. Biomol. Chem. 8 (2010) 3244-3250. DOI:10.1039/b927421a |
| [11] |
D. Akalay, G. Dürner, M.W. Göbel, Eur. J. Org. Chem. (2008) 2365-2368. DOI:10.1002/ejoc.200800179 |
| [12] |
M. Shi, Z.Y. Lei, M.X. Zhao, J.W. Shi, Tetrahedron Lett. 48 (2007) 5743-5746. DOI:10.1016/j.tetlet.2007.06.107 |
| [13] |
O. Riant, H.B. Kagan, Tetrahedron Lett. 30 (1989) 7403-7406. DOI:10.1016/S0040-4039(00)70709-X |
| [14] |
J. Shen, T.T. Nguyen, Y.P. Goh, et al., J. Am. Chem. Soc. 128 (2006) 13692-13693. DOI:10.1021/ja064636n |
| [15] |
C.C.J. Loh, X. Fang, B. Peters, M. Lautens, Chem. Eur. J. 21 (2015) 13883-13887. DOI:10.1002/chem.201502718 |
| [16] |
Z.L. Zhao, Q. Gu, X.Y. Wu, S.L. You, Chin. Chem. Lett. 27 (2016) 619-622. DOI:10.1016/j.cclet.2016.02.017 |
| [17] |
W.P. Baik, C.H. Yoon, S.H. Koo, et al., Korean Chem. Soc. 25 (2004) 491-500. DOI:10.5012/bkcs.2004.25.4.491 |
| [18] |
Z.L. Li, G.C. Fang, Q.S. Gu, X.Y. Liu, Chem. Soc. Rev. 49 (2020) 32-48. DOI:10.1039/C9CS00681H |
| [19] |
S.W. Roh, K. Choi, C. Lee, Chem. Rev. 119 (2019) 4293-4356. DOI:10.1021/acs.chemrev.8b00568 |
| [20] |
J. Loup, U. Dhawa, F. Pesciaioli, et al., Angew. Chem. Int. Ed. 58 (2019) 12803-12818. DOI:10.1002/anie.201904214 |
| [21] |
F. Wang, P. Chen, G. Liu, Acc. Chem. Res. 51 (2018) 2036-2046. DOI:10.1021/acs.accounts.8b00265 |
| [22] |
F. Zhou, Q. Cai, Beilstein J. Org. Chem. 11 (2015) 2600-2615. DOI:10.3762/bjoc.11.280 |
| [23] |
S. Liao, X.L. Sun, Y. Tang, Acc. Chem. Res. 47 (2014) 2260-2272. DOI:10.1021/ar800104y |
| [24] |
R. Narayan, M. Potowski, Z.J. Jia, et al., Acc. Chem. Res. 47 (2014) 1296-1310. DOI:10.1021/ar400286b |
| [25] |
V.A. Peshkov, O.P. Pereshivko, E.V. Van der Eycken, Chem. Soc. Rev. 41 (2012) 3790-3807. DOI:10.1039/c2cs15356d |
| [26] |
G. Desimoni, G. Faita, K.A. Jørgensen, Chem. Rev. 106 (2006) 3561-3651. DOI:10.1021/cr0505324 |
| [27] |
Y. Gu, T. Wang, M. Gao, Z.J. Yao, Chin. Chem. Lett. 32 (2021) 380-384. DOI:10.1016/j.cclet.2020.02.015 |
| [28] |
H. Wu, N. Ma, M. Song, G. Zhang, Chin. Chem. Lett. 31 (2020) 1580-1583. DOI:10.1016/j.cclet.2019.10.043 |
| [29] |
J. Liu, Z. Fang, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 1332-1336. DOI:10.1016/j.cclet.2019.10.035 |
| [30] |
X.H. Hu, Z.T. Liu, L. Shao, X.P. Hu, Synthesis 47 (2015) 913-923. DOI:10.1055/s-0034-1379968 |
| [31] |
D.Y. Zhang, X.P. Hu, Tetrahedron Lett. 56 (2015) 283-295. DOI:10.1016/j.tetlet.2014.11.112 |
| [32] |
Y. Nishibayashi, Synthesis 44 (2012) 489-503. DOI:10.1055/s-0031-1290158 |
| [33] |
C.H. Ding, X.L. Hou, Chem. Rev. 111 (2011) 1914-1937. DOI:10.1021/cr100284m |
| [34] |
R.J. Detz, H. Hiemstra, J.H. van Maarseveen, Eur. J. Org. Chem. (2009) 6263-6276. DOI:10.1002/ejoc.200900877 |
| [35] |
N. Ljungdahl, N. Kann, Angew. Chem. Int. Ed. 48 (2009) 642-644. DOI:10.1002/anie.200804114 |
| [36] |
Y.W Xu, L. Li, X.P Hu, Org. Lett. 22 (2020) 9534-9538. DOI:10.1021/acs.orglett.0c03587 |
| [37] |
Z.J. Zhang, L. Zhang, R.L. Geng, et al., Angew. Chem. Int. Ed. 58 (2019) 12190-12194. DOI:10.1002/anie.201907188 |
| [38] |
X. Gao, R. Cheng, Y.L. Xiao, X.L. Wan, X. Zhang, Chem 5 (2019) 2987-2999. DOI:10.1016/j.chempr.2019.09.012 |
| [39] |
