b Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou 350207, China;
c CNRS UMR 6014 COBRA, Normandie Université, Mont Saint Aignan 76821, France
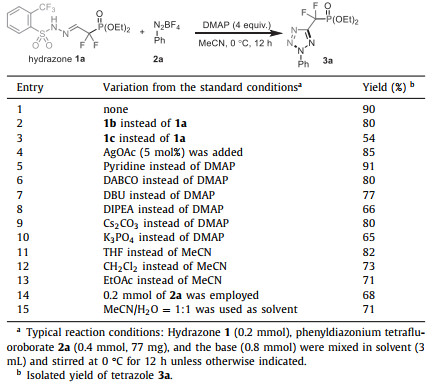
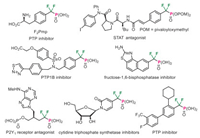
Difluoromethylene phosphonates [DFMP, CF2PO(OR)2] could mimic the naturally occurring phosphates and phosphonates, thus having emerged as a frequently utilized structural motif in the study of various biochemical processes [1, 2]. For example, phosphonodifluoromethyl phenylalanine (F2Pmp) has been developed as one of the most powerful nonhydrolyzable phosphotyrosine mimetics to modulate the corresponding protein-protein interactions and used as protein tyrosine phosphatases (PTPs) inhibitors (Fig. 1) [3-5]. Furthermore, the difluoromethylene phosphonate moiety has been also utilized in the design and development of signal transducer and activator of transcription (STAT) antagonists, fructose-1, 6-bisphosphatase (FBPase) inhibitors, P2Y1 receptor antagonists, and cytidine triphosphate synthetase inhibitors (Fig. 1) [6-10]. Therefore, the design and development of new difluoromethylene phosphonate-containing molecular frameworks has emerged as an increasingly important task in synthetic organic chemistry. In this context, while simple aryl-substituted difluoromethylene phosphonates have been extensively studied in the past few decades [11-17], the construction of heterocyclic difluoromethylene phosphonates remains far less explored. More importantly, most previous studies have mainly focused on the use of metalated CF2PO(OR)2 derivatives to undergo cross-coupling-type transformations, while the development of new reactive DFMP-containing 1, 3-dipoles to be used in convergent cycloadditions remains elusive. Very recently, Han, Röschenthaler, and co-workers reported the design of aryl-substituted difluoromethylene phosphonate-containing diazo reagents (DFMP-Diazo, Scheme 1a) [18, 19]. The [3 + 2] cycloaddition reaction of DFMP-Diazo with vinyl sulfones enabled the synthesis of difluoromethylene phosphonate-containing pyrazolines with good efficiency. However, due to the instability of DFMP-containing diazo compounds, the utilization of unsubstituted DFMP-functionalized 1, 3-dipolar species to produce aromatic cycloadducts is an unsolved problem. As part of our long interest in fluorinated diazoalkanes [20-28]and the construction of tetrazoles (Scheme 1b) [29-33], herein we report the preparation of three bench-stable DFMP-containing diazo precursors (DFMP-Hydrazones) and their application in the regioselective synthesis of DFMP-tetrazoles via [3 + 2] cycloaddition reactions with aryldiazonium salts (Scheme 1c). Note that this study represents the first example of introducing a difluoromethylene phosphonate motif onto the tetrazole scaffold. These two chemical entities are both metabolically stable to many of the biological transformations and have widespread implications in pharmaceuticals and bioconjugations [34-36].
|
Download:
|
| Fig. 1. Selected bio-active difluoromethylene phosphonates. | |
|
Download:
|
| Scheme 1. Cycloaddition reactions for the preparation of difluoromethylene phosphonate heterocycles. | |
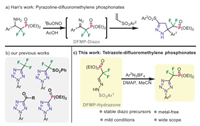
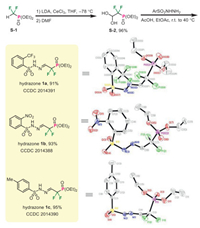
At the outset, we synthesized three DFMP-hydrazones via the condensation of DFMP-aldehyde precursor S-2 with the corresponding benzenesulfonyl hydrazides. As outlined in Scheme 2, treating commercially available diethyl (difluoromethyl)-phosphonate S-1 with LDA in the presence of CeCl3, followed by addition of N, N-dimethylformamide (DMF) in one-pot, the desired dihydrate S-2 was smoothly obtained in 96% yield [37, 38]. Subsequently, condensation of this masked aldehyde S-2 with different hydrazides under acidic conditions proved to be viable, thus giving the target DFMP-hydrazones 1a–1c in 91%–95% yields [39, 40]. Importantly, these diazo precursors were found to be quite stable under bench conditions, and all of them have been successfully crystallized for X-ray diffraction analysis.
|
Download:
|
| Scheme 2. Preparation of difluoromethylene phosphonate hydrazones. | |
With the three difluoromethylene phosphonate hydrazones in hand, we then proceeded to optimize the [3 + 2] cycloaddition reaction with phenyldiazonium salt 2a (Table 1) [41]. Pleasingly, by just using 4-dimethylaminopyridine (DMAP) as the base, acetonitrile as the solvent, the desired 2, 5-disubstituted tetrazole 3a was obtained in up to 90% yield from CF3-hydrazone 1a without the detection of any regioisomer (entry 1). Employing NO2-hydrazone 1b or Me-hydrazone 1c could also permit the formation of tetrazole 3a, albeit in decreased yields (entries 2 and 3). The significantly lower yield observed when using Me-hydrazone 1c could be the result of a lower leaving-group ability of its benzenesulfonyl moiety with respect to 1a and 1b. Notably, this reaction does not require the use of an organometallic species and thus should not involve a carbenoid intermediate that is clearly indicative of a different mechanism compared with our previous studies on the silver-catalyzed synthesis of differently decorated tetrazoles (see Supporting Information for a proposed mechanism) [42]. Addition of silver acetate to the reaction mixture was not beneficial (entry 4). Changing DMAP to a series of organic and inorganic bases resulted in no obvious improvement (entries 5–10). This cycloaddition transformation was also found to be compatible with different solvents such as THF, CH2Cl2, and EtOAc (entries 11–13). Reducing the amount of diazonium salt 2a to 1 equiv. could still generate 3a at the expense of a relatively lower yield (entry 14). Using water as a co-solvent was found be viable, albeit in slightly decreased yield (entry 15).
|
|
Table 1 Optimization of reaction conditions. |
This DMAP-promoted [3 + 2] cycloaddition protocol proved to be quite general with respect to the scope of aryldiazonium salts (Scheme 3). Electron-donating groups substituted at the benzene ring at different positions, including alkyl, alkoxyl, and amino groups, all reacted well with hydrazone 1a under identical conditions (products 3b–3i). Incorporation of another phenyl group turned out be no problem (products 3j–3k). Fluorine, bromine, and iodine atoms substituted at the para, ortho, and meta locations on the phenyl ring were all well tolerated and afforded the corresponding tetrazoles 3l–3u in up to 92% yield. Furthermore, strong electron-withdrawing substituents such as -Ac, -CN, -CO2Me and -NO2, had no obvious influence on the reaction performance (products 3v–3y). 1-Naphthyl-, 3-thienyl-, and 8-quinolinyl-derived tetrazol-5-yl difluoromethylene phosphonates 3z–3b′ were also obtained with exclusive regioselectivity, albeit with low yield in the case of basic 8-quinolinyl product 3b′. 7-Coumarin-diazonium salt also underwent the cycloaddition with hydrazone 1a under standard conditions, thus delivering the coumarin-tetrazole 3c′ in 72% yield (X-ray confirmed). Importantly, this cycloaddition reaction also tolerates alkynyl and alkenyl moieties as exemplified by the smooth generation of cycloadducts 3d′ and 3e′ in good yields. The presence of protected amino or free carboxylic group in aryldiazonium salts could be also tolerated, giving the corresponding tetrazoles 3f′ and 3g′ with good result.
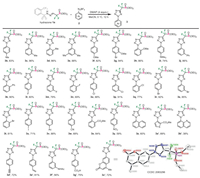
|
Download:
|
| Scheme 3. Substrate scope of 2-aryl-2H-tetrazol-5-yl difluoromethylene phosphonates. | |
To further illustrate the utility of this protocol, we conducted cycloaddition reactions with several pharmacophore-derived aryldiazonium salts (Scheme 4). Aniline derivative featuring the Lapatinib's substructure was easily converted to the diazonium salt and cyclized with hydrazone 1a with good result (product 3h′). Despite the presence of free amide group, Pomalidomide-derived diazonium salt was also a feasible substrate for this reaction and gave access to the corresponding difluoromethylene phosphonate 3i′ in 66% yield. More noteworthy is that phenylalanine-derived diazonium salt 2j′ smoothly participated in the cycloaddition process, thereby providing the noncanonical amino acid 3j′ in good yield with maintained complete regio-selectivity. Finally, the phosphonate group in 3a was successfully hydrolyzed with the aid of TMSBr, affording the free phosphonic acid 4a in 92% yield (Scheme 5).