F.D. Lu, D. Liu, L. Zhu, et al., J. Am. Chem. Soc. 141 (2019) 6167-6172. DOI:10.1021/jacs.9b02338 |
| [40] |
Q. Zhu, B. Meng, C. Gu, et al., Org. Lett. 21 (2019) 9985-9989. DOI:10.1021/acs.orglett.9b03894 |
| [41] |
L. Yang, X. Pu, D. Niu, Z. Fu, X. Zhang, Org. Lett. 21 (2019) 8553-8557. DOI:10.1021/acs.orglett.9b03032 |
| [42] |
Y.W. Xu, X.P. Hu, Org. Lett. 21 (2019) 8091-8096. DOI:10.1021/acs.orglett.9b03081 |
| [43] |
Z. Fu, N. Deng, S.N. Su, et al., Angew. Chem. Int. Ed. 57 (2018) 15217-15221. DOI:10.1002/anie.201809391 |
| [44] |
Y.C. Zhang, Z.J. Zhang, L.F. Fan, J. Song, Org. Lett. 20 (2018) 2792-2795. DOI:10.1021/acs.orglett.8b01101 |
| [45] |
K. Zhang, L.Q. Lu, S. Yao, et al., J. Am. Chem. Soc. 139 (2017) 12847-12854. DOI:10.1021/jacs.7b08207 |
| [46] |
K. Tsuchida, Y. Senda, K. Nakajima, Y. Nishibayashi, Angew. Chem. Int. Ed. 55 (2016) 9728-9732. DOI:10.1002/anie.201604182 |
| [47] |
L. Shao, Y.H. Wang, D.Y. Zhang, J. Xu, X.P. Hu, Angew. Chem. Int. Ed. 55 (2016) 5014-5018. DOI:10.1002/anie.201510793 |
| [48] |
R.Z. Li, D.Q. Liu, D. Niu, Nat. Catal. 3 (2020) 672-680. DOI:10.1038/s41929-020-0462-9 |
| [49] |
J.E. Gómez, À. Cristòfol, A.W. Kleij, Angew. Chem. Int. Ed. 58 (2019) 3903-3907. DOI:10.1002/anie.201814242 |
| [50] |
J.E. Gómez, W. Guo, S. Gaspa, A.W. Kleij, Angew. Chem. Int. Ed. 56 (2017) 15035-15038. DOI:10.1002/anie.201709511 |
| [51] |
R.Z. Li, H. Tang, K.R. Yang, et al., Angew. Chem. Int. Ed. 56 (2017) 7213-7217. DOI:10.1002/anie.201703029 |
| [52] |
K. Nakajima, M. Shibata, Y. Nishibayashi, J. Am. Chem. Soc. 137 (2015) 2472-2475. DOI:10.1021/jacs.5b00004 |
| [53] |
R.J. Detz, M.M.E. Delville, H. Hiemstra, J.H. van Maarseveen, Angew. Chem. Int. Ed. 47 (2008) 3777-3780. DOI:10.1002/anie.200705264 |
| [54] |
J.T. Xia, X.P. Hu, Org. Lett. 22 (2020) 1102-1107. DOI:10.1021/acs.orglett.9b04621 |
| [55] |
J. Zhang, T. Ni, W.L. Yang, W.P. Deng, Org. Lett. 22 (2020) 4547-4552. DOI:10.1021/acs.orglett.0c01594 |
| [56] |
L. Shao, X.P. Hu, Chem. Comm. 53 (2017) 8192-8195. DOI:10.1039/C7CC03034G |
| [57] |
Q. Wang, T.R. Li, L.Q. Lu, et al., J. Am. Chem. Soc. 138 (2016) 8360-8363. DOI:10.1021/jacs.6b04414 |
| [58] |
W. Shao, H. Li, C. Liu, C.J. Liu, S.L. You, Angew. Chem. Int. Ed. 54 (2015) 7684-7687. DOI:10.1002/anie.201503042 |
| [59] |
L. Zhao, G. Huang, B. Guo, et al., Org. Lett. 16 (2014) 5584-5587. DOI:10.1021/ol502615y |
| [60] |
G. Hattori, K. Sakata, H. Matsuzawa, et al., J. Am. Chem. Soc. 132 (2010) 10592-10608. DOI:10.1021/ja1047494 |
| [61] |
F.L. Zhu, Y. Zou, D.Y. Zhang, et al., Angew. Chem. Int. Ed. 53 (2014) 1410-1414. DOI:10.1002/anie.201309182 |
| [62] |
J. Huang, H.H. Kong, S.J. Li, et al., Chem. Comm. 57 (2021) 4674-4677. DOI:10.1039/D1CC00663K |
| [63] |
S.J. Li, J. Huang, J.Y. He, et al., RSC Adv. 10 (2020) 38478-38483. DOI:10.1039/D0RA07698H |
| [64] |
H. Xu, L. Laraia, L. Schneider, et al., Angew. Chem. Int. Ed. 56 (2017) 11232-11236. DOI:10.1002/anie.201706005 |
| [65] |
R.J. Detz, M.M.E. Delville, H. Hiemstra, J.H. van Maarseveen, Angew. Chem. Int. Ed. 47 (2008) 3777-3780. DOI:10.1002/anie.200705264 |
| [66] |
G. Hattori, K. Sakata, Y. Nishibayashi, et al., J. Am. Chem. Soc. 132 (2010) 10592-10608. DOI:10.1021/ja1047494 |
 2022, Vol. 33
2022, Vol. 33