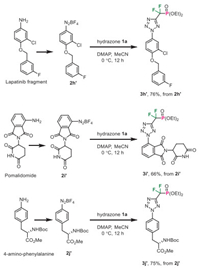
|
Download:
|
| Scheme 4. Preparation of pharmacophore-derived tetrazolyl difluoromethylene phosphonates. | |
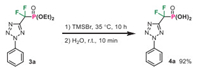
|
Download:
|
| Scheme 5. Hydrolysis of difluoromethylene phosphonate 3a. | |
In summary, we have developed a bench-stable difluoromethylene phosphonate hydrazone to serve as the corresponding diazo precursor and established a metal-free [3 + 2] cycloaddition transformation with aryldiazonium salts. A number of 2-aryl-2H-tetrazol-5-yl difluoromethylene phosphonates were obtained in good yields with single regioselectivity under mild conditions. Future studies will focus on the reaction mechanism and products applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21772142, 21901181 and 21961142015), the National Key Research and Development Program of China (No. 2019YFA0905100), Tianjin Municipal Science & Technology Commission (No. 19JCQNJC04700), and the CNRS in France.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.007.
| [1] |
M. Shevchuk, Q. Wang, R. Pajkert, et al.
, Adv. Synth. Catal. 363 (2021) 2912-2968. DOI:10.1002/adsc.202001464 |
| [2] |
J. A. Ma, D. Cahard, Emerging Fluorinated Motifs: Synthesis, Properties and Applications, Wiley-VCH, Weinheim, 2020
|
| [3] |
T.R. Burke, H.K. Kole, P.P. Roller, Biochem. Biophys. Res. Commun. 204 (1994) 129-134. DOI:10.1006/bbrc.1994.2435 |
| [4] |
A.P. Combs, J. Med. Chem. 53 (2010) 2333-2344. DOI:10.1021/jm901090b |
| [5] |
I.G. Boutselis, X. Yu, Z.Y. Zhang, R.F. Borch, J. Med. Chem. 50 (2007) 856-864. DOI:10.1021/jm061146x |
| [6] |
C.P. Holmes, X. Li, Y. Pan, et al.
, Bioorg. Med. Chem. Lett. 18 (2008) 2719-2724. DOI:10.1016/j.bmcl.2008.03.007 |
| [7] |
T. Tsukada, M. Takahashi, T. Takemoto, et al.
, Bioorg. Med. Chem. Lett. 19 (2009) 5909-5912. DOI:10.1016/j.bmcl.2009.08.081 |
| [8] |
H.S. Kim, D. Barak, T.K. Harden, et al.
, J. Med. Chem. 44 (2001) 3092-3108. DOI:10.1021/jm010082h |
| [9] |
S.D. Taylor, F. Mirzaei, A. Sharifi, S.L. Bearne, J. Org. Chem. 71 (2006) 9420-9430. DOI:10.1021/jo0617666 |
| [10] |
P.K. Mandal, P. Morlacchi, J.M. Knight, et al.
, J. Med. Chem. 58 (2015) 8970-8984. DOI:10.1021/acs.jmedchem.5b01321 |
| [11] |
H.Q. Cao, J.K. Li, F.G. Zhang, D. Cahard, J.A. Ma, Adv. Synth. Catal. 363 (2021) 688-729. DOI:10.1002/adsc.202001345 |
| [12] |
F.G. Zhang, X.Q. Wang, Y. Zhou, et al.
, Chem. Eur. J. 26 (2020) 15378-15396. DOI:10.1002/chem.202003416 |
| [13] |
L. Ruyet, T. Besset, Beilstein J. Org. Chem. 16 (2020) 1051-1065. DOI:10.3762/bjoc.16.92 |
| [14] |
M.V. Ivanova, A. Bayle, T. Besset, X. Pannecoucke, T. Poisson, Chem. Eur. J. 23 (2017) 17318-17338. DOI:10.1002/chem.201703542 |
| [15] |
M.V. Ivanova, A. Bayle, T. Besset, T. Poisson, X. Pannecoucke, Angew. Chem. Int. Ed. 54 (2015) 13406-13410. DOI:10.1002/anie.201507130 |
| [16] |
Z. Feng, Q.Q. Min, Y.L. Xiao, B. Zhang, X. Zhang, Angew. Chem. Int. Ed. 53 (2014) 1669-1673. DOI:10.1002/anie.201309535 |
| [17] |
L. Wang, X.J. Wei, W.L. Lei, et al.
, Chem. Commun. 50 (2014) 15916-15919. DOI:10.1039/C4CC07925F |
| [18] |
H. Mei, L. Wang, R. Pajkert, et al.
, Org. Lett. 23 (2021) 1130-1134. DOI:10.1021/acs.orglett.1c00150 |
| [19] |
J. Liu, J. Xu, R. Pajkert, et al.
, Acta Chim. Sin. 79 (2021) 747-750. DOI:10.6023/a21030096 |
| [20] |
P.K. Mykhailiuk, Chem. Rev. 120 (2020) 12718-12755. DOI:10.1021/acs.chemrev.0c00406 |
| [21] |
F. Li, J. Nie, L. Sun, Y. Zheng, J.A. Ma, Angew. Chem. Int. Ed. 52 (2013) 6255-6258. DOI:10.1002/anie.201301870 |
| [22] |
F.G. Zhang, Y. Wei, Y.P. Yi, J. Nie, J.A. Ma, Org. Lett. 16 (2014) 3122-3125. DOI:10.1021/ol501249h |
| [23] |
Z. Chen, Y. Zheng, J.A. Ma, Angew. Chem. Int. Ed. 56 (2017) 4569-4574. DOI:10.1002/anie.201700955 |
| [24] |
J.L. Zeng, Z. Chen, F.G. Zhang, J.A. Ma, Org. Lett. 20 (2018) 4562-4565. DOI:10.1021/acs.orglett.8b01854 |
| [25] |
Z.Q. Zhang, M.M. Zheng, X.S. Xue, et al.
, Angew. Chem. Int. Ed. 58 (2019) 18191-18196. DOI:10.1002/anie.201911701 |
| [26] |
Y. Ouyang, F.L. Qing, Chin. J. Org. Chem. 40 (2020) 806-807. DOI:10.6023/cjoc202000011 |
| [27] |
Z. Chen, N. Ren, X. Ma, et al.
, ACS Catal. 9 (2019) 4600-4608. DOI:10.1021/acscatal.9b00846 |
| [28] |
Z. Chai, J.P. Bouillon, D. Cahard, Chem. Commun. 48 (2012) 9471-9473. DOI:10.1039/c2cc35246j |
| [29] |
Z. Chen, S.Q. Fan, Y. Zheng, J.A. Ma, Chem. Commun. 51 (2015) 16545-16548. DOI:10.1039/C5CC07324C |
| [30] |
X. Peng, M.Y. Xiao, J.L. Zeng, F.G. Zhang, J.A. Ma, Org. Lett. 21 (2019) 4808-4811. DOI:10.1021/acs.orglett.9b01697 |
| [31] |
S.J. Zhai, X. Peng, F.G. Zhang, J.A. Ma, Org. Lett. 21 (2019) 9884-9888. DOI:10.1021/acs.orglett.9b03800 |
| [32] |
M.Y. Xiao, M.M. Zheng, X. Peng, et al.
, Tetrahedron 76 (2020) 131063. DOI:10.1016/j.tet.2020.131063 |
| [33] |
X.Y. Liu, S.J. Zhai, F.F. Feng, F.G. Zhang, J.A. Ma, ChemCatChem 12 (2020) 5623-5626. DOI:10.1002/cctc.202001143 |
| [34] |
F.G. Zhang, X. Peng, J.A. Ma, Chin. J. Org. Chem. 39 (2019) 109-116. DOI:10.6023/cjoc201808007 |
| [35] |
C.G. Neochoritis, T. Zhao, A. Dömling, Chem. Rev. 119 (2019) 1970-2042. DOI:10.1021/acs.chemrev.8b00564 |
| [36] |
R.K.V. Lim, Q. Lin, Acc. Chem. Res. 44 (2011) 828-839. DOI:10.1021/ar200021p |
| [37] |
T.P. Lequeux, J.M. Percy, J. Chem. Soc. Chem. Commun. 1995 (1995) 2111-2112. |
| [38] |
R. Pajkert, G.V. Röschenthaler, J. Org. Chem. 78 (2013) 3697-3708. DOI:10.1021/jo400198a |
| [39] |
Y. Ning, X. Zhang, Y. Gai, et al.
, Angew. Chem. Int. Ed. 59 (2020) 6473-6481. DOI:10.1002/anie.202000119 |
| [40] |
X. Zhang, Z. Liu, X. Yang, et al.
, Nat. Commun. 10 (2019) 284-292. DOI:10.1038/s41467-018-08253-z |
| [41] |
F.G. Zhang, Z. Chen, C.W. Cheung, J.A. Ma, Chin. J. Chem. 38 (2020) 1132-1152. DOI:10.1002/cjoc.202000270 |
| [42] |
M.Y. Xiao, M.M. Zheng, X. Peng, et al.
, Org. Lett. 22 (2020) 7762-7767. DOI:10.1021/acs.orglett.0c03025 |
 2022, Vol. 33
2022, Vol. 